- Academic Editor
†These authors contributed equally.
Background: To evaluate the effect of carbohydrate antigen 125 (CA125)
and CA19-9 in distinguishing stage Ⅲ and Ⅳ endometriosis from benign and
malignant tumors, and to explore whether it is related to the clinical features
of the disease. Methods: In a retrospective cohort study based on
clinical data from hospitals, a total of 183 patients with pathologically
confirmed diagnosis of ovarian endometriotic cysts (OEC) in Hainan Provincial
People’s Hospital for surgical treatment from January 2019 to August 2022 were
selected as the case group, and a total of 276 cases of benign diseases,
including 184 cases of benign ovarian tumors, 94 cases of gynecological common
diseases, and 102 cases of malignant ovarian tumors were selected as the control
group, with a total of 276 cases of benign diseases, including 184 cases of
benign ovarian tumors, 94 cases of gynecological common diseases, and 102 cases
of malignant ovarian tumors. There were also 23 cases of ruptured ectopic cysts.
We compared the clinical characteristics (age of onset, fertility, dysmenorrhea,
preoperative CA125 and CA19-9 values) of the patients in the OEC group with those
of the other control groups; analyzed the serum CA125 and CA19-9 values in
relation to the pathological characteristics of OEC (recurrence, unilateral and
bilaterality, multilocularity and unilocularity, rupture, dysmenorrhea,
fertility, and staging); and analyzed the CA125 and CA19-9 values by unordered
logistic regression, CA19-9 to predict OEC; sensitivity, specificity and cut-off
values of CA125, CA19-9 and their combined indexes to diagnose OEC.
Results: The symptoms of dysmenorrhea and infertility in OEC group were
significantly higher than those in the other three groups. The preoperative CA125
value in OEC group was higher than that in benign tumor and other gynecological
diseases group, and significantly lower than that in malignant tumor group. There
was no significant difference in the value of CA19-9 and CA125 in the degree of
dysmenorrhea, recurrence and infertility. The values of CA19-9 and CA125 of
multilocular cysts were higher than those of unicameral cysts, bilateral cysts
were higher than unilateral cysts, and ruptured cysts were significantly higher
than unruptured cysts. The value of CA125 in the dysmenorrhea group was higher
than that in the non-dysmenorrhea group, and that in the fourth stage was higher
than that in the third stage, and the difference was statistically significant
(p
Endometriosis (EMT), is an estrogen-dependent disease in which functional endometrial tissue exists and grows outside the uterine cavity. Common symptoms include infertility, dysmenorrhea, chronic pelvic pain, sexual discomfort and defecation pain, affecting 10%–15% of women of childbearing age. EMT is a risk factor for ovarian cancer and some reports suggest that ovarian endometrioid adenocarcinoma and ovarian clear cell adenocarcinoma originate from ovarian endometriosis [1, 2]. There are three main types of EMT: ovarian endometriotic cyst (OEC), superficial peritoneal endometriosis and deep invasive endometriosis (DIE) [3, 4, 5]. OEC is the most common clinical type, accounting for 17%–44% of all endometriosis [6]. Laparoscopy is the gold standard for the diagnosis of EMT [7]. It is an invasive examination with high cost, surgical risk and the possibility of postoperative adhesion. Transvaginal ultrasound and magnetic resonance imaging can diagnose ectopic disease. The sensitivity and specificity of transvaginal ultrasound and magnetic resonance imaging are similar to those of surgery, and the accuracy of examination is highly related to the personal skills of doctors [8, 9]. Carbohydrate antigen 125 (CA125) and CA19-9 are commonly used tumor markers in clinic. The purpose of this study was to review the expression of CA125 and CA19-9 in stage Ⅲ and Ⅳ endometriosis, and to evaluate the effect of these two tumor markers in differentiating stage Ⅲ and Ⅳ endometriosis from benign and malignant tumors.
This was a retrospective cohort study conducted in Hainan Provincial People’s
Hospital, and a total of 183 patients with pathologically confirmed diagnosis of
OEC in surgical treatment at Hainan Provincial People’s Hospital, China, from
January 2019 to August 2022 were selected as the case group; a total of 276 cases
of benign diseases, including 184 benign ovarian tumors, 94 cases of
gynecological general diseases (57 cases of uterine fibroids, 14 cases of
endometrial polyps, and 23 cases of inflammatory diseases of the pelvis) and 102
cases of malignant ovarian tumors were selected as the control group. There were
also 23 cases of OEC rupture. The staging method proposed by the American
Fertility Society (r-AFS) was used as a criterion for staging the group of cases.
