- Academic Editor
†These authors contributed equally.
Background: This study aimed to investigate the relationship between
coronavirus disease 2019 (COVID-19) disease and maternal and neonatal outcomes.
Methods: This is a retrospective cohort study. This study analyzed 3615
participants from Fujian Provincial Maternity and Child Health Hospital Fujian
Obstetrics and Gynecology Hospital from November 1, 2022, to January 31, 2023.
All pregnant women must provide a 24-hour nucleic acid test report when they are
admitted to the hospital to deliver babies. Chi-square test and linear regression
analyses were used to evaluate the risk of COVID-19 infection with the maternal
outcome and neonatal outcomes. Results: Finally, 3615 patients were
included in the cohort study. 549 (15.2%) were diagnosed with COVID-19
infection. The most common symptom is fever, cough, sore throat. 51 (9.2%)
newborns had positive test results. In addition, the mother with COVID-19
infection were significantly associated with a higher rate of premature rupture
of membranes (PPROM) and postpartum hemorrhage. Furthermore, a mother with
COVID-19 infection was significantly associated with a higher rate of low birth
weight infant (LBW) and macrosomia in newborns, higher rate of respiratory
distress syndrome (RDS), higher rate of intro-ventricular hemorrhage (IVH),
higher rate of neonatal pneumonia, and a higher rate of aspiration of amniotic
fluid and meconium syndrome (AAFMS) (all p
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused coronavirus disease 2019 (COVID-19), posing a massive threat to public health [1]. It has led to more than 6.3 million deaths by midway through 2022 [2]. SARS-CoV-2 took the respiratory tract as the leading invasion site, with cough, shortness of breath, and fever as the main symptoms. In addition, it can also cause signs in the digestive system, cardiovascular system, nervous system, reproductive system, immune system, and urinary system [3, 4, 5, 6]. The severity of COVID-19 varies by population. Studies have indicated that the elderly, men, and individuals with other cardiovascular commodities are at increased risk of developing critical illness [7, 8]. Pregnancy is considered an independent risk factor for severe COVID-19 due to immunological and physical changes during pregnancy [9]. Therefore, studies about COVID-19 in pregnancy and the pregnant outcomes provide essential information for obstetric care.
With the spread of the epidemic, for the particular population of pregnant women, the public is paying more attention to whether the novel coronavirus can lead to vertical transmission from mother to child and the impact of the virus on pregnancy complications and adverse pregnancy outcomes in pregnant women. Some studies demonstrated that the risk of severe disease after COVID-19 infection was not significantly higher than in pregnant women-to-non-pregnant women in the early days of the COVID-19 pandemic [10, 11, 12]. However, recent studies revealed COVID-19 infection had a higher risk of developing severe maternal disease and the fetus [13, 14, 15, 16]. Papageorghiou et al. [17] found that COVID-19 in pregnancy is strongly associated with preeclampsia, especially in non-parturient women. Pre-pregnancy vaccination also has an impact on the outcome of a pregnancy infected with COVID-19. Villar et al. [18] found that infection with COVID-19 of pregnancy is associated with an increased risk of severe maternal morbidity and death, especially in symptomatic and unvaccinated women, and vaccination reduces the risk of serious complications and death in pregnant women. Pregnant women may still need to be vaccinated. In addition, diabetes mellitus and overweight or obesity were risk factors for COVID-19 diagnosis in pregnancy [19]. Currently, there is a controversy about the symptoms of COVID-19 infection and its impact on pregnancy outcomes. Optimal management of the mother and newborn remains unknown. Therefore, it is still necessary to carry out research on COVID-19 infection and pregnancy outcome.
There is growing evidence that COVID-19 also poses a higher risk of severe illness in pregnant women, and threats to this population can, unfortunately, be overlooked. In addition, a few studies are comparing pregnant women with COVID-19 and without COVID-19 in China mainland due to the government’s strict prevention and control policies [20, 21]. Under prevention and control protection, the COVID-19 infection rate of pregnant women is shallow, so research on pregnant women cannot be provided. Unfortunately, at the end of 2022, with the national development and adjustment of the epidemic prevention and control policy, the COVID-19 infection rate of the population increased, and the number of pregnant women also increased significantly. Therefore, it is urgent to study the symptoms of pregnant women after COVID-19 infection and the impact on pregnancy outcomes in China.
