- Academic Editor
Background: Endometriosis is common in reproductive age women, which contributes to infertility. This study aims to build a model including antimüllerian hormone (AMH) to predict spontaneous pregnancy within one year after laparoscopy combined with hysteroscopy in women with minimal to mild endometriosis-associated infertility. Methods: There were 220 women included in the study, and a generalized linear model was constructed. The women enrolled in the study were experienced symptoms of endometriosis, and underwent combined laparoscopy and hysteroscopy between January and September 2016. All participants were diagnosed with minimal to mild endometriosis following surgery. Results: The predictive power (sensitivity, specificity, area under the curve (AUC)) of the model for spontaneous pregnancy rate after surgery was measured and compared with the endometriosis fertility index (EFI). The AUC for prediction model of postoperative spontaneous pregnancy was 0.697 (95% confidence interval (95% CI): 0.626–0.768). The best cut-off point was 0.629 (sensitivity: 78.4%; specificity: 54.5%). While the AUC for EFI as the spontaneous pregnancy predictor was 0.573 (95% CI: 0.498–0.648). The best cut-off point was 7.5 (sensitivity: 42.3%; specificity: 74.8%). Conclusions: We suggest that laparoscopy combined with hysteroscopy may offer better fertility outcomes to patients with minimal to mild endometriosis-associated infertility. The nomogram visualized the points of variate in the generalized linear model may provide a simple and convenient method for clinicians in making decisions for individual patients.
Endometriosis is common in reproductive age women, characterized by the presence
of ectopic endometrial implants [1]. The stages of endometriosis were assessed by
the revised American Fertility Society (rAFS) scores, with total score
Currently, there are many clinical prediction models and biomarkers for women with infertility or subfertility to explore a reliable method to assess the probability of pregnancy [5, 6]. Among them, the most commonly used method for estimating the pregnancy rate of women with endometriosis-associated infertility is biomarker interleukin-6 (IL6) [7] and the endometriosis fertility index (EFI) [8, 9, 10], which includes age, duration of infertility, prior pregnancy, least function (LF) score, rAFS endometriosis lesion and total score. However, declined ovarian reserve is an increasing consideration of reduced fertility [11]. Antimüllerian hormone (AMH) is considered a reliable independent marker of ovarian reserve [12]. To date, only a few publications have focused on the relationship between AMH and spontaneous conception [13, 14, 15]. Previous studies have shown a significantly higher frequency of endometrial polyps in women with endometriotic infertility [16, 17]. It has been revealed in a meta-analysis that identifying and removing endometrial polyps via hysteroscopy will be clinically helpful to treat endometriosis-related infertility [18].
Therefore, a new model including AMH and endometrial polyps is needed to assess the spontaneous pregnancy rate after surgery in women suffering from minimal/mild endometriosis associated infertility.
This retrospective study reviewed infertile women with one or more symptoms of
endometriosis such as dysmenorrhea, dyspareunia, and chronic pelvic pain, or with
the finding of a posterior fornix tenderness nodule found by gynecologic
examination. They were diagnosed with minimal/mild endometriosis by laparoscopy
combined with hysteroscopy and been treated during the operation between January
and September 2016 in West China Second University Hospital of Sichuan
University. The basic information from medical history and operative documents
were reviewed and collected. According to intraoperative findings, we assessed
the stages of endometriosis by rAFS, with a score of
Venous blood samples were collected within 3 months before surgery. Serum AMH
levels were determined by enzyme-linked immunosorbent assay (Kang Run
biotechnology Co., Ltd., Guangzhou, Guangdong, China). The variation coefficient between and
within batches were
The enrolled 220 patients were further divided into 3 subgroups according to
their serum AMH levels: Group 0: AMH
Data were analyzed using R software package version 3.4.4 (https://cran.r-hub.io/bin/windows/base/old/3.4.4/). We used package “rms” for nomogram and “pROC” for ROC plots. Student’s t-tests were used for continuous values and 83 Chi-square tests were used to compare categorical values. Moreover, a generalized linear 84 model was made. AMH levels were projected to interact with age in the generalized linear model. A nomogram was constructed to visualize the results of the generalized linear model.