Stage Ⅰ (Minimal) 1–5, stage II (Mild) 6–15, stage III (Moderate) 16–40, stage
IV (Severe)
| Age | Dysmenorrhea | Infertility | CA19-9 | CA125 | ||
| OEC n = 183 | 32 (28–38) | 69 (37.7%) | 19 (10.4%) | 30.67 (9.54–68.35) | 54 (31–108.3) | |
| 253.676/0.000 | 22.602/0.000 | |||||
| Benign tumor n = 182 | 31 (26–40) | 12 (6.6%) | 2 (1.1%) | 11.96 (4.47–31.69) | 16.55 (12.82–21.43) | |
| OEC vs. p value | 1.0 | 0.000 | 0.000 | |||
| Ovarian teratoma: a report of 97 cases | 22.63 (6.81–47.75) | 15.9 (13.3–21.0) | ||||
| 10 cases of sex cord stromal tumor | 9.64 (4.19–13.63) | 19.8 (14.97–28.02) | ||||
| Serous mucinous cystadenoma: a report of 12 cases | 7.63 (2.0–19.07) | 16.1 (11.15–24.27) | ||||
| Serous cystadenoma: a report of 25 cases | 7.76 (4.34–14.94) | 15.0 (10.45–23.75) | ||||
| Mucinous cystadenoma: a report of 38 cases | 8.46 (2.42–18.9) | 16.6 (11.9–23.57) | ||||
| Malignant tumor n = 102 | 51 (43–59) | 6 (5.9%) | 2 (2.0%) | 10.47 (3.32–32.09) | 540.1 (144.5–1000) | |
| OEC vs. p value | 0.000 | 0.000 | 0.000 | |||
| 70 cases of serous carcinoma of ovary | 7.27 (3.12–19.71) | 980.0 (374.5–1000) | ||||
| Mucinous ovarian carcinoma: a report of 5 cases | 16.15 (2.13–22.56) | 113.2 (14.85–333.2) | ||||
| 12 cases of endometrioid carcinoma of ovary | 111.7 (13.22–788.7) | 229.8 (141.0–691.6) | ||||
| Borderline ovarian tumors: a report of 15 cases | 24.2 (3.99–52.44) | 64.7 (26.4–265.9) | ||||
| Other gynaecology n = 94 | 36 (30.75–40) | 5 (5.4%) | 2 (2.1%) | 6.69 (2.71–14.46) | 13.88 (10.6–19.67) | |
| OEC vs. p value | 0.84 | 0.000 | 0.000 | |||
| 57 cases of uterine leiomyoma | 7.34 (2.69–14.55) | 13.96 (11.85–19.95) | ||||
| 14 cases of endometrial polyps | 3.91 (2.0–10.96) | 12.3 (9.77–15.42) | ||||
| 23 cases of pelvic inflammatory diseases | 6.9 (3.67–13.15) | 16.0 (9.7–23.1) | ||||
OEC, ovarian endometriotic cysts; CA125, carbohydrate antigen 125.
| Break time * | CA19-9 | CA125 |
| 512.44 (310.88–1200) | 963.7 (542.2–1000) | |
| 437.08 (79.25–857.2) | 210.6 (147.15–900) | |
| 72.91 (26.61–191.67) | 217.7 (104.1–366.4) |
*Break time: the time from the onset of acute abdominal pain to admission for surgery. All of the 23 patients had occasional acute severe abdominal pain and were found to have a tear in the cyst or a large amount of cyst fluid in the abdominal peritoneum.
Inclusion criteria: ① operated in our hospital and confirmed by postoperative pathology; ② the case group was mainly diagnosed as OEC; ③ the medical history is complete; ④ the patients in the case group did not use corticosteroids within 6 months before operation; ⑤ both the case and control groups were operated on electively, and the patients were in the proliferative stage of endometrium.
Exclusion criteria: ① the case and the control group were complicated with severe chronic diseases, accompanied by severe systemic diseases such as heart, brain, lung, kidney, liver insufficiency and thyroid dysfunction; ② those with incomplete medical history; ③ the disease occurred in the same case in the case group and the control group. This study was approved by the Ethics Committee of Hainan Provincial people’s Hospital (approval number: Med-Eth-Re [2023] 145).
