- Academic Editor
Objective: This review aims to summarize the association between insulin resistance (IR) and symptoms of Polycystic Ovary Syndrome (PCOS) while explaining how nutritional interventions, specifically ketogenic diets, help manage PCOS. Mechanism: The effect of IR on diagnostic criteria for PCOS is first described, followed by how a standard diet exacerbates IR. Afterward, nutritional interventions, specifically for women with PCOS, are described. Findings in Brief: IR is associated with ovulatory dysfunction, hyperandrogenism, and polycystic ovarian morphology, which leads to metabolic abnormalities and loss of fertility. Activation of the polyol pathway, advanced glycation end-product accumulation, and hexosamine flux by hyperglycemia and IR are involved in the PCOS phenotypes and reproduction alterations. IR affects oocytes, ovaries, and the endometrium among women with PCOS, leading to infertility. However, nutritional interventions, specifically ketogenic diets, were shown to lower serum cholesterol, triglycerides, androstenedione, testosterone and attenuate IR. At the same time, high-density lipoprotein increased, promoting menstrual regularity and, eventually, providing a better environment for in vitro fertilization. Conclusion: For women with PCOS, managing IR is essential for managing their symptoms and improving fertility. Resolving glucotoxicity caused by excessive dietary glucose with a ketogenic diet is crucial for the prevention and correction of the damage associated with hyperinsulinemia and hyperglycemia, contributing to fertility.
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy affecting approximately 10% to 13% of women of reproductive age, usually characterized by ovulatory dysfunction (OD), hyperandrogenism (HA), and polycystic ovarian morphology (PCOM) [1]. Additionally, between 40% and 80% of women with PCOS typically will endure poor insulin action, resulting from hyperinsulinemia and insulin resistance (IR) [2]. Women who suffer from PCOS have an increased risk of infertility; however, the causes of infertility are numerous, and the mechanisms overlap. Nevertheless, obesity and IR are two factors associated with decreased fertility that can be attenuated with medication, exercise, and diet [3]. In this review, we examine the association between infertility and IR in women with PCOS and assess potential nutritional interventions.
Using the modified Rotterdam criteria, PCOS may be diagnosed if any two of the following are present: (1) clinical or biochemical hyperandrogenism, (2) evidence of oligo-anovulation, (3) polycystic appearing-ovarian morphology on ultrasound (12 or more follicles with 2–9 mm in diameter) with exclusion of other relevant disorders, and (4) Anti-Müllerian Hormone (AMH) as serum AMH levels are significantly higher in women with PCOS compared with normal ovulatory women [4]. The European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine/Rotterdam/PCOS 2023 guidelines established that PCOS should be diagnosed using the consensus Rotterdam criteria and include AMH levels only in adults and as an alternative to ultrasound. Cardiovascular risk, via a metabolic assessment, ethnicity, pre-menopause risk, and sleep disorders are also recommended criteria for the new diagnostic guidelines [5, 6]. With these diagnostic criteria (OD, HA, and PCOM), PCOS can be categorized into four phenotypes: (1) Phenotype A (HA + OD + PCOM); (2) Phenotype B (HA + OD); (3) Phenotype C (HA + PCOM); (4) Phenotype D (OD + PCOM). Phenotypes A and B have more irregular menstrual patterns and are more likely to have IR. With Phenotype D, women have normal androgen levels and are associated with better insulin sensitivity, resulting in a lower prevalence of IR [7].