The demographics of 220 patients are shown in Table 1, including their age, previous pregnancy history, duration of infertility, serum AMH levels, intraoperative finding as LF score, revised American Fertility Society (rAFS) score, and existence of endometrial polyps which located in the middle and upper part of the uterine cavity, with a number of 1–6 and a diameter of 2–10 mm, most of them present with tongue-like or inflammatory protrusions. The mean duration of follow-up was 14.7 months (10–22 months). There were 123 spontaneous pregnancies (55.90%, 123/220) noted during follow-up with 4 being ectopic pregnancies. There were 101 full term deliveries and 6 preterm births secondary to of twin pregnancy or premature rupture of membrane.
| Variable | Pregnant | Non-pregnant | ||
| (n = 123) | (n = 97) | |||
| Basic information | ||||
| Age (years), mean |
29.39 |
30.79 | ||
| 101 | 61 | |||
| 22 | 36 | |||
| Serum AMH levels (ng/mL), mean |
6.20 |
5.55 | ||
| 46 | 45 | |||
| 4–6 | 24 | 21 | ||
| 53 | 31 | |||
| Medical history | ||||
| Duration of infertility (years), mean |
2.22 |
2.88 | ||
| 107 | 72 | |||
| 16 | 25 | |||
| Previous pregnancy history | ||||
| Yes | 66 | 48 | ||
| No | 57 | 49 | ||
| Intraoperative findings | ||||
| Endometrial polyps | ||||
| Yes | 40 | 30 | ||
| No | 83 | 67 | ||
| LF-Score (mean |
5.16 |
5.24 | ||
| rAFS-Score (mean |
4.91 |
4.99 | ||
Note: Values are expressed as mean
In women
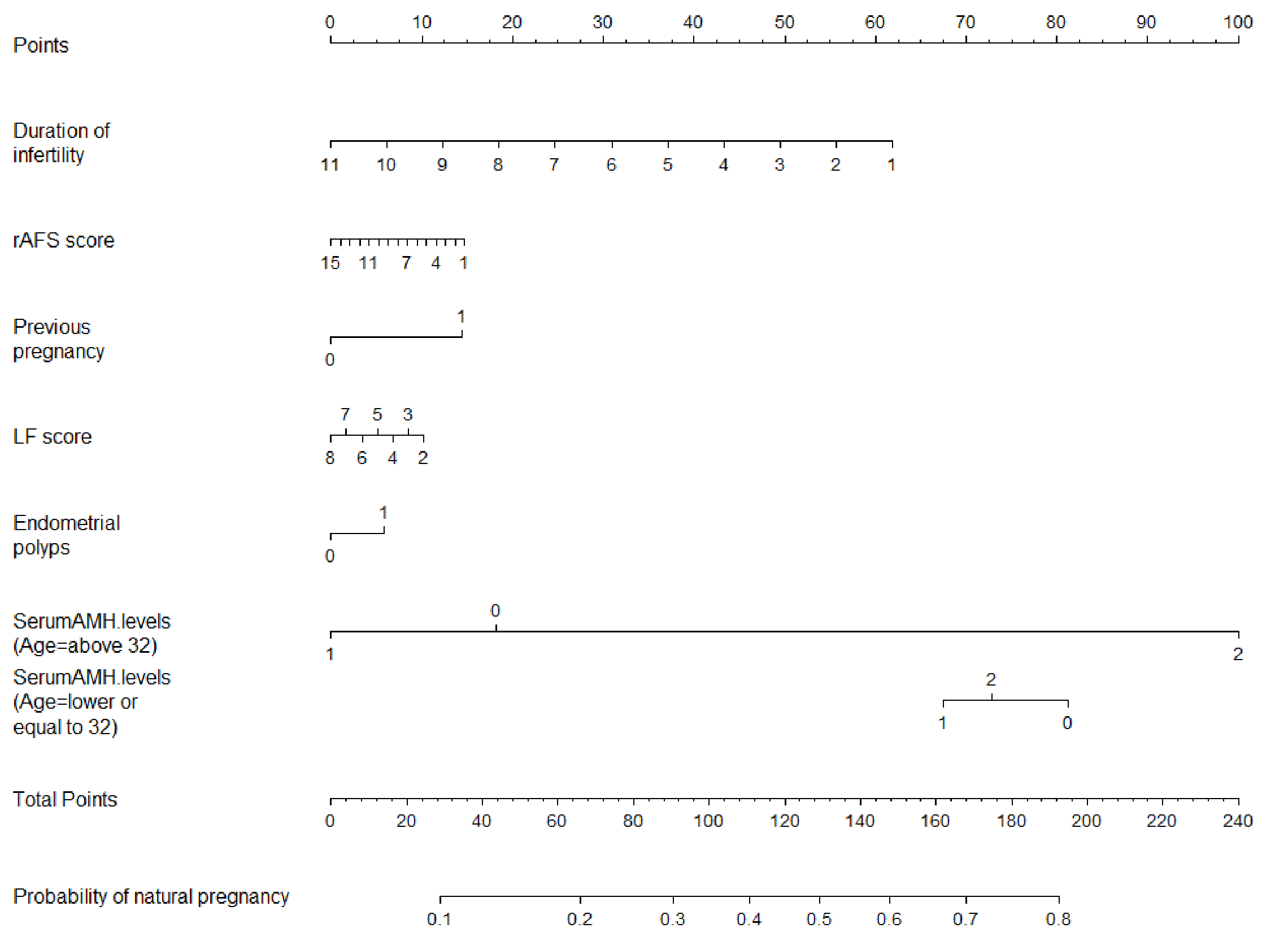 Fig. 1.