Whole blood was collected from surgical patients 1 day before surgery and sent to the Laboratory Department, then CA125 and CA19-9 were detected by enzyme-linked immunosorbent assay (ELISA) luminescence. Comparison of clinical characteristics (age of onset, fertility, dysmenorrhea, preoperative CA125 and CA19-9 values) between patients in the OEC group and other control patients; analysis of serum CA125 and CA19-9 values and pathological characteristics of OEC (recurrent, unilateral, bilaterally, multilocular unilocular, rupture, dysmenorrhea, fertility, and staging); analysis of age, dysmenorrhea, infertility, CA125, and CA19-9 to predict OEC by unordered logistic regression; and sensitivity, specificity, and cut-off value of CA125 to diagnose OEC.
Statistical processing SPSS 25.0 software (IBM Corp., Armonk, NY, USA) for data
analysis. The measurement data did not conform to the normal distribution in
terms of median and quartile spacing. Mann-Whitney U rank sum test was used for
comparison between the two groups, and Kruskal-Wallis test was used for
comparison between multiple groups. The counting data were expressed by the
number of cases, and the comparison between groups was made by
The CA19-9 levels in the OEC group were 30.67 (9.54–68.35) U/mL, and CA125 levels were 54 (31–108.3) U/mL. The CA19-9 levels in other benign tumors, malignant tumors, and other gynecological groups were 11.96 (4.47–31.69) U/mL, 10.47 (3.32–32.09) U/mL, and 6.69 (2.71–14.46) U/mL, respectively; CA125 is 16.55 (12.82–21.43) U/mL, 540.1 (144.5–1000) U/mL, and 13.88 (10.6–19.67) U/mL, respectively. The symptoms of dysmenorrhea and infertility in OEC group were significantly higher than those in the other three groups (p = 0.000), and the preoperative CA19-9 value in OEC group was significantly higher than that in the other three groups (p = 0.000). The preoperative CA125 value in OEC group was higher than that in benign tumor group and other gynecological disease group, but significantly lower than that in malignant tumor group (p = 0.000). See Table 1.
The age of onset in the malignant tumor group was significantly higher than that in the other three groups, and the difference was statistically significant (p = 0.000). After adjusting the age of patients in malignant tumor group and OEC group, malignant tumors were divided into three subgroups and compared with OEC group. In serous ovarian cancer, CA19-9 was lower than OEC group, CA125 was higher than OEC group, the difference was statistically significant. CA19-9 and CA125 in ovarian endometrioid carcinoma were higher than those in OEC group, and the difference was statistically significant. There was no significant difference in CA19-9 and CA125 between borderline tumors and OEC group. See Table 3.
| Age | p value | CA19-9 | p value | CA125 | p value | ||
| OEC n = 35 | 46 (42–48) | 43.71 (10.9–126.9) | 46.8 (24.7–109.9) | ||||
| Serous carcinoma, n = 43 | 48 (43–51) | 0.196 | 9.89 (2.41–22.2) | 0.001 | 913.3 (300–1000) | 0.000 | |
| OEC n = 18 | 48 (46.7–50) | 40.29 (9.42–89.8) | 44.5 (24.7–120.9) | ||||
| Endometrioid carcinoma, n = 12 | 50.5 (46.2–58.7) | 0.305 | 111.7 (13.2–788.7) | 0.15 | 229.8 (141.05–691.6) | 0.001 | |
| OEC n = 10 | 43 (26.25–50.25) | 44.72 (18.58–89.69) | 77.7 (35.25–112.27) | ||||
| Mucinous ovarian carcinoma, n = 5 | 43 (25–54) | 0.951 | 16.15 (2.13–22.56) | 0.075 | 113.2 (14.85–333.2) | 0.540 | |
| OEC n = 73 | 32 (28–38.5) | 36.87 (9.45–71.29) | 54.7 (29.35–105.05) | ||||