In this study, we conducted a large cohort study to analyze the relationship between pregnant women with or without COVID-19 infection and the clinical outcomes with maternal-neonatal outcomes in China. We aimed to evaluate whether COVID-19 infection is a risk factor for pregnancy complications and adverse neonatal effects to provide best practices regarding infection control in China.
This is a retrospective cohort study. This study analyzed 3615 participants from Fujian Provincial Maternity and Child Health Hospital and Fujian Obstetrics and Gynecology Hospital from November 1, 2022, to January 31, 2023 (Fig. 1). Pregnant women must meet the following criteria: (1) single pregnancy; (2) with nucleic acid or antigen test; (3) complete clinical data. Those women were excluded due to the following criteria: (1) the functions of vital organs such as heart, liver and kidney are severely deficient; (2) fetal anomalies; (3) people who do not have a fever at admission but are at risk of intrapartum fever. Maternal and newborn data were obtained from outpatient and inpatient records. The study was approved by the Hospital Ethics Committee of Fujian Provincial Maternity and Children’s Health Hospital, an affiliated hospital of Fujian Medical University (2023KY005).
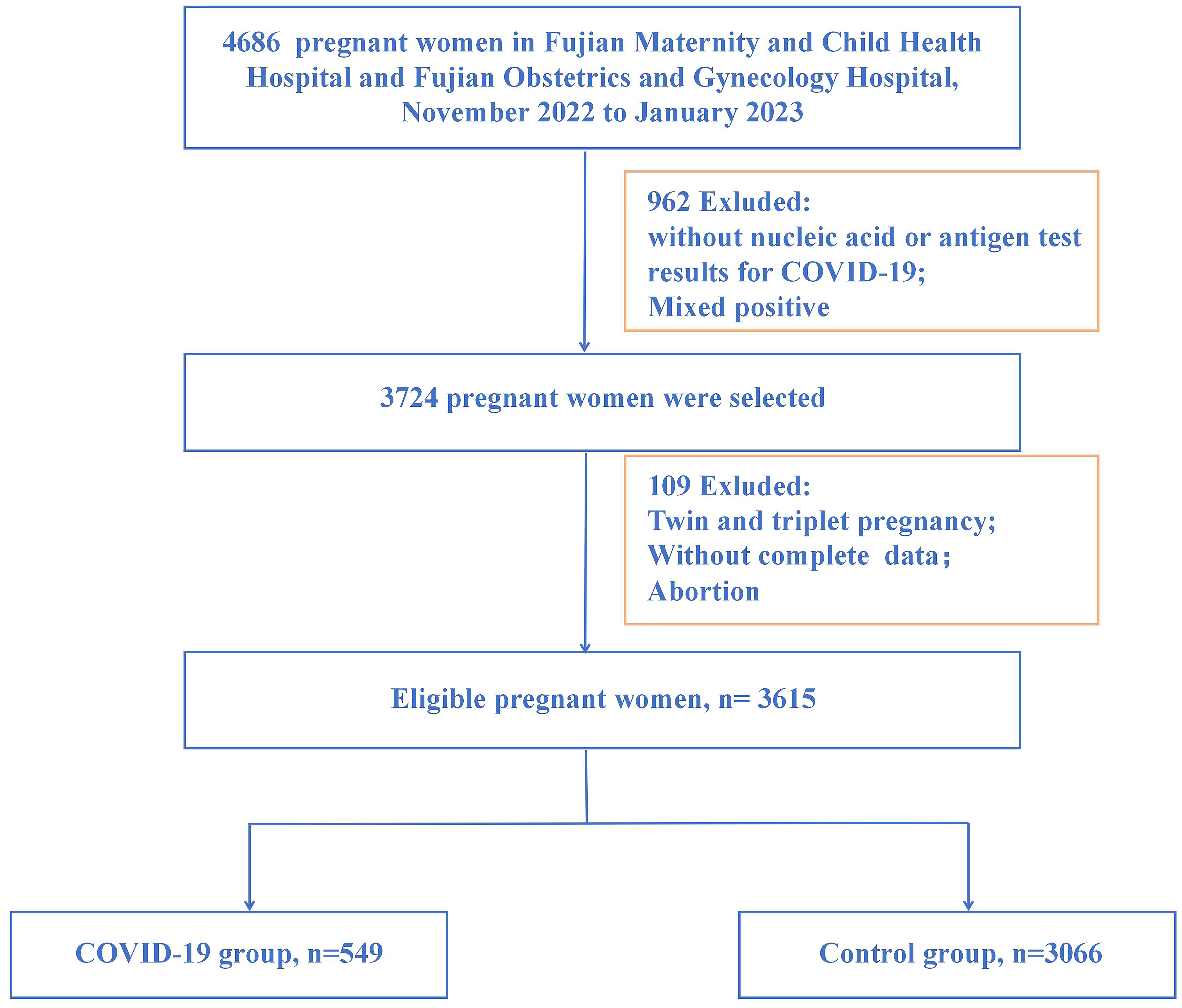 Fig. 1.