Fig. 1.Nomogram for predicting the individual probability of
spontaneous pregnancy in infertile women with minimal to mild endometriosis.
Note: Group 0: AMH
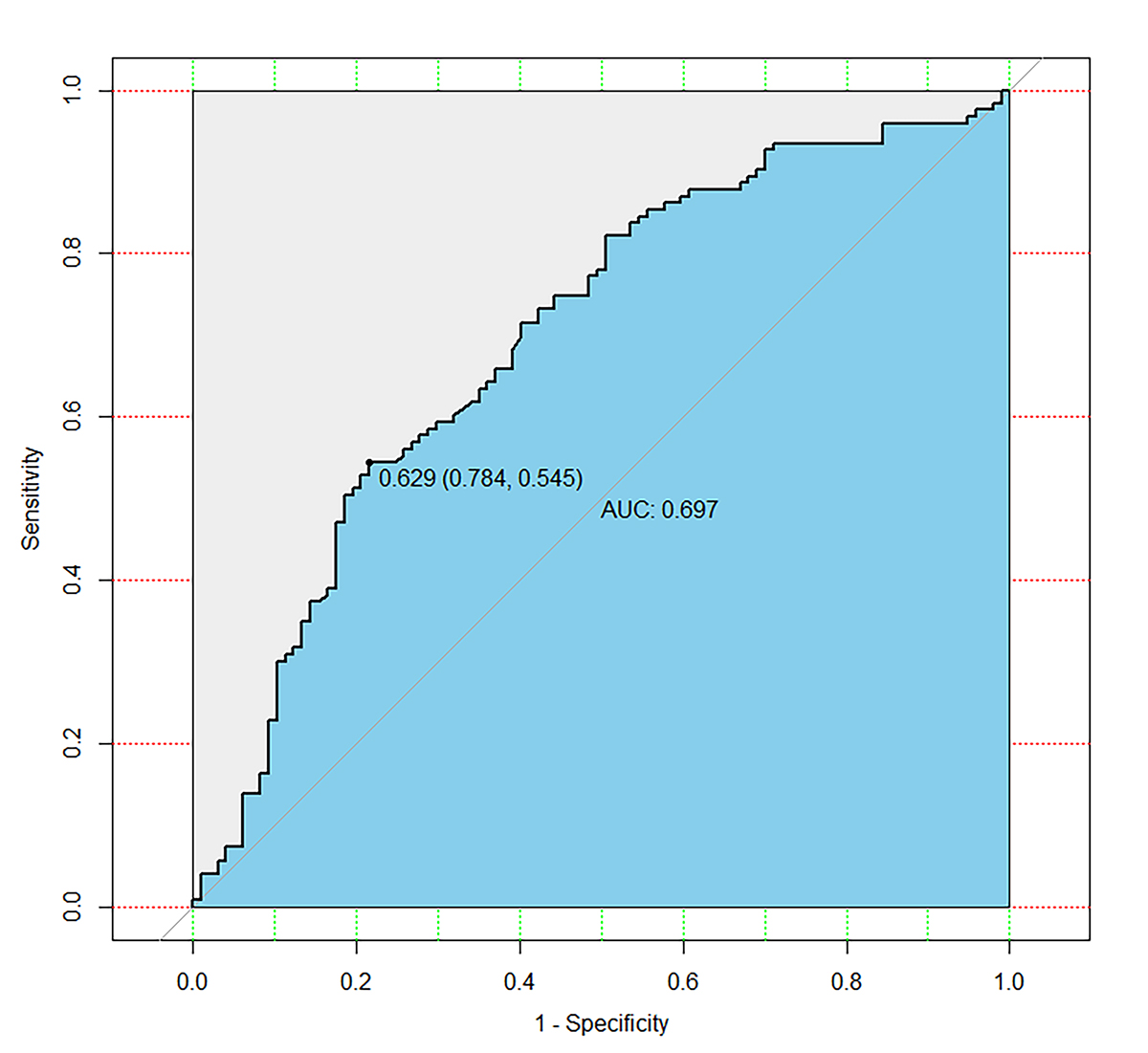 Fig. 2.