PCOS comprises OD, HA, and PCOM; interestingly, each of these criteria is associated/exacerbated with IR. Concerning OD, insulin promotes ovarian steroidogenesis and the primordial to primary follicle transition [8]. Under the development of hyperinsulinemia, luteinizing hormone (LH) causes small follicles’ granulosa cells to respond, as well as premature theca cell differentiation, resulting in anovulation [9]. In patients with PCOS, treatment with thiazolidinediones decreased IR while improving ovulation as well as menstrual cycles became more regular [10, 11]. Concerning HA, the link between IR and HA is well documented [12, 13]; however, Ding et al. [14] describe the “vicious cycle” that explains the association between insulin levels, IR, and HA. Here, hyperinsulinemia promotes the secretion of androgens, and androgens augment insulin production, leading to IR. Unluhizarci et al. [12] pointed out that the ovaries and the adrenal glands are two tissues that remain sensitive to insulin; however, eventually, the ovaries do become IR, resulting in OD. Lastly, Lee et al. [15] demonstrated that fasting IR parameters were positively correlated with the total antral follicular count. This evidence gives just the tip of the iceberg in the complex interactions/pathways that lead to the mechanisms of PCOS development and its associated disorders, especially infertility.
When dietary glucose consumption is high and prolonged, serum glucose concentrations remain unresolved, leading to hyperglycemia. Uncontrolled hyperglycemia induces inflammation (Fig. 1A) and disturbs other metabolic pathways, overcoming the antioxidant defense system with the overproduction of free radicals and promoting oxidative stress (OS) [16]. In women with PCOS, OS has been correlated with IR, HA, and a pro-inflammatory state [17]. Together, they create a continuous pro-inflammatory environment that contributes to PCOS development. For example, pro-inflammatory stimuli can upregulate theca cell steroidogenic enzyme production of Cytochrome P450 (CYP17), responsible for androgen production, resulting in HA [18].
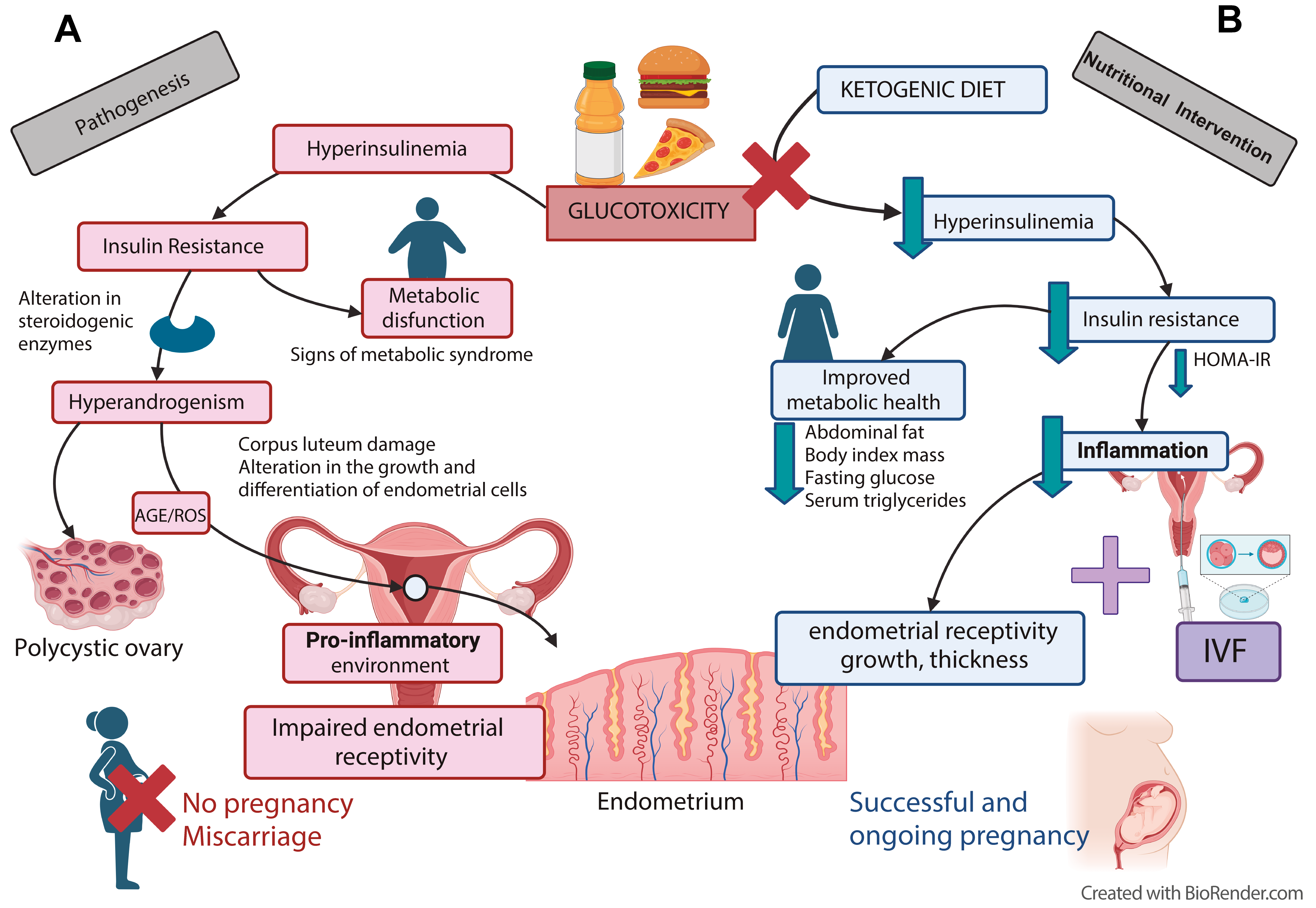 Fig. 1.
Fig. 1.Nutritional intervention to avoid glucotoxicity and
hyperinsulinemia in PCOS women improves fertility. (A) Excess consumption of
foods associated with a higher glycemic load results in elevated serum glucose
(hyperglycemia), leading to elevated serum insulin (hyperinsulinemia).
Glucotoxicity, prolonged and sustained hyperglycemia, and hyperinsulinemia lead
to IR, in which insulin loses its physiological effect over targeted organs, such