| Borderline tumor, n = 15 | 33 (25–51) | 0.356 | 24.27 (3.99–52.44) | 0.328 | 64.7 (26.4–265.9) | 0.495 | |
There was no significant difference in the value of CA19-9 and CA125 in the
degree of dysmenorrhea, recurrence and infertility (p
| CA19-9 | p | CA125 | p | ||
| Dysmenorrhea | |||||
| Yes (n = 69) | 25.4 (6.64–74.58) | 74.5 (35.1–126.9) | |||
| None (n = 111) | 35.14 (10.78–67.64) | 0.647 | 50.5 (31–87.3) | 0.016* | |
| Degree of dysmenorrhea | |||||
| Bearable (n = 42) | 23.60 (5.6–75.74) | 67.75 (30.15–134.72) | |||
| Intolerable (n = 27) | 40.16 (11.03–74.24) | 0.423 | 75.8 (44.5–119.2) | 0.740 | |
| Relapse★ | |||||
| Yes (n = 11) | 22.63 (7.45–48.1) | 59.7 (35.2–94.7) | |||
| No (n = 172) | 31.41 (9.55–69.52) | 0.499 | 53.5 (30.85–109.5) | 0.587 | |
| Package block | |||||
| Single room (n = 158) | 26.03 (9.15–65.17) | 49.05 (29. 42–88.52) | |||
| multi room (n = 25) | 55.29 (22.63–140.75) | 0.030* | 90.5 (65.15–127.25) | 0.000* | |
| Unilateral (n = 121) | 25.19 (8.93–51.5) | 44 (27.55–82.4) | |||
| Both sides (n = 62) | 49.25 (15.05–116.16) | 0.032* | 80.05 (48.37–152.2) | 0.000* | |
| Rupture (n = 23) | 191.67 (59.71–514.47) | 347.9 (143.1–800) | |||
| Unbroken (n = 183) | 30.67 (9.54–68.35) | 0.000* | 54 (31–108.3) | 0.000* | |
| Infertility | |||||
| Yes (n = 19) | 26.7 (6.89–43.65) | 60.0 (31.8–108.3) | |||
| No (n = 163) | 30.67 (9.54–69.92) | 0.421 | 53.1 (30.8–109.9) | 0.64 | |
| Staging | |||||
| Ⅲ (n = 88) | 25.29 (9.41–44.73) | 44.2 (28.22–83.37) | |||
| Ⅳ (n = 95) | 41.53 (9.58–84.77) | 0.105 | 65.6 (35.7–119.2) | 0.005* | |
*p value
In the comparison of OEC with benign diseases: CA19-9 was not statistically significant; higher CA125 had a statistically significant higher risk of developing endometriosis [odds ratio (OR) = 0.966; 95% confidence interval (95% CI): 0.958–0.974].
In the comparison of OEC with malignancy: CA19-9 was not statistically significant; higher CA125 had a statistically significant higher risk of malignancy [OR = 1.007; 95% CI: 1.005–1.010], see Table 5.
| Disease type | Variable | Standard error | Wald | p | OR | 95% CI | ||
| Benign disease | CA19-9 | 0.000 | 0.001 | 0.100 | 0.751 | 1.000 | 0.998 | 1.001 |
| CA125 | –0.034 | 0.004 | 63.616 | 0.000* | 0.966 | 0.958 | 0.974 | |
| Malignant tumor | CA19-9 | 0.001 | 0.001 | 0.522 | 0.470 | 1.001 | 0.999 | 1.002 |
| CA125 | 0.007 | 0.001 | 39.329 | 0.000* | 1.007 | 1.005 | 1.010 | |
*p value
Sensitivity, specificity and cut-off values were calculated by using receiver operating characteristic (ROC) curves, see Table 6. In the benign disease control group the cut-off value of CA125 was 23.1 IU/mL with an AUC value of 0.90 (0.869–0.926), a sensitivity of 89.62% and a specificity of 81.52%; the ROC curve is shown in Fig. 1.
| AUC (95% CI) | Sensitivity % | Specificity % | Truncation value | Yoden index | p | ||
| Benign diseases | |||||||
| CA125 | 0.90 (0.869–0.926) | 89.62 | 81.52 | 0.7114 | |||
| CA19-9 | 0.678 (0.633–0.721) | 63.39 | 69.20 | 0.3259 | |||
| Benign OEC | 0.899 (0.868–0.925) | 89.62 | 81.16 | 0.7078 | |||
| Malignancy | |||||||
| CA125 | 0.859 (0.813–0.897) | 95.08 | 71.57 | 0.6665 | |||
| CA19-9 | 0.635 (0.576–0.691) | 65.03 | 64.71 | 0.2973 | 0.0001 | ||
| Malignant OEC | 0.859 (0.814–0.898) | 95.08 | 72.55 | 0.6763 | |||
Benign OEC is a combined indicator of CA125 and CA19-9 in the benign control group. Malignant OEC is a combined indicator of CA125 and CA19-9 in the malignant control group. AUC, area under the curve.