Fig. 2.Receiver operator characteristic curve. The area under the curve (AUC) for prediction model for spontaneous pregnancy was 0.697 (95% confidence interval (95% CI): 0.626–0.768). The best cut-off point was 0.629 (sensitivity: 78.4%; specificity: 54.5%).
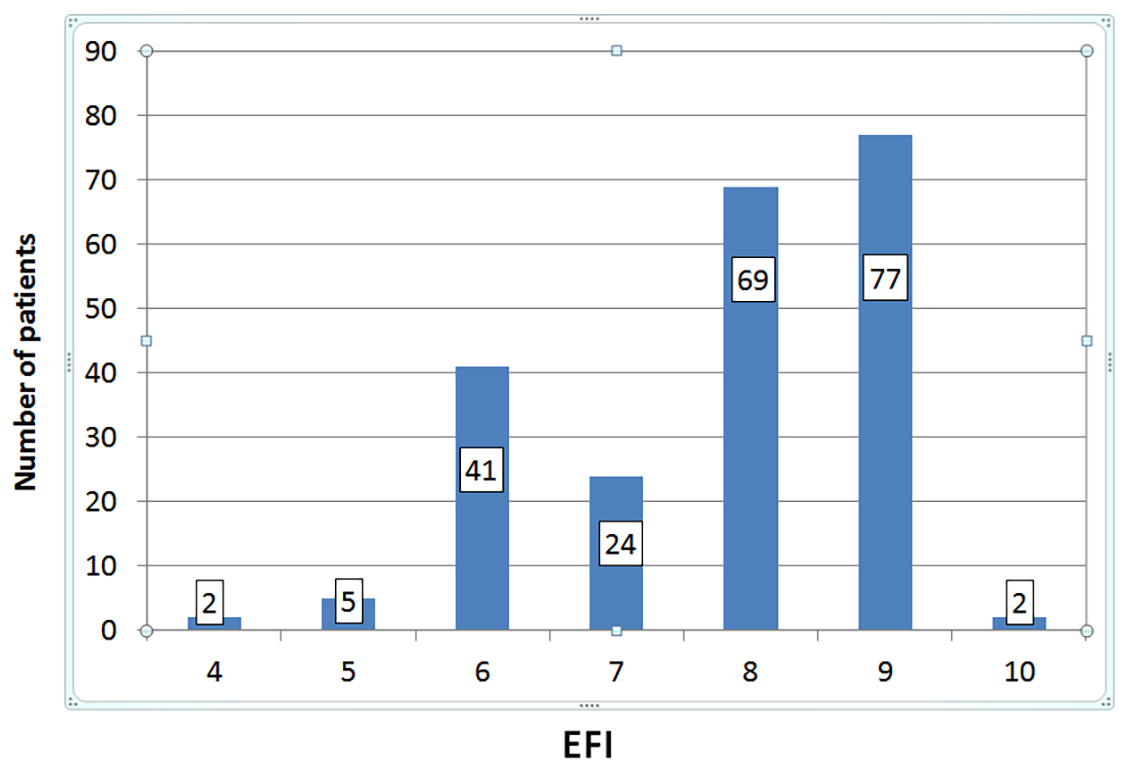 Fig. 3.
Fig. 3.Distribution of patients according to their endometriosis fertility index (EFI) score. The median EFI score was 8.