Fig. 1.Flowchart of participants in the study. COVID-19, coronavirus disease 2019.
According to the local prevention and control policies, all pregnant women must provide a 24-hour nucleic acid test report to deliver babies when admitted to the hospital. When the mother occurred symptoms of fever, sore throat, cough, shortness of breath, anosmia, ageusia, diarrhea, rhinorrhoea, myalgias, and vomiting, nucleic acid testing is required again.
Testing for SARS-CoV-2 was done using real-time polymerase chain reaction (RT-PCR); RealStar SARS-CoV-2 RT-PCR Kit, cobas SARS-CoV-2 Test (DAAN GENE, Guangzhou, Guangdong, China). Turnaround time from specimen collection to result from reporting was 24 h. Neonates with mother under COVID-19 infection were tested for SARS-CoV-2 by RT-PCR on a nasopharyngeal swab sample at 12–24 h, 2–7 days, and 8–28 days of life and as indicated at subsequent visits. These time points were chosen to allow repeat testing and routine neonatal care.
Symptomatic COVID-19 pregnancies were defined as the pregnancies had COVID-19 positive tests and the common symptom, such as fever, cough, sore throat, pain, and fatigue. Non-symptomatic COVID-19 pregnancies were defined as the pregnancies had positive tests but not the symptom [22]. Newborn COVID-19 refers to the period between delivery of the mother and 28 days after birth when the fetus tests positive for COVID-19. Pregnancy complication was defined as the adverse events that occurred during pregnancy or delivery times, including gestational diabetes mellitus (GDM), gestational hypertension, preterm premature rupture of membranes (PPROM), abnormal amniotic fluid (such as oligohydramnios and polyhydramnios), placental abruption, postpartum hemorrhage, and perineal laceration. An adverse neonatal outcome was defined as the presence of any of the following: neonatal death, intro-ventricular hemorrhage (IVH), patent ducts arterioles (PDA), respiratory distress syndrome (RDS), neonatal pneumonia, neonatal jaundice and aspiration of amniotic fluid and meconium syndrome (AAFMS).
The counting data used t-tests, and the measurement data were analyzed
with Pearson chi-squared tests by SPSS version 26.0 (IBM, Armonk, NY, USA).