as adipose tissue, the liver, and organs associated with the reproductive system.
The mechanism involves the polyol and hexosamine pathways and the production of
inflammation, OS, and advanced glycation end-products (AGEs). Besides the
characteristic hormonal disturbances in PCOS, such as androgenism, this can
affect the reproductive system, causing damage to the oocytes and ovarian cells
and altering ovulation. (B) A nutritional intervention, a non-pharmacological
treatment, to control carbohydrate (CH) consumption (
Pathophysiologic processes associated with uncontrolled hyperglycemia, such as polyol pathway activity, advanced glycation end-products (AGEs) accumulation, and hexosamine flux, are involved in free radical overproduction [19]. Overproduction of AGEs is one of the consequences of hyperglycemia [20]. AGEs, formed under a non-enzymatic protein glycation process, interfere with intrinsic protein properties and functions [21]. Besides being associated with vascular complications [20], augmented levels of AGEs exert toxic effects on ovarian morphology, contributing to the pathophysiology changes observed in ovarian granulosa cells (Fig. 1A) [22]. Moreover, AGEs were shown to affect fertility in women. For example, methylglyoxal is a reactive AGE precursor and, under hyperglycemic conditions, is overproduced from upregulation of the fructose/aldose B pathway in vascular smooth muscle cells [23]. In addition, methylglyoxal-dependent glycate stress contributes to the ovarian PCOS phenotype in dehydroepiandrosterone mice [24].
The hexosamine pathway contributes to cardiovascular complications [25]. Under hyperglycemic conditions, the enzymatic functions of aldose reductase and sorbitol dehydrogenase are augmented. In the polyol pathway, both enzymes participate in the conversion of glucose to sorbitol. The excessive aldose reductase activity accumulates polyols, followed by osmotic damage and cell lesions [26]. In diabetic mice, sorbitol or the activators of the polyol pathway led to reduced cell-cell communication between the oocyte and the cumulus cells. This leads to compromised follicle-stimulating hormone (FSH)-mediated cyclic adenosine monophosphate (cAMP) production and de novo purine synthesis. The suppression of FSH-induced meiotic maturation observed in oocytes from diabetic mice may result from the shunting of glucose through the polyol pathway [27]. Thus, activation of the polyol pathway, AGEs accumulation, and hexosamine flux by hyperglycemia and IR are involved in the PCOS phenotypes and alterations in fertility.