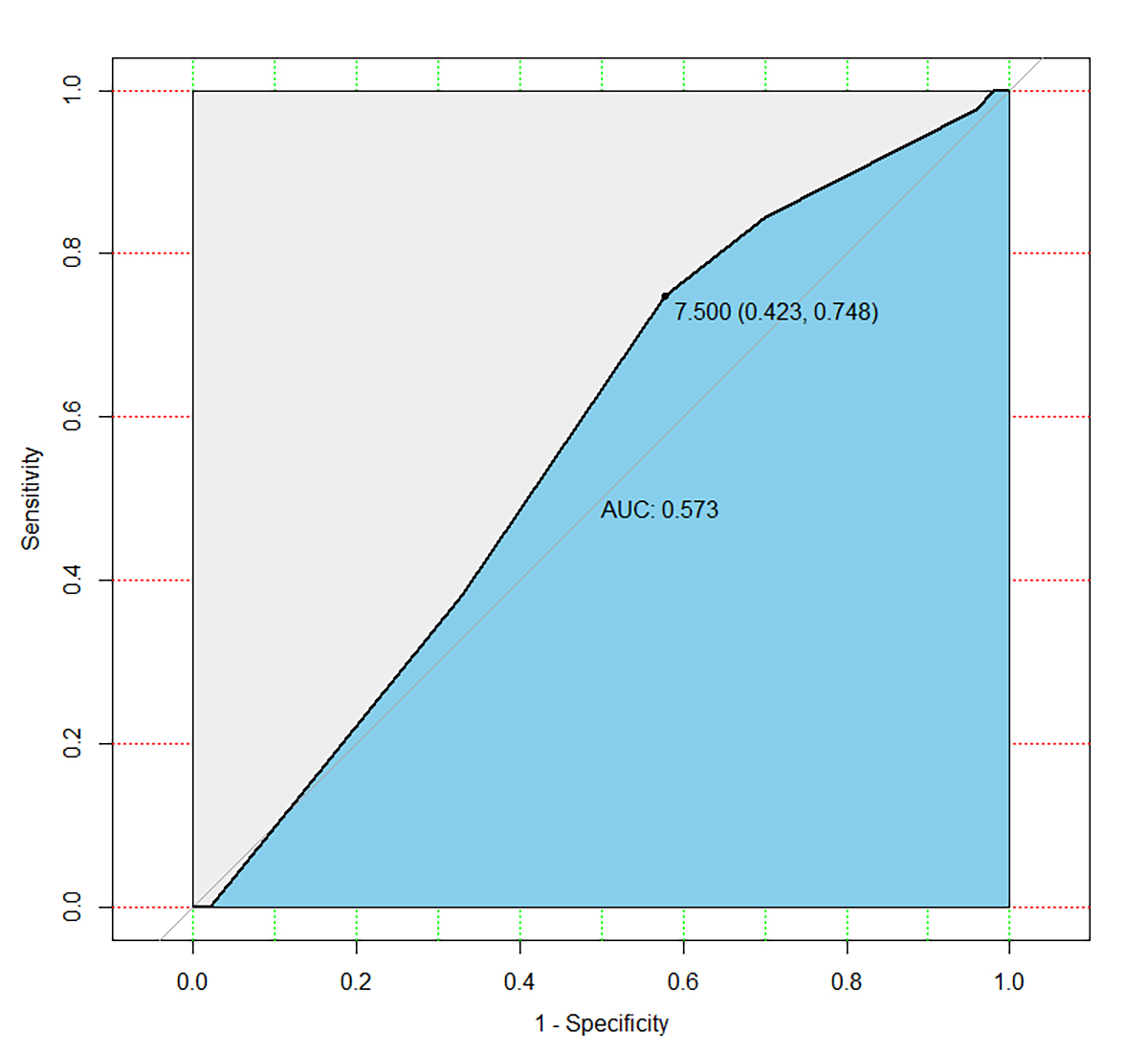 Fig. 4.