Pearson chi-squared tests were used to assess the differences in demographic and
pregnancy characteristics by COVID-19 infection. Spearman rank correlation
coefficients were analyzed to evaluate the relationships between the symptoms of
COVID-19 disease and maternal and neonatal outcomes. In all statistical tests,
the differences were considered statistically significant at p-values
Finally, a total of 3615 patients were included in the cohort study. The
maternal outcomes and neonatal outcomes are shown in Table 1. The mean maternal
age was 30.2
| Characteristic | Total (N = 3615) | COVID-19 (N = 549) | No COVID-19 (N = 3066) | p-value | |
| Maternal age (years) | 30.2 |
30.4 |
30.2 |
0.319 | |
| Gravida | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.617 | |
| Parity | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | 0.637 | |
| Pre-BMI (kg/m |
0.740 | ||||
| Underweight | 510 (14.1) | 82 (14.9) | 428 (14.0) | ||
| Normal weight | 2440 (67.5) | 363 (66.1) | 2077 (67.7) | ||
| Overweight | 665 (18.4) | 104 (19.0) | 561 (18.3) | ||
| Education | 0.208 | ||||
| Junior school or under | 393 (10.9) | 71 (12.9) | 322 (10.5) | ||
| High school | 444 (12.3) | 62 (11.3) | 382 (12.5) | ||
| Undergraduate or higher | 2778 (76.8) | 416 (75.8) | 2362 (77.0) | ||
| Occupation | 0.160 | ||||
| White-collar workers and civil servant | 563 (15.6) | 80 (14.6) | 483 (15.8) | ||
| Professionals | 893 (24.7) | 155 (28.2) | 738 (24.1) | ||
| Domestic works | 472 (13.1) | 69 (12.6) | 403 (13.1) | ||
| Laborers | 321 (8.9) | 36 (6.6) | 285 (9.3) | ||
| Student | 15 (0.4) | 2 (0.4) | 13 (0.4) | ||
| Servant | 162 (4.5) | 30 (5.5) | 132 (4.3) | ||
| Other | 1189 (32.9) | 177 (32.2) | 1012 (33.0) | ||
| Hypertension | 221 (6.1) | 39 (7.1) | 182 (5.9) | 0.293 | |
| Diabetes mellitus | 41 (1.1) | 9 (1.6) | 32 (1.0) | 0.225 | |