In response to dietary glucose, pancreatic
Hyperinsulinemia leads to cell alterations and the development of IR [32]. The relationship between chronic hyperglycemia and IR development is well documented [33]. Proposed mechanisms of IR development include but are not limited to, mutations in the insulin receptor or the insulin response element, diminished expression of the insulin receptor or the glucose transporter (GLUT)-4, and liver dysfunction (review by Petersen and Shulman [34]). Cells respond differently to a hyperinsulinemic/hyperglycemic state. The most accepted disadvantage of IR is impaired glucose uptake, typically performed by the muscles. However, insulin-resistant liver cells do not stop gluconeogenesis in the presence of insulin, resulting in increased glucose release [35]. Insulin-resistant adipose tissue has reduced insulin-mediated inhibition of lipolysis, leading to an increase in circulating free fatty acids that further inhibit the antilipolytic effect of insulin [36]. Therefore, IR affects many different organs.
IR affects the oocytes, the ovaries, and the endometrium and appears to be associated with infertility, especially for PCOS. In the ovaries, hyperinsulinemia’s pathological effects occur at several levels. In cumulus cells from obese, infertile women with PCOS, insulin signaling was dysregulated even without clinical evidence of IR, pointing to the long-lasting effects of constant hyperinsulinemia [37]. IR affects the steroidogenic function of the ovaries through increased CPY17 Subfamily A Member 1 (CYP17A1) activity in theca cells, resulting in a HA state [34]. Granulosa-lutein cells from anovulatory women with PCOS are resistant to insulin-stimulated glucose uptake, probability due to the impairment of the insulin signaling pathway [38]. At least ten genes are overexpressed in obese women compared with normal-weight women, of which the BCL2L1, Mechanistic Target of Rapamycin Kinase (mTOR), and Phosphoenolpyruvate Carboxykinase 2 (PCK2) genes were responsible for the proliferation and differentiation of the cumulus cells during oocyte maturation as well as the development of IR, regulation of apoptosis, and glucose metabolism during early embryogenesis [37]. A literature review indicates that these alterations may be associated with a worse prognosis of follicular development and oocyte maturation observed in obese women with PCOS [37]. Again, one of PCOS’s main characteristics is HA, which has been correlated with hyperinsulinemia [39, 40]. Androgen levels are increased by the interaction between insulin and LH in theca cells, which upregulates mRNA expression of Steroidogenic Acute Regulatory Protein (StAR) and CYP17A1 [41]. In the ovary, insulin increases the expression and activity of CYP17A1 and insulin growth factor 1 (IGF-1) [42]. In theca cells, exposed to insulin, the PI3K pathway is induced as a mediator in regulating androgen production [43].
Infertility results from the arrest of follicular development and poor oocyte production in PCOS [44]. Hyperinsulinemia can decrease the production of Sex Hormone Binding Globulin (SHBG) at the hepatic level, which is responsible for transporting androgens and estrogens in the blood, including testosterone [45]. Insulin-resistant women with PCOS correlate with reduced ovarian sensitivity to exogenous gonadotropins (Gn), leading to poor ovarian function. There was an inverse correlation between the homeostasis model assessment of insulin resistance (HOMA-IR) and the ovarian sensitivity index (OSI) during controlled ovarian stimulation in women with PCOS [46].
The endometrium is also compromised when IR is present. Decidualization is impaired due to a high-insulin environment, decreasing glucose uptake by GLUT-1 [47]. Obese women with PCOS are in a state of chronic (low-grade) inflammation; that is to say, these women have high TNF-alpha levels. Under these conditions, the endometrium could induce several alterations, such as the expression of molecules associated with the adiponectin pathway or lower GLUT-4 levels [48]. It has also been shown that decidualization damage is related to decreased expression of insulin-like growth factor binding protein-1 (IGFBP1) [49].