 Fig. 1.
Fig. 1.The receiver operating characteristic (ROC) curve of the combined indicators of CA19-9 and CA125 in the diagnosis of benign diseases and OEC.
In the malignant control group the cut-off value of CA125 was
 Fig. 2.
Fig. 2.Comparison of ROC curves between CA19-9, CA125, and combined indicators in the diagnosis of benign diseases and OEC.
EMT is a recognized chronic inflammatory disease, the common symptoms are dysmenorrhea, infertility, chronic pelvic pain, sexual discomfort, etc., the infertility rate is as high as 50% [5]. Dysmenorrhea and infertility in OEC group are higher than those in the other three groups, which may be due to ovarian dysfunction caused by chronic abdominal inflammation, changes in fertilization process and pelvic adhesion in EMT patients [11]. Chronic inflammation leads to prostaglandin overdose, peripheral and central sensitization, and abnormal stress response leading to secondary dysmenorrhea and severe symptoms [11, 12].
We determined that all five predictors, age, dysmenorrhea, infertility, CA125, and CA19-9, differed among the three groups of diseases, and then determined, by unordered multiclassified logistic regression analysis, that CA125 was a predictor of CA19-9 in the comparison of OEC with benign and malignant diseases; CA19-9 was not statistically significant as a predictor.
CA19-9 is synthesized in pancreas and bile duct cells, stomach, colon, endometrium and saliva epithelial cells, and can be overexpressed in some benign and malignant gastrointestinal diseases. Serum levels can also be significantly increased [13]. An increase was also found in the serum of patients with EMT, and some previous studies suggested that CA19-9 could be used as a diagnostic marker for EMT [14, 15]. In our study, the value of CA19-9 in OEC group was significantly higher than that in benign diseases, but the CA19-9 value in malignant diseases varied greatly with the nature of tumors. Some studies have pointed out that the increase of serum CA19-9 is related to tumor pathology and tumor size, mainly in mucinous tumors, which is often used as a tumor marker of gastrointestinal tract [16], and also reported a significant increase in ovarian mucinous tumors (borderline and malignant) [17]. In our study, the expression of CA19-9 is low in serous ovarian carcinoma and high in ovarian endometrioid carcinoma. These results are consistent with the previous experimental results [18]. Because borderline and malignant mucinous tumors are less included in our cases, it is impossible to further compare the expression of OEC with borderline and malignant mucinous tumors, which limits our study. We analyzed that CA19-9 was not statistically significant as a predictor of OEC by logistic regression [19].
CA125 is a mature tumor marker, which is produced in the epithelial cells of the
body cavity during embryonic development. The role of ovarian cancer in the
diagnosis of ovarian cancer has been widely recognized, and the serum levels of
patients with EMT are also increased in varying degrees [20]. A large number of
studies have recommended the use of CA125 to assist in the diagnosis of patients
suspected of having EMT [21, 22, 23]. However, the conclusions of the study on the
sensitivity of biomarkers are not consistent, and the key is to select the
appropriate cut-off value [24]. At present,
In our study, we also found that CA125 is elevated in borderline tumors, which makes the accuracy of CA125 as a diagnostic criterion for distinguishing benign or potential malignant diseases very challenging [25].