Continuous variables are presented as mean
Of the 549 with COVID-19 infection, 541 (98.5%) had symptoms. The specific symptoms and distribution are shown in Fig. 2. Fever is the most common of these 541 women with symptoms, affecting 489 (89.1%) women. 352 (64.1%) of 541 women had a cough, followed by 218 (39.7%) with a sore throat, 107 (19.5%) with pain, 46 (8.4%) with fatigue, and 19 (3.5%) with other symptoms, such as vomiting, diarrhea, weight change, and insomnia.
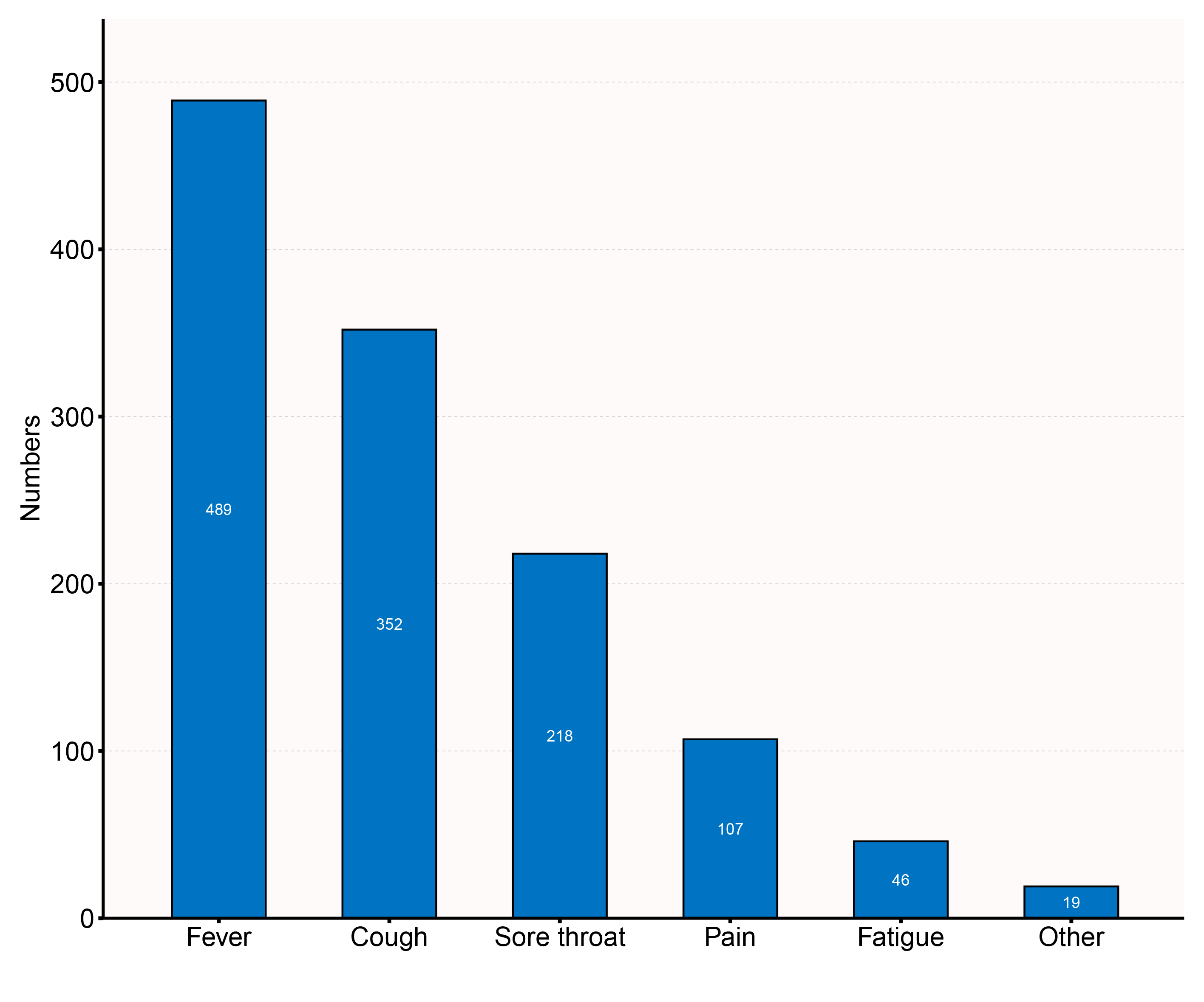 Fig. 2.
Fig. 2.Percentage of women with symptoms under COVID-19.
The RT-PCR results from a nasopharyngeal swab obtained at birth were available for all 549 neonates initially identified (Table 2). 545 (99.3%) neonates had a negative RT-PCR result, and 4 (0.7%) neonates had positive results. 545 neonates had repeat RT-PCR testing at 2–7 days of life, and 13 (2.4%) neonates had positive results. 532 neonates had recurrence RT-PCR testing at 8–28 days of life, of whom 34 (6.4%) had a positive test result.
| 24 h of life (N = 549) | 2–7 days of life (N = 545) | 8–28 days of life (N = 532) | ||
| RT-PCR done | ||||
| Yes | 549 (100%) | 545 (100%) | 532 (100%) | |
| No | 0 | 0 | 0 | |
| Result | ||||
| Positive | 4 (0.7%) | 13 (2.4%) | 34 (6.4%) | |
| Negative | 545 (99.3%) | 532 (97.6%) | 498 (93.6%) | |
Data are n (%) or n/N (%). RT-PCR, real-time polymerase chain reaction.
The relationships between COVID-19 infection and pregnancy outcomes are shown in
Table 3. Some maternal outcomes were significantly associated with COVID-19
disease. Mothers with COVID-19 infection were significantly associated with a
higher rate of PPROM and a higher rate of postpartum hemorrhage. In addition,
some neonatal outcomes were significantly associated with COVID-19 infection.