Assisted reproduction technology (ART) remains the best option for infertile women. Independent if the cause of infertility is low oocyte reserve, immature endometrium, uterine deformities, an overactive immune system, or unknown, when IVF is applied, IR decreases the success rate compared to women without IR [50]. Moreover, when women with PCOS undergo IVF, there are more immature oocytes, production of low-quality embryos, an increased risk of miscarriages, and a higher incidence of Ovarian Hyperstimulation Syndrome (OHSS) when compared with non-PCOS women [51]. With IVF, for non-PCOS women, the implantation rate is around 60–70%, but for women with PCOS, the chance of getting pregnant is between 20% and 40%. Moreover, a lower pregnancy rate was observed in women with PCOS diagnosed as obese, metabolically compromised, or IR when compared to their controls [52]. Therefore, addressing the IR component of PCOS, by either diet or by pharmacological intervention, presents a method to aid IVF for these women.
There is limited information that focuses specifically on reducing IR in women with PCOS and pharmacological treatments. Nevertheless, as seen with diabetes, treatment with biguanides, mainly Metformin and thiazolidinediones, can sufficiently reduce IR by increasing insulin sensitivity, leading to Metformin being an adjuvant treatment for PCOS [53]. Considered more like supplements, Myoinositol (MI) and D-chiro-inositol (DCI) have a significant role in glucose metabolism. Both MI and DCI show insulin-mimetic properties and decrease postprandial serum glucose. Glucose metabolism is shifted toward glycogen synthesis by DCI and towards glucose catabolism by MI. The available clinical data suggest that their use could be beneficial for improving metabolic, oxidative, hormonal, and reproductive parameters of PCOS [54]. Pharmacological interventions, as mentioned above, have shown promise in reducing symptoms associated with PCOS; however, pharmacological interventions have been reviewed before [53, 55, 56].
Evidence has confirmed the relationship between a high-calorie, high-carbohydrate diet, and obesity, as well as other common degenerative diseases [57]. Furthermore, like many other chronic degenerative diseases, PCOS has been strongly connected with a high-carbohydrate diet [58]. The standard American diet is carbohydrate-based, including frequent eating and excessive food intake, promoting hyperglycemia and hyperinsulinemia [59]. Furthermore, sugar is a significant component of processed foods and beverages in Western society. Therefore, constant exposure and consumption of sugar-sweetened foods are favored, as these foods are very palatable, readily available, cheap, and continuously advertised [60].
Diet is the most relevant environmental factor in managing PCOS [61]. Evidence has shown the relationship between dietary carbohydrate consumption and PCOS, as the risk of developing PCOS is associated with a diet high in glycemic index and glycemic load [62]. In addition, female rats fed with high-refined carbohydrates showed PCOS-like features, impaired ovarian reserve, and other reproductive abnormalities [1]. Lifestyle interventions based on dietary modification resulted in improved metabolic parameters, better glycemic control, decreased androgenic symptoms, and favorable anthropometric outcomes [63]. For example, women with PCOS on a low-carbohydrate, low-fat diet had reduced body mass index (BMI), HOMA-IR, low-density lipoprotein cholesterol (LDL-C), and testosterone levels but increased FSH [3]. A low-carbohydrate, high-fat diet for women with PCOS reduced body fat accumulation and day-long insulin concentrations. The diet lowered glycemic levels while improving IR and lipid parameters, such as triglycerides and LDL-C [64, 65]. Therefore, diets are often shown to be beneficial for women with PCOS; however, the optimal diet remains elusive.
