1 Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, 100191 Beijing, China
2 National Clinical Research Center for Obstetrics and Gynecology, 100191 Beijing, China
3 Key Laboratory of Assisted Reproduction, Ministry of Education, Peking University Third Hospital, 100191 Beijing, China
4 Bejing Key Laboratory of Reproductive Endocrinology and Assisted Reproductive Technology, Peking University Third Hospital, 100191 Beijing, China
5 Department of Gynecology and Obstetrics, Tianjin Medical University General Hospital, 300052 Tianjin, China
6 Tianjin Key Laboratory of Female Reproductive Health and Eugenics, Tianjin Medical University General Hospital, 300052 Tianjin, China
†These authors contributed equally.
Abstract
Background: The incidence of ectopic pregnancy (EP) is purportedly elevated among individuals with a history of EP (referred to as the EP group) compared to those with no previous ectopic pregnancy (non-EP group). Nevertheless, the question of whether an EP history represents an autonomous risk factor for subsequent ectopic pregnancy of in vitro fertilization-embryo transfer (IVF-ET) patients remains a subject of debate. Methods: This study is a retrospective cohort study conducted at a single center. A total of sixty-seven patients with a prior ectopic pregnancy (EP) who underwent bilateral salpingectomy were included, and they were age-matched with a control group of 201 patients who did not have a history of EP but underwent bilateral salpingectomy during the period from January 2011 to April 2017. In all cases, laparoscopic salpingectomy was performed, followed by subsequent IVF-ET and frozen-thawed embryo transfer (FET) cycles. Results: The cumulative clinical pregnancy rates in the EP group and non-EP group were 65.7% and 73.6%, respectively, demonstrating no significant difference. Likewise, the cumulative live birth rates between the two groups were comparable (50.7% in the EP group vs. 63.6% in the non-EP group, p = 0.2). However, the incidence of ectopic pregnancy was significantly higher in the EP group compared to the non-EP group (15.9% vs. 3.4%, p = 0.003). Subsequent regression analyses revealed a significant association between a history of EP and an elevated risk of ectopic pregnancy. Conclusions: Women with a history of ectopic pregnancy even if they have had bilateral salpingectomies are at a significantly higher risk of subsequent ectopic pregnancies, indicating that a prior ectopic pregnancy is an independent risk factor for this condition, even if fallopian tubes have been removed.
Keywords
- bilateral salpingectomy
- ectopic pregnancy
- assistive reproductive technology
Ectopic pregnancy (EP) refers to the implantation and development of the embryo outside the endometrial cavity. The incidence rate of EP is approximately 2%–3% in women during early pregnancy [1, 2], with a higher occurrence observed in cases involving assisted reproductive technology (ART). Despite continuous improvements in diagnosis and treatment, EP remains the leading cause of pregnancy-related mortality during the first trimester [3, 4]. This can be life-threatening if it is not diagnosed or treated in time, which remains a global medical problem that cannot be prevented. The prevalence of EP is on the rise due to various risk factors, including the increased utilization of ART, a history of prior EP, tubal disease, tubal injury or surgery, pelvic inflammatory disease (PID), endometriosis, smoking, and advanced maternal age [5, 6, 7]. Pelvic infections play a central role in tubal pathology, resulting in alterations in tubal function, tubal blockage, and the development of pelvic adhesive disease. Consequently, these factors increase the odds of experiencing EP, with reported odds ratios ranging from 2.1 to 4.5 [8, 9].
Tubal surgeries, such as tubal sterilization, bipolar coagulation, and tubal reconstructive procedures are widely recognized as risk factors for EP [10, 11]. Additionally, cesarean section and endometriosis have been identified as independent risk factors for EP [12, 13, 14]. Furthermore, excessive ovarian response and multiple embryo transfer during in vitro fertilization (IVF) have also been associated with an elevated risk of EP, with reported incidence rates as high as 11% [15, 16]. Previous study has also indicated that a history of EP is also a risk factor, with a probability of recurrence of approximately 10% for individuals with one prior EP and over 25% for those with two or more ectopic pregnancies [17]. Tubal pregnancy is the most common type of ectopic pregnancy, it was previously believed that after a oviduct fenestration for ectopic pregnancy, the likelihood of recurrent ectopic pregnancy would increase, and that bilateral salpingectomy would reduce the risk of another ectopic pregnancy, but currently, there are no studies showing whether a history of ectopic pregnancy is an independent high-risk factor for ectopic pregnancy after bilateral salpingectomy or if inflammation of the fallopian tube and endometrium after EP treatment plays a role. Thus, the direct association between prior EP and subsequent EPs remains uncertain.