In the analysis of the relationship between clinical characteristics, there was no significant difference in the recurrence and initial onset of CA125 between the two groups, which was different from the results of previous studies [26, 27]. It may be because fewer patients in the relapse group in our study do not really reflect the differences between the two groups. In staging comparison, stage Ⅳ was significantly higher than stage III, which was the same as the previous study [23]. CA125 was more sensitive in severe patients [28, 29]. Previously, most of them used CA125 to distinguish between mild (Ⅰ, Ⅱ) and severe (Ⅲ, Ⅳ). But our study only compared between stage Ⅲ and Ⅳ, lack of stage Ⅰ and Ⅱ, which is another limitation of our results. In our results, the CA125 value of bilateral cysts was higher than that of unilateral cysts, and that of multilocular cysts was higher than that of single cysts. It is suggested that the larger the cystoma is, the higher the CA125 is. The size of ectopic cystoma is also related to the stage [16, 30]. The CA125 value of patients with dysmenorrhea is significantly higher than that of patients without dysmenorrhea, which is consistent with the results of Liu et al. [31]. Dysmenorrhea symptoms combined with increased CA125 can provide some evidence for the diagnosis of dysmenorrhea related to ectopic menstruation. CA125 and CA19-9 increased significantly when OEC ruptured, and the combination of them has obvious significance in the diagnosis of OEC rupture [32]. One of the advantages of our study is that all the selected cases are surgical cases, which can completely exclude the patients with endometriosis in the control group. One of the strengths of our study is that all the cases selected were post-surgical cases with pathologic findings, which made the diagnosis more convincing and allowed complete exclusion of patients with endometriosis from the control group. The controls I chose were all our common and frequent diseases and our study used benign controls as well as malignant controls in order to explore the critical values of CA125 and CA19-9 used in OEC. See Fig. 3. and Fig. 4.
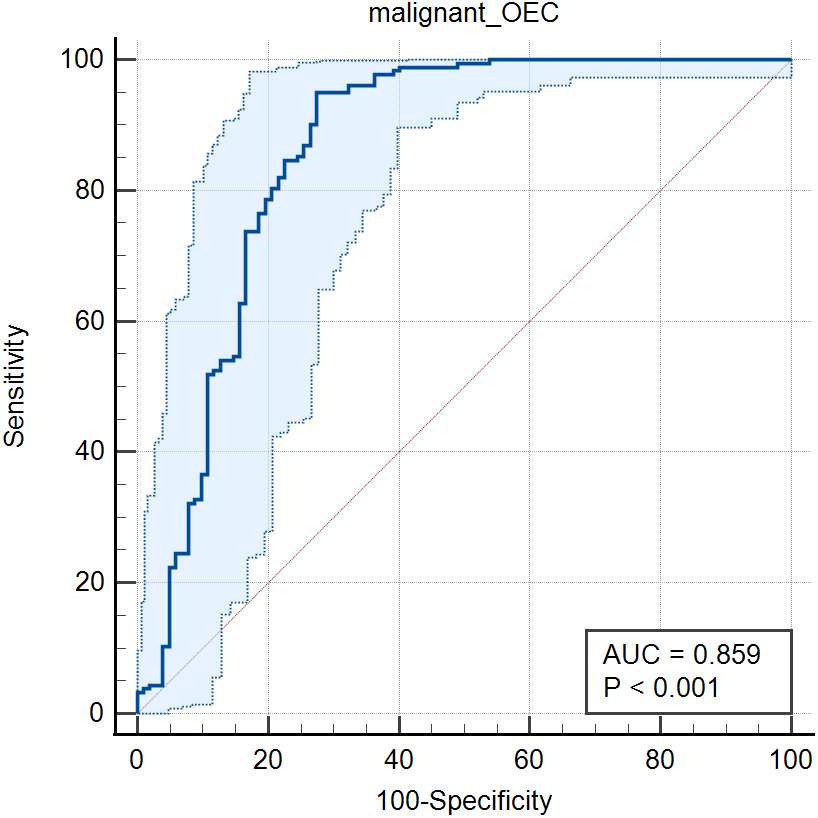 Fig. 3.
Fig. 3.The ROC curve of the combined indicators of CA19-9 and CA125 in the diagnosis of malignant diseases and OEC.
 Fig. 4.
Fig. 4.Comparison of ROC curves between CA19-9, CA125, and combined indicators in the diagnosis of malignant diseases and OEC.
We concluded that the effect of serum CA19-9 in the diagnosis of EMT is not
ideal. CA125 has a certain value in the diagnosis of endometriosis, but it is
necessary to explore the range of cut-off value. When serum 23.1 U/mL
The effect of serum CA19-9 in the diagnosis of EMT is not ideal. CA125 has a certain value in the diagnosis of endometriosis, but it is necessary to explore the range of cut-off value.
All data points generated or analyzed during this study are included in this article and there are no further underlying data necessary to reproduce the results.
GZ and JC designed the research study. WZ performed the research. HT provided help and advice on the ELISA experiments. QJ analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Hainan Provincial People’s Hospital (approval number: Med-Eth-Re [2023] 145).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest. Jiming Chen is serving as one of the Guest editors of this journal. We declare that Jiming Chen had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Valerio Gaetano Vellone.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