Reducing carbohydrate consumption during a nutritional intervention has positive results for women with PCOS [66]. A recent meta-analysis demonstrated that women with PCOS showed improved IR when following a diet with lower carbohydrate concentrations [67]. Moreover, women with PCOS, who combined a nutritional intervention with metformin, the beneficial effect was mainly due to the diet, with an additive null impact from metformin [68]. Evidence suggests that following a four-week low-carb intervention can show positive effects, such as lower BMIs, HOMA-IR scores, total cholesterol, testosterone, and FSH serum levels [3]. This is most likely due to the reduced endocrine problems in PCOS, allowing spontaneous pregnancy to be achieved after several months [69]. This indicates that a low-carb diet intervention may be helpful to women with PCOS and may be an effective addition to IVF procedures.
Nutritional interventions have been used before, independent of PCOS, for IVF, particularly in obese patients, primarily for weight loss. The nutritional interventions mainly consisted of either the Mediterranean diet or a traditional medicine-oriented diet without caloric restriction, typically for a 3-month period, where whole and nutritious foods, such as vegetables, fish, shrimp, chicken, lamb, bread, milk, honey, olive oil, and eggs are recommended [70, 71]. For caloric restriction diets, the results are inconclusive, going from no effect in healthy, normal-weight women to better reproductive and pregnancy rates in obese women [72]. Caloric restriction diets typically reduce the total daily calorie intake by at least 500–800 kcal, ideally reducing the total carbohydrate content; however, patients are still advised to keep a 50% carbohydrate consumption. Nevertheless, alternative diets still need to be assessed for women with PCOS, in which caloric restriction or Mediterranean or medicine-oriented diets fail to improve fertility.
The “ketogenic diet”, which is composed of low-carbohydrate, moderate/adequate-protein, and high-fat consumption, has been successfully used for therapeutic purposes in patients suffering from seizures and epilepsy [73] as well as diabetic patients [74]. Carbohydrate consumption must be less than 15% of the total calories (50 g maximum per day), with moderate protein consumption, which is crucial for avoiding a rise in insulin (1 g/kg of ideal weight), and an abundance of fat, coming from naturally fat foods for energy completion. Fat has the lowest impact on insulin levels and slows the absorption of carbohydrates, contributing to maintaining/controlling blood glucose levels [75]. Fat also creates a satiety that lasts longer, thus reducing eating [76]. Quality fats contain fat-soluble vitamins such as A, D, E, and K [77].
The ketogenic diet could be an excellent intervention for women with PCOS. Few studies have shown that ketogenic diets improve clinical and metabolic parameters as well as fertility in women with PCOS [67, 69]. A literature review illustrates how women with PCOS undergoing a ketogenic diet for six months improved their total body weight, free testosterone, LH/FSH ratio, glucose, insulin levels, estradiol and progesterone levels while decreasing triglycerides, total cholesterol, and LDL-C [69]. The control of carbohydrate intake in women with PCOS may represent an essential intervention to improve these patients’ clinical symptoms [3]. In a comparison study, Meneghini et al. [78] demonstrated that ketogenic diets were superior to the Mediterranean diet for women with PCOS. Ketogenic diets were more effective at decreasing cholesterol, triglycerides, IR, androstenedione, and testosterone, while High-Density Lipoprotein-cholesterol (HDL-C) increased. Moreover, ketogenic diets were better at promoting menstrual regularity and lowering the risk of OHSS. Published nutritional interventions demonstrated that when ketogenic diets were applied, after months of adherence, there was an improvement in spontaneous conception [61]. Moreover, a recent report, in which a ketogenic diet was combined with IVF for PCOS, showed that a nutritional intervention diminished glucotoxicity, improved insulin control, and reduced IR, which resulted in increased clinical pregnancy rates [65] (Fig. 1B).