This retrospective matched-pair study aimed to investigate whether a history of EP was an independent risk factor for the occurrence of EP after embryo transfer in in vitro fertilization-embryo transfer (IVF-ET) treatment.
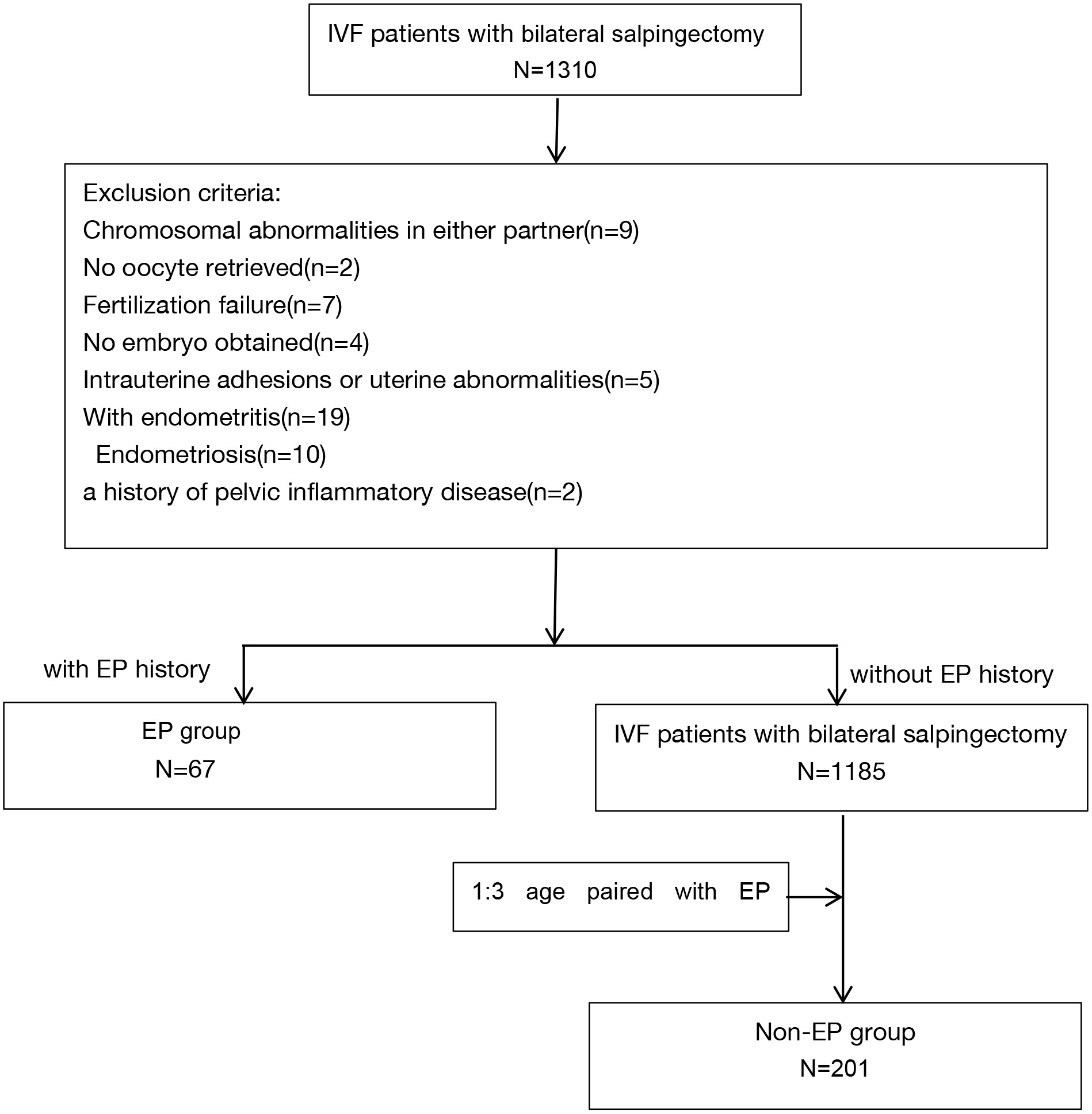
This retrospective matched-pair study utilized a 1:3 ratio. The study participants were selected from a pool of patients who received in vitro fertilization (IVF) treatment at the Center of Reproductive Medicine, Peking University Third Hospital (PUTH), China, during the period from January 2011 to April 2017. The study received approval from the ethics committee of PUTH. In total, 67 women with a previous occurrence of fallopian ectopic pregnancy (excluded special and rare ectopic pregnancy sites, such as rectal ectopic pregnancy, heteroectopic pregnancy, and retroperitoneal ectopic pregnancy) who had undergone bilateral salpingectomy were included in the study, constituting the EP group, and age-matched women with bilateral salpingectomy due to tubal obstruction, hydrosalpinx, or fallopian tube adhesion as the control group (non-EP group), which was showed in Fig. 1. Laparoscopic surgery was used for all cases.