Fig. 4.Receiver operator characteristic curve. The area under the curve for EFI as a spontaneous pregnancy predictor was 0.573 (95% CI: 0.498–0.648). The best cut-off point was 7.5 (sensitivity: 42.3%; specificity: 74.8%).
| Variable | Odds ratio | 95% CI | p | ||
| Lower | Higher | ||||
| History of pregnancy | 1.370 | 0.766 | 2.451 | 0.289 | |
| Duration of infertility | 0.873 | 0.744 | 1.025 | 0.096 | |
| LF score | 0.963 | 0.743 | 1.249 | 0.779 | |
| rAFS score | 0.977 | 0.859 | 1.112 | 0.727 | |
| Endometrial polyps | 1.138 | 0.615 | 2.106 | 0.680 | |
| Age group (1) | 3.984 | 1.587 | 10.006 | 0.003 | |
| AMH group | |||||
| AMH group (1) | 0.672 | 0.114 | 3.979 | 0.661 | |
| AMH group (2) | 6.011 | 1.305 | 27.684 | 0.021 | |
| Age group * AMH group | |||||
| Age group (1) by AMH group (1) | 1.100 | 0.150 | 8.070 | 0.095 | |
| Age group (1) by AMH group (2) | 0.138 | 0.025 | 0.761 | 0.023 | |
Note: *Refers to interaction. 95% CI, 95% confidence interval.
Formula = History of pregnancy + Duration of infertility + LF score + rAFS
score + Endometrial polyps + Age
The probability of spontaneous pregnancy after surgery is calculated by drawing a line to the point on the axis for each of the following variables: duration of infertility, previous pregnancy history, LF score, rAFS score, endometrial polyps and age interacting with AMH. First, the points for each variable are summed and located on the total points line. Next, a vertical line is projected from the total points line to the predicted probability bottom scale to obtain the individual probability of spontaneous pregnancy.
To our knowledge, the prediction model and the nomogram designed in this study has not been previously reported to evaluate individual probability of postoperative pregnancy rate in women with mild/minimal endometriosis. In addition to factors included in commonly used EFI score, our prediction model contains ovarian reserve and endometrial polyps, which have been suggested to have influence on pregnancy. Our results suggest that the individual probability of postoperative spontaneous pregnancy in women with minimal to mild endometriosis can be predicted more accurately by a nomogram than EFI. This is of particular interest for clinicians who are increasingly interested in such tools to support their decision about treatment options and medical advice for the patients. According to the consensus on diagnosis and evaluation of female infertility by ASRM (American Society of Reproductive Medicine), if patients have signs and symptoms of endometriosis or risk factors for tubal obstruction, laparoscopy should be considered [19]. In our study, the surgery for each patient was performed in the same hospital, ensuring that the surgical indications and treatment options are similar. Of the 21 couples who were lost to follow-up, 8 couples divorced, 10 couples lost contact, and 3 couples did not desire for pregnancy for other reasons. The advantages of our study can be presented as follows: accurate clinical data, prospective collection of data, consecutive incorporation of 220 patients and lower loss of patients to follow-up which was only 8.7%.
Currently, other clinical models of predicting pregnancy outcomes have been
built. The most commonly used prediction rule for endometriosis-associated
infertility is EFI, which includes age, duration of infertility, previous
pregnancy history, LF score, rAFS endometriosis lesion score and rAFS
endometriosis total score. EFI has been proved to be an efficient way to predict
pregnancy rate in various studies [8, 9, 10, 20, 21]. However, ovarian reserve should
not be ignored in predicting the pregnancy probability of women with minimal to
mild endometriosis. Since intrauterine factors such as endometrial polyps are of
high prevalence in endometriosis-associated infertility [16], it should be taken
into consideration. Our study was focused on the post-operative spontaneous
pregnancy rate, and aimed to develop a model including AMH levels and other
pregnancy related factors, statistically or clinically, to predict the
spontaneous pregnancy rate of women with minimal to mild endometriosis. Previous
study suggested that AMH levels were associated with age, and that AMH levels
decrease steadily with the increase of age from 24 to 50 years [11]. A recent
study has suggested that women in their late thirties experience a significant
reduction in fecundity and an increase in the probability of infertility [22].
Our study demonstrated similar results in that inpatients
We presented data that combined laparoscopy with hysteroscopy may offer better fertility outcomes to patients with minimal to mild endometriosis-associated infertility. The nomogram visualized the points of variate in the generalized linear model and may provide a simple and convenient method for clinicians in making decisions for individual patients.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
WH and BL designed the research study. BL, TJP and XH performed the research. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of West China Second University Hospital of Sichuan University (approval number: 2019-067).
We would like to express our gratitude to all those who helped us during the writing of this manuscript. Thanks to all the peer reviewers for their opinions and suggestions.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