Mothers with COVID-19 infection were significantly associated with a higher rate
of low birth weight infant (LBW) and macrosomia in newborns, a higher rate of
RDS, a higher rate of IVH, a higher rate of neonatal pneumonia, and a higher rate
of AAFMS (all p
| Variable | COVID-19 (N = 549) n (%) | No COVID-19 (N = 3066) n (%) | p-value | |
| Maternal outcomes | ||||
| PPROM | 173 (31.5%) | 766 (25.0%) | 0.001 | |
| Placental abruption | 14 (2.6%) | 85 (2.8%) | 0.769 | |
| Abnormal amniotic fluid | 32 (5.8%) | 191 (6.2%) | 0.719 | |
| GA at delivery (weeks) | 38.2 |
38.3 |
0.114 | |
| Cesarean delivery | 215 (39.2%) | 1119 (36.5%) | 0.233 | |
| Postpartum hemorrhage | 24 (4.4%) | 62 (2.0%) | 0.001 | |
| Perineal laceration | 244 (44.4%) | 1349 (44.0%) | 0.846 | |
| Neonatal Mortality | 7 (1.3%) | 54 (1.8%) | 0.415 | |
| Birth weight | 0.022 | |||
| LBW | 52 (9.5%) | 231 (7.5%) | ||
| Normal birth weight | 469 (85.4%) | 2736 (89.2%) | ||
| Macrosomia | 28 (5.1%) | 99 (3.2%) | ||
| Apgar score (1 min) | 9.8 |
9.7 |
0.865 | |
| Apgar score (5 min) | 9.8 |
9.8 |
0.699 | |
| Apgar score (10 min) | 9.7 |
9.8 |
0.633 | |
| NICU admission | 39 (7.2%) | 174 (5.8%) | 0.200 | |
| RDS | 17 (3.1%) | 43 (1.4%) | 0.004 | |
| IVH | 25 (4.6%) | 87 (2.8%) | 0.033 | |
| PDA | 13 (2.4%) | 108 (3.5%) | 0.166 | |
| Neonatal pneumonia | 60 (10.9%) | 236 (7.7%) | 0.011 | |
| Neonatal jaundice | 47 (8.6%) | 336 (11.0%) | 0.093 | |
| AAFMS | 47 (8.6%) | 127 (4.1%) | ||
Notes: COVID-19, coronavirus disease 2019; GA, gestational age; PPROM, preterm premature rupture of membranes; LBW, low birth weight infant; NICU, neonatal intensive care unit; RDS, respiratory distress syndrome; IVH, intraventricular haemorrhage; PDA, patent ductus arteriosus; AAFMS, aspiration of amniotic fluid and meconium syndrome.
According to Table 4, we found that the symptoms of COVID-19 infection, such as
fever, cough, sore throat, pain, and fatigue, were significantly associated with
maternal outcomes. It suggested that positive symptoms indicated a higher rate of
gestational hypertension (r = –0.141, p = 0.001). The mothers with
pain, sore throat, and fatigue had a higher rate of PPROM (r = 0.091, p
= 0.033; r = 0.114, p = 0.008; r = 0.204, p
| Variable | Symptoms | Fever | Pain | Sore throat | Cough | Fatigue | |
| GDM | r | 0.015 | –0.001 | 0.045 | 0.012 | 0.066 | –0.039 |
| p | 0.731 | 0.973 | 0.293 | 0.774 | 0.120 | 0.360 | |
| Gestational hypertension | r | –0.141** | –0.043 | –0.033 | –0.031 | –0.035 | 0.027 |
| p | 0.001 | 0.310 | 0.442 | 0.470 | 0.414 | 0.534 | |
| PPROM | r | –0.063 | 0.021 | 0.091* | 0.114** | –0.042 | 0.204** |
| p | 0.143 | 0.630 | 0.033 | 0.008 | 0.327 | ||
| Placental abruption | r | 0.018 | 0.019 | –0.051 | –0.013 | –0.048 | 0.034 |
| p | 0.667 | 0.658 | 0.237 | 0.753 | 0.261 | 0.425 | |
| Abnormal amniotic fluid | r | 0.028 | –0.042 | –0.101* | –0.054 | 0.018 | –0.046 |
| p | 0.515 | 0.322 | 0.018 | 0.209 | 0.675 | 0.284 | |
| Cesarean delivery | r | –0.018 | 0.008 | –0.082 | –0.039 | –0.021 | –0.059 |
| p | 0.676 | 0.855 | 0.055 | 0.359 | 0.631 | 0.172 | |
| Postpartum hemorrhage | r | 0.024 | 0.074 | 0.097* | 0.118** | 0.104* | 0.450** |
| p | 0.570 | 0.082 | 0.023 | 0.006 | 0.015 | ||
| Perineal laceration | r | 0.069 | 0.039 | –0.034 | 0.030 | 0.025 | –0.058 |