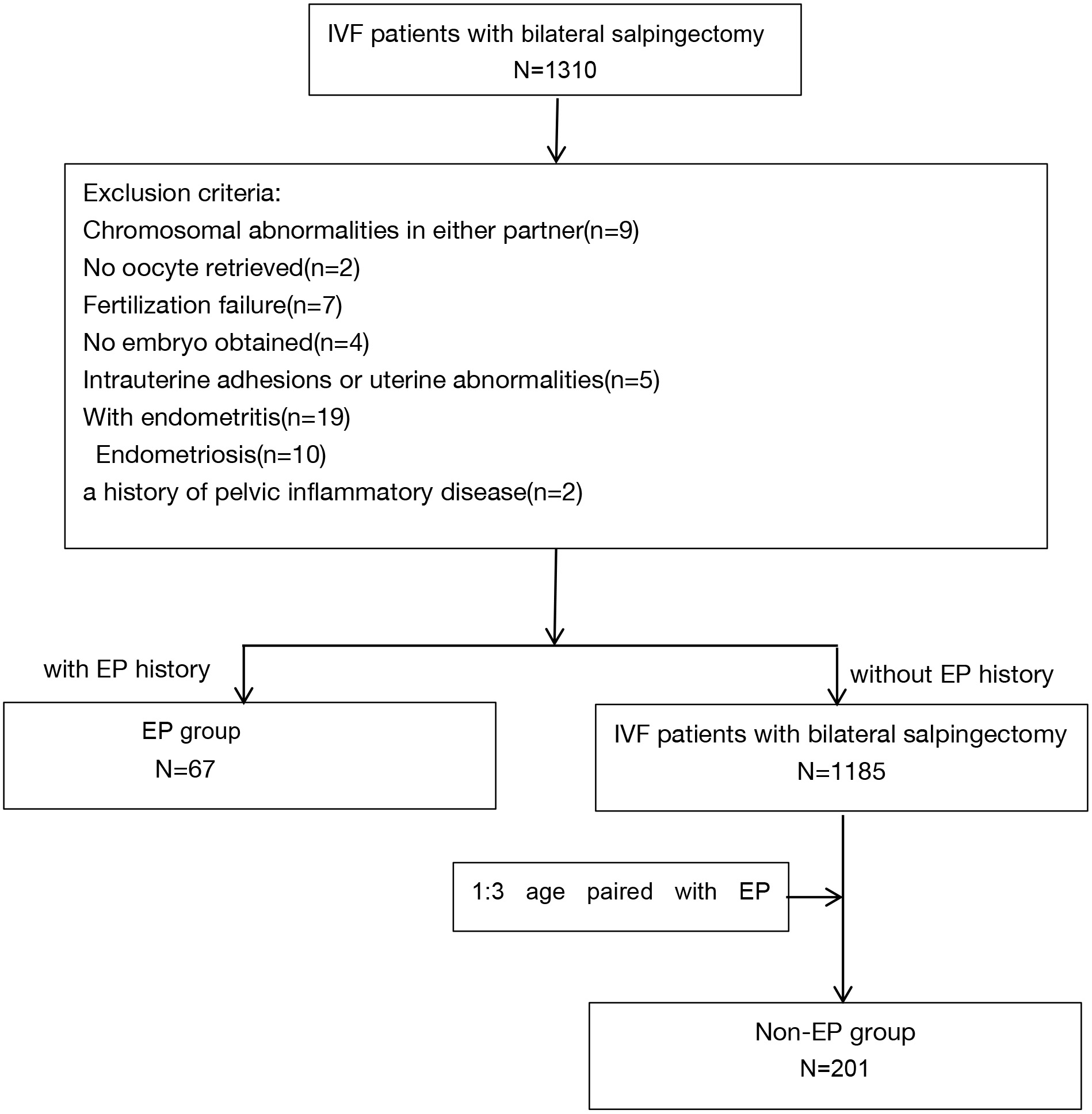 Fig. 1.