Alterations in energy metabolism are evident in a high percentage of women with PCOS, with more than 80% showing decreased insulin sensitivity and manifestations of the components of MetS [79]. Moreover, approximately 50% of women with PCOS are obese. Many reports show that specific diets can reduce IR and hyperglycemia [3]. Therefore, it would be reasonable to speculate that a nutritional intervention should improve IR, improving women with PCOS’s fertility. Unlike previous interventions focusing on weight loss [80, 81], caloric restriction must be avoided [82]. Long-term caloric restriction contributes to leptin resistance, IR, low testosterone, and thyroid problems, which impact IVF [80]. Therefore, a ketogenic diet aimed at reducing hyperglycemia and hyperinsulinemia will primarily improve fertility in women with PCOS, if a nutritional balance is kept while avoiding starvation. The ketogenic diet mitigates the development of IR’s harmful effects and may offer benefits related to insulin sensitivity in several organs: (a) improved blood sugar control by limiting carbohydrates: the ketogenic diet can help stabilize blood sugar levels and reduce insulin spikes, thereby potentially improving insulin sensitivity; (b) weight loss: ketogenic diets often result in weight loss, which can reduce the burden on insulin production and improve insulin sensitivity; (c) heart health: some studies propose that the ketogenic diet might positively affect heart health markers, such as Low Density Cholesterol (LDL-C) and triglycerides, that often are affected by IR; (d) brain health: ketones produced during ketosis are a preferred energy source for the brain and might offer neuroprotective effects, potentially benefiting individuals with IR-related cognitive issues; (e) liver and kidneys: emerging evidence suggests that a ketogenic diet might help mitigate fatty liver disease and improve kidney function in some cases, both of which can be affected by IR [83, 84]. A word of caution, ketogenic diet must be closely supervised, as side effects have been recorded. Some adverse effects at the initiation of the diet may be secondary to the fast itself, such as light nausea, vomiting, constipation, and dehydration were reported in a few clinical studies, which can be corrected after adapting to the diet. LDL-C can be elevated in the early stages of the diet or under prolonged conditions; therefore, they should be carefully monitored [83]. Elevated LDL-C is associated with cardiovascular disease risks and women with PCOS have intrinsic elevated risk for cardiovascular disease. Serious side effects could be a problem in cases in which there are other metabolic deficiencies that have not yet been previously recognized, such as defects in fatty acid oxidation, carnitine deficiency, and pyruvate mitochondrial disorders. Kidney stones and pancreas failure have also been reported in rare cases and almost exclusively in children treated for epilepsy where ketogenic diet is more restrictive, with respect to protein and carbohydrate consumption [85, 86].
Shifting the focus to a multilevel approach by diminishing glucose overload, while controlling hyperinsulinemia through a well-focused nutritional intervention for women with PCOS, can improve the positive outcomes of their infertility treatment. As a proof of concept, for women with PCOS trying to become pregnant by undergoing IVF, a carefully designed and monitored ketogenic nutritional intervention was implemented that focused on controlling glucose load (quantity, frequency, and time of exposure) to decrease insulin concentrations (hyperinsulinemia). IR and metabolic parameters improved while the ovarian and endometrial environments were enriched. This promoted an adequate response to controlled ovarian stimulation, producing better oocytes for fertilization and a better endometrium for embryo implantation [65].
Managing IR is an essential factor when trying to control PCOS symptoms. Due to the persistently high and chronic increases in blood glucose and insulin levels, a nutritional intervention is crucial for the prevention of, as well as correction of, the damage associated with glucotoxicity and hyperinsulinemia. Therefore, a carefully designed and monitored nutritional intervention that is focused on controlling glucose load will help to decrease IR, while enhancing fertility. A ketogenic diet is proposed as the ideal intervention.
LRM and CPG performed the literature review and wrote the first draft of the manuscript. LMP and ELB revised, critically appraised, and edited the manuscript. All authors contributed to editorial changes in the manuscript. All authors have read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
We would like to acknowledge the editorial help from Bruno López-Bayghen (MSc), Lucero Cervantes (BS), and Martha Elba Gonzalez-Mejia (MD, PhD).
The study was funded by Consejo Nacional de Humanidades, Ciencia y Tecnología (Conahcyt grant number: 250768 to ELB and the scholarship to CPG number 756245). The funding source had not contributed to the manuscript’s writing and the decision of where to submit the manuscript for publication.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