| p | 0.106 | 0.365 | 0.431 | 0.491 | 0.558 | 0.174 | |
| Mortality | r | 0.013 | 0.040 | 0.026 | 0.040 | 0.017 | –0.035 |
| p | 0.762 | 0.356 | 0.544 | 0.346 | 0.690 | 0.420 | |
| NICU admission | r | –0.032 | –0.020 | 0.011 | –0.073 | –0.036 | 0.153** |
| p | 0.453 | 0.651 | 0.800 | 0.089 | 0.402 | ||
| Apgar score (1 min) | r | 0.037 | 0.010 | –0.010 | 0.001 | 0.049 | –0.031 |
| p | 0.391 | 0.817 | 0.816 | 0.977 | 0.249 | 0.467 | |
| Apgar score (5 min) | r | 0.082 | 0.017 | –0.008 | 0.012 | –0.001 | 0.008 |
| p | 0.054 | 0.691 | 0.854 | 0.773 | 0.984 | 0.857 | |
| Apgar score (10 min) | r | 0.087* | 0.022 | –0.014 | 0.028 | 0.008 | 0.047 |
| p | 0.042 | 0.607 | 0.743 | 0.519 | 0.851 | 0.269 | |
| RDS | r | –0.263** | –0.174** | –0.030 | –0.049 | –0.018 | –0.018 |
| p | 0.486 | 0.250 | 0.675 | 0.668 | |||
| IVH | r | 0.025 | –0.009 | 0.091* | 0.198** | 0.127** | –0.003 |
| p | 0.561 | 0.839 | 0.033 | 0.003 | 0.938 | ||
| PDA | r | 0.018 | 0.015 | –0.047 | –0.029 | 0.016 | –0.004 |
| p | 0.679 | 0.718 | 0.277 | 0.503 | 0.704 | 0.923 | |
| Neonatal pneumonia | r | –0.013 | 0.026 | 0.022 | 0.042 | 0.050 | 0.001 |
| p | 0.763 | 0.549 | 0.608 | 0.321 | 0.239 | 0.988 | |
| Neonatal jaundice | r | –0.081 | 0.022 | 0.030 | 0.004 | –0.016 | 0.024 |
| p | 0.057 | 0.603 | 0.484 | 0.925 | 0.706 | 0.569 | |
| AAFMS | r | 0.034 | 0.020 | 0.050 | 0.090* | 0.061 | 0.005 |
| p | 0.421 | 0.637 | 0.242 | 0.035 | 0.153 | 0.907 | |
**p
According to Table 4, the mothers with the sign of fatigue had a higher rate of
neonatal intensive care unit (NICU) admission (r = 0.153, p
Pregnancy is considered an independent risk factor for severe COVID-19 due to immunological and physical changes during pregnancy [9]. The progression of COVID-19 mainly depends on the viral entry into the host cells after binding to the angiotensin-converting enzyme 2 (ACE2). This factor may make pregnant women more susceptible to COVID-19 because ACE 2 is highly expressed in the placenta throughout the pregnancy [23]. During the COVID-19 pandemic, concerns arose about whether infection by the SARS-CoV-2 virus adversely affects pregnancy outcomes. Under the prevention and control protection in China Mainland, the COVID-19 infection rate among pregnant women is shallow. Therefore, few studies about COVID-19 infection in pregnancy and pregnancy outcomes exist. Unfortunately, at the end of 2022, with the national development and adjustment of the epidemic prevention and control policy, the COVID-19 infection rate of the population increased, and the number of pregnant women also increased significantly. Therefore, it is urgent to study the symptoms of pregnant women after COVID-19 infection and the impact on pregnancy outcomes in China.
In the present study, 549 (15.2%) were diagnosed with COVID-19 infection. Among them, 489 (89.1%) fever is the most common symptom, followed by 352 (64.1%) cough, 218 (39.7%) sore throat, 107 (19.5%) pain, 46 (8.4%) fatigue and 19 (3.5%) other symptoms. Similarly, studies have indicated that the most common clinical signs of COVID-19 are fever, fatigue, cough, expectoration, anorexia, sputum production, and shortness of breath [24, 25]. Thus, the symptoms of pregnant women with COVID-19 infection are similar to those of non-pregnant women.