Fig. 1.Flow chart of the patient cohort selection. IVF, in vitro fertilization; N, number; EP, ectopic pregnancy.
Patients with no oocyte retrieved, fertilization failure, no embryo obtained, chromosomal abnormalities in either partner, endometritis, intrauterine adhesions, uterine malformations, endometriosis and a history of pelvic inflammatory disease were excluded from the study.
All study participants underwent a standardized ovarian stimulation protocol
during their IVF cycles. The ovarian stimulation regimen involved the use of
recombinant follicle-stimulating hormone (FSH) (Gonal-F from Merck-Serono, Darmstadt, Hessen, Germany) in
either gonadotrophin-releasing hormone agonist (GnRH-agonist) protocols or
gonadotrophin-releasing hormone antagonist (GnRH-antagonist) protocols. When at
least three dominant follicles
IVF procedures were performed utilizing either conventional insemination or intracytoplasmic sperm injection, with the choice of method determined by the couple’s medical history. Embryo transfers were carried out either on day 3 or day 5 of embryo development.
Demographic and basal characteristics, including age, type of infertility, body mass index (BMI) were analyzed. IVF/FET (frozen-thawed embryo transfer, FET) cycle characteristics including basal follicle-stimulating hormone (FSH) level, basal estrogen (E2) level, peak endometrial thickness, number of oocytes retrieved, number of embryos transferred, average transfer cycels were also analyzed.
The primary outcomes assessed in this study is ectopic pregnancy rate (EPR), the secondary outcomes included the cumulative clinical pregnancy rates (CCPR) and cumulative live birth rates (CLBR) within the context of IVF/FET. CCPR was calculated by dividing the total number of clinical pregnancy cycles by the total number of patients included in the analysis. Clinical pregnancy was defined as the visualization of a gestational sac accompanied by the detection of fetal cardiac activity through ultrasound examination within 28 to 30 days post-embryo transfer. CLBR, on the other hand, was determined by dividing the number of live birth cycles by the total number of patients. Ectopic pregnancy was operationally defined based on the detection of the embryo’s location during laparoscopic surgery. For the purpose of this study, any birth occurring after reaching 26 weeks gestational age was considered a live birth.
The statistical analysis in this study was conducted utilizing the IBM SPSS Statistics Package version 20 (IBM Corporation Inc., Armonk, NY, USA). Descriptive statistics for the baseline characteristics were compared using either Student’s t-test (for normally distributed data) or the Mann-Whitney U-test (for skewed data). The CCPR and EPR were assessed using the paired chi-squared test. Furthermore, logistic regression analysis was employed to identify the factors that influenced the occurrence of ectopic pregnancy. A significance threshold of 0.05 was set for all statistical analyses.
Table 1 displays demographic and basal characteristics of 67 women with a prior ectopic pregnancy (EP group) and 201 women without a history of EP (non-EP group) who underwent IVF/FET. The two groups exhibited comparable age and BMI distributions. However, a notable distinction was observed in terms of basal FSH levels, which were significantly higher in the EP group compared to the non-EP group (6.37 vs. 5.58, p = 0.045). No significant differences were identified between the groups in relation to cycle characteristics, including the number of retrieved oocytes, transferable embryos, endometrial thickness, and the number of embryos transferred.
| EP group (n = 67) | Non-EP group (n = 201) | p-value | ||
| Age (years) | 31.45 | 31.97 | NS (0.763) | |
| Type of infertility | 0.001 | |||
| Primary n (%) | 0% | 47.80% | ||
| Secondary n (%) | 100% | 52.20% | ||
| BMI (kg/m |
22.67 | 22.62 | NS (0.295) | |
| Basal FSH (mIU/mL) | 6.37 | 5.85 | 0.045 | |
| Basal E2 (mIU/mL) | 154.04 | 156.57 | NS (0.663) | |
| Number of oocytes retrieved (n) | 12.6 |
13.4 |
NS (0.848) | |
| Number of transferable embryos (n) | 5 [1, 23] | 5 [1, 24] | NS (0.93) | |
| Endometrial thickness (mm) | 9.97 | 10.87 | NS (0.532) | |
| Number of embryo transfer (n) | 2 [1, 3] | 2 [1, 3] | NS (0.744) | |
| Average transfer cycles (n) | 1.6 |
1.9 |
NS (0.484) | |
BMI, body mass index; NS, not statistically significant; EP, ectopic pregnancy; FSH, follicle-stimulating hormone; E2, estrogen.
To further determine the accociation between EP history and cycle outcomes, the study assessed a total of 112 IVF/ET cycles in the EP group and 395 IVF/ET cycles in the non-EP group. There were no statistically significant differences observed between the two groups in terms of the CCPR and CLBR ( 65.7%, 50.7% in the EP group; 73.6%, 63.6% in the non-EP). However, among women with clinical pregnancy, a higher rate of EP was observed in the EP group (15.9% vs. 3.4%, p = 0.003) (Table 2). It is noteworthy that among the study participants, a total of 12 cases were diagnosed with EP, and it is worth mentioning that the sites of recurrence of ectopic pregnancy were exclusively in the interstitial tubal pregnancy or cornual pregnancy. Specifically, within the EP group, seven out of 44 patients experienced an EP, while within the non-EP group, five out of 148 patients were diagnosed with EP.
| EP group (n = 67) | non-EP group (n = 201) | p-value | |
| Cumulative clinical pregnancy rate | 65.7 (44/67) | 73.6 (148/201) | NS (0.76) |
| Ectopic pregnancy rate | 15.9 (7/44) | 3.4 (5/148) | 0.003 |
| Cumulative live birth rate | 50.7 (34/67) | 63.6 (128/201) | NS (0.21) |
NS, not statistically significant; Measurements are presented as number (%).
To examine the potential influence of EP history as an independent factor on the occurrence of EP, logistic regression analysis was conducted. In the univariate logistic regression model, each candidate factor affecting EP in infertility patients was examined and presented in Table 3. The analysis revealed that a history of ectopic pregnancy (EP) was a significant and independent risk factor for subsequent occurrence of EP (p = 0.01), as shown in Table 3.
| Odds ratio | 95% CI | p-value | |
| EP history | 18.7 | (2.0, 175) | 0.01 |
| Age | 0.9 | (0.8, 1.1) | 0.97 |
| Type of infertility | 0.16 | ||
| Primary n (%) | 5.1 | (0.5, 49) | |
| BMI | 1.2 | (0.9, 1.4) | 0.09 |
| Basal FSH | 1.2 | (0.9, 1.6) | 0.19 |
| Endometrial thickness | 1.2 | (0.8, 1.8) | 0.33 |
| Number of embryo transferred | 1.6 | (0.3, 7.9) | 0.59 |
95% CI, 95% confidence interval.
The principal aim of this case-control study was to examine the effects of previous tubal disease necessitating bilateral salpingectomy on IVF outcomes, as well as to evaluate the potential association between prior EP and the risk of recurrent EP. This study represents the first investigation to demonstrate that a history of EP serves as a significant risk factor for recurrent EP in infertile women who have undergone bilateral salpingectomy.
Infertility has long been established as a crucial risk factor for EP and other adverse pregnancy outcomes [18]. Multiple studies [18, 19] have reported higher EP rates in ART cycles due to the transfer of multiple embryos. Although embryos are directly transferred into the uterine cavity during IVF-ET, a significant proportion of pregnancies still localize ectopically [20]. Numerous literature reviews have documented that the occurrence of ectopic pregnancy subsequent to IVF varies within the range of 2.1% to 8.6% of all clinical pregnancies [19, 21]. Consistent with previous reports, the EP rate in the present study was 3.4% in the non-EP group, while the rate was markedly higher (15.9%) in women with a history of EP. This finding suggests that a history of EP is a significant risk factor for recurrent EP, and that EP history should be taken into account when predicting the probability of EP in infertile women undergoing bilateral salpingectomy and IVF.