We found that mothers with COVID-19 infection had slightly higher cesarean
section rates than non-infected mothers, but the difference was not statistically
significant. The current findings are different. The research of Jafari
et al. [26] shows that the rate of cesarean delivery in
pregnant women infected with COVID-19 infection varied from 42.9%–85%,
significantly higher than that of without COVID-19 disease pregnant women. And, a
survey of obstetricians by the Japanese Obstetrics Association showed that
obstetricians are more inclined to use cesarean section for pregnant women with
COVID-19 [27]. However, Eleje et al. [28] found that the cesarean
section rate during the COVID-19 period was significantly less than the period
prior to the pandemic. Moreover, we revealed a higher rate of postpartum
hemorrhage with COVID-19 infection. The study by
Auger et al. [29] also shows an increased risk of COVID-19 infection and
postpartum hemorrhage. In addition, we found pregnancies with COVID-19 infection
were significantly associated with higher PPROM. It is similar to previous
studies. Zhu et al. [30] reported a study with 9 cases of pregnant women
with COVID-19 infection and 10 newborns delivered, among which 3 cases had PPROM.
Also, Du et al. [31] reported an 11% increased risk of premature
rupture of membranes and fetal distress during the covid-19 pandemic (95% confidence interval (95% CI),
1.04 to 1.18; p
We demonstrated that some neonatal outcomes were significantly associated with COVID-19 infection. Mothers with COVID-19 infection were significantly associated with higher rates of LBW and macrosomia in newborns, higher rate of RDS, higher rate of IVH, higher rate of neonatal pneumonia, and a higher rate of AAFMS. Similarly, there is a rise in preterm and low birth weight cases due to COVID-19 infection [32]. Symptomatic illness in pregnancy is related to adverse maternal and neonatal outcomes such as higher cesarean delivery rates, preterm delivery, and low birth weight. In addition, 4 (0.7%) newborns are positive at the 12–24 h. However, the four newborns could not confirm whether it was caused by vertical transmission. There is still debate regarding the rate of vertical transmission of COVID-19 infection to newborns and its possible mechanisms-transplacental or during passage through the birth canal. In a study conducted by Edlow et al. [33] in 2020, no evidence of placental infection or standard vertical transmission of COVID-19 was found in the 64 patients who tested positive for COVID-19. Also, a retrospective cohort [34] analysis reported data for 101 newborns from mothers that tested positive for SARS-CoV-2. There was no evidence of vertical transmission during the first 25 days of life, despite the newborns rooming in with mothers and direct breastfeeding practices.
This is a large sample of data in South China, which is representative. However, there are some limitations to this study. Firstly, this study did not include data on vaccination, so it is impossible to assess the impact of vaccination on pregnant women. Secondly, though the case number of the present study was over the previous studies, it is a retrospective single-center study, rather than a prospective study. Multi-center or randomized controlled trial is necessary for the future.
In conclusion, our data prove that specific symptoms of pregnant women were similar to non-pregnant, including fever, cough, sore throat, pain, and fatigue. In addition, some maternal complications and neonatal outcomes were significantly associated with COVID-19 infection. It suggests that pregnancy with COVID-19 disease in China has some adverse consequences for pregnant women and newborns. Therefore, infected pregnant women need to be alert to these pregnancy complications, such as PPROM and postpartum hemorrhage. Clinicians should strengthen clinical monitoring to provide better care for mothers and children, and provide infected pregnant women with appropriate counseling and advice on PPROM and postpartum hemorrhage. The COVID-19 epidemic continues, requiring targeted public health measures to reduce the infection rate in pregnant women and the poor prognosis of mothers and children.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
YL, ZC, LX and XX designed the research study. ZW and YX collected the data and performed the research. YL analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee Fujian Provincial Maternity and Children’s Hospital, an affiliated hospital of Fujian Medical University (approval number: 2023KY005). This study is a retrospective study, the data are anonymous, and the requirement for informed consent was therefore waived.
We would like to thank all the women who kindly agreed to participate in this study.
Joint Funds for the innovation of science and Technology, Fujian province (Grant number 2020Y9147). Joint Funds for the innovation of science and Technology, Fujian province (2020Y9165).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