Numerous factors have been identified to contribute to the occurrence of ectopic pregnancy, including endometritis, intrauterine adhesion, uterine malformation, endometriosis, and smoking. Previous investigations have indicated that a history of EP raises the probability of experiencing another EP in subsequent pregnancies. It is widely recognized that tubal disease represents a significant risk factor for EP, contributing to approximately one-third of all cases [22]. The resulting damage to the fallopian tube may lead to changes in embryo-fallopian transport and the fallopian tube microenvironment, thereby constituting one of the primary factors contributing to the heightened incidence of recurrent EP. However, the exclusion of confounding factors following treatment of EP has been a challenge for follow-up studies due to multiple treatment options such as expectant management, conservative medication, and surgery (e.g., salpingectomy or salpingostomy).
Surgery is the preferred treatment in clinic for women with tubal EP and different surgical methods and decisions regarding retaining the contralateral fallopian tube can lead to the higher risk for subsequent EP recurrence. Salpingotomy (retain the fallopian tubes and only remove the trophoblast layer) is an optional treantment for those couples with conceiving needs naturlly, while it has been associated with an elevated risk of subsequent EP occurrences [22, 23]. More study groups have been reported the similar EP rate after different surgical methods of tubal EP. Mol et al. [24] have conducted a randomised controlled trial with 215 allocated to salpingotomy and 231 to salpingectomy. They reported 8% repeat ectopic pregnancy rate in the salpingotomy group and 5% in the salpingectomy group, which supported by the results in Xue et al. [25] group. Moreover, Xue et al. [25] demonstrated that women with a prior single ectopic pregnancy (6.8%) exhibit a greater risk of experiencing EP following IVF compared to women with no history of EP (2.1%) and those with a history of recurrent EP (2.4%). Notably, the salpingectomy in the former studies contained both unilateral and bilateral salpingectomy, which may result in the distinct EP occurrence rate. This study is strengthened by its inclusion of patients who underwent laparoscopic bilateral salpingectomy, which minimized unequal comparisons and bias between case-control groups. Furthermore, this study attempted to eliminate other EP-related confounding factors by including women who underwent assisted reproductive technology. The study found that a history of EP was an independent risk factor leading to an increased likelihood of subsequent EP.
There are several limitations to this study that need to be acknowledged. Firstly, due to the sample size, fresh and frozen-thawed cycles were not analyzed separately. All participants in this study underwent one stimulation cycle followed by IVF-ET and FET cycles until they had a live birth. The aim of the study was to focus on the individual-level probability of EP occurrence. Despite a prior investigation that observed no disparity in the occurrence of ectopic pregnancy between fresh cycles and frozen-thawed cycles in a substantial IVF patient cohort [26], this finding has been challenged by other research groups. Emerging evidence indicates that frozen-thawed embryo transfer (FET) may exhibit a lower incidence of ectopic pregnancy (EP) when compared to fresh cycles, as documented in recent studies [27, 28, 29]. Therefore, larger sample sizes and more comprehensive studies are necessary to address this issue.
In conclusion, our study found that a prior EP is a significant independent risk factor for recurrent EP among infertile women undergoing bilateral salpingectomy and IVF. This finding highlights the importance of considering EP history when predicting the probability of EP in infertile women undergoing bilateral salpingectomy and IVF. Despite certain limitations in this study, such as linited sample size and the absence of considering embryo quality, it provides valuable insights for clinicians to improve the prognosis of infertile patients and to avoid occurrence of emergency and complications. Further investigation involving larger-scale and comprehensive studies is warranted to validate our findings and furnish clinicians with more comprehensive information.
All generated data are incorporated into the article. The original data of individual participants underlying this article will be shared on reasonable request to the corresponding author.
MLin and WX, MLiang and RL conceived the idea and designed the study; MLin, WX, MLiang collected and analysed the data, designed the figures and tables, and wrote the first draft of the manuscript. SY and RL interpreted the data and revised the manuscript. All authors participated in the discussion of analyses and interpretation of data in this article. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Peking University Third Hospital (approval number: IRB-2022-596-01).
We thank Miss. Lixue Chen in Peking University Third Hospital for helping us to collect the data and thank Editage for English language editing.
This study was supported by the Project funded by Peking University Third Hospital Key clinical projects (Ming-Mei Lin, Grant nos. BYSYZD2023022); The National Key Research and Development Program of China (no. 2022YFC2702500).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.