Aims: to evaluate changes in clinical periodontal parameters, salivary
levels of MMP-8 and MMP-9, in individuals taking Isotretinoin (INN), and compare
with individuals not taking the medication and to compare findings among
different stages of periodontal disease and healthy periodontium.
Material and methods: A case-control study was conducted with a total of
180 human adults divided into six groups. Clinical parameters, including pocket
depth (PD), clinical attachment level (CAL), and bleeding on probing (BOP) were
measured at six sites per tooth. Whole unstimulated saliva samples were collected
from all subjects to detect salivary level of MMP-8, MMP-9 using Enzyme-linked
immunosorbent assay (ELISA). Data were analyzed using IBM SPSS Software. Kruskal
Wallis test and Mann-Whitney U-tests were used to test any significant
differences in any of the groups on all parameters. Pearson Chi-square test was
used to compare the distribution of categorical responses across the study
groups. All tests were compared at a significance level of 0.05.
Results: In Gingivitis cases, INN group was found to have significantly
less BOP (P
Isotretinoin (INN); which is also known as 13-cis-Retinoic acid (brand name; Roaccutanne/Accutane-Roche, Switzerland), is an oral administrative drug that belongs to a class of drugs known as retinoids [1]. It is mostly used to treat severe nodular acne that has not responded to other treatment [2]. It has been approved by FDA to be used as a treatment for acne in the United States since 1982 [3]. In terms of mechanism of action; INN is the only therapy that targets all major etiological factors implicated in acne [4]. This is achieved by influencing cell-cycle progression, cellular differentiation, cell survival, and apoptosis. It results in a significant reduction in sebum production from sebaceous glands, influences comedogenesis, lowers surface and ductal Propionibacterium acnes, and has anti-inflammatory properties [5, 6, 7, 8, 9].
In terms of the anti-inflammatory action, INN inhibits the action of the matrix metalloproteinase-9 (MMP-9) in facial sebum without influencing the action of tissue inhibitors of metalloproteinases-1 and 2 (TIMP-1 and TIMP-2) [10]. In addition to its anti-inflammatory effects, Papakonstantinou et al. [10] reported an anti-bacterial effect of INN in cases of facial sebum devoid of Propionibacterium acnes and Staphylococcus epidermidis. It is well-known that MMPs are a group of enzymes that are responsible for the degradation of most extracellular matrix proteins during organogenesis, growth, and normal tissue turnover [11]. They have shown a significant role in tissue destruction during the path of periodontal diseases [12]. Several studies have reported the presence of multiple MMPs in the gingival tissue, such as MMP-2, MMP-7, MMP-14 [13].
In the Gingival Crevicular Fluid (GCF) and saliva, the most widely investigated MMPs are MMP-8, MMP-9, and MMP-13 [14, 15, 16, 17]. Both MMP-8 and MMP-9 have been used as markers for active periodontal destruction or disease activity in the GCF, saliva, and serum [12, 18, 19]. Both collagenases MMP-8 and MMP-9 are mainly responsible for collagen degradation in inflamed tissue during gingivitis and adult periodontitis [20]. The destroying activity of MMPs can be controlled by inhibiting their action, which can be mediated by the four members of the tissue inhibitor of metalloproteinase (TIMP) family [20].
The objective of this study was to evaluate changes in: (1) clinical periodontal parameters including: pocket depth (PD), clinical attachment level (CAL), bleeding on probing (BOP); and (2) salivary levels of MMP-8, MMP-9 during treatment with oral INN in patients receiving the drug for a minimum of 3 months for cutaneous acne, and to compare these findings among patients with different stages of periodontal disease and healthy periodontium.
The study was registered after receiving approval from the Institutional Review Board (IRB) related to King Saud University institute (E-19-3856). The study was conducted in accordance with the Helsinki Declaration of 1975, revised in 2013. The sample size was determined using G-Power software based on a previous pilot study done. Where a confidence level was set at 95%, a power level of 80% was set with a moderate effect size with a final sample size of 180 subjects. These subjects were divided in a total of six groups with equal sample sizes (n = 30).
Inclusion criteria for participants includes (A) Human subjects who are
All non-smoker subjects who were receiving 0.5 or 1.0 mg/kg/day dose of INN (Roaccutane®) and not receiving any periodontal treatment or antibiotic therapy for medical or dental reasons 3 months prior to the investigation were included. After periodontal examination for these subjects, which was held in Dental University Hospital at King Saud University, they were divided into three groups. The first group was composed of subjects with Healthy Periodontium (HINN) (n = 30), the second group was composed of subjects with Generalized Plaque induced Gingivitis (GINN) (n = 30), and the third group was composed of subjects with Generalized Periodontitis Stage I (PINN) (n = 30). This was defined as presence of interproximal clinical attachment loss not exceeding 2 mm associated with maximum probing depth of 4 mm and mostly horizontal bone loss that is less than 15% of the coronal third of related teeth roots with an extend of 30% or more and no tooth loss related to periodontal condition.
Regarding to the negative control groups, which include subjects who were not taking the medication (INN), but categorized in the same manner. The first group was composed of subjects with Healthy Periodontium (HC) (n = 30), the second group includes subjects diagnosed with Generalized Plaque induced Gingivitis (GC) (n = 30), and finally the third group includes subjects diagnosed with Generalized Periodontitis Stage I (PC) (n = 30) [21]. Subjects were excluded from the study if they underwent any long-term use of medications affecting periodontal status, had a history of metabolic bone diseases, autoimmune diseases, diabetes, or postmenopausal osteoporosis. Pregnant women were also excluded from the study.
All teeth except third molars were assessed for periodontal clinical parameters by two calibrated examiners (A.Z. and M.K.) blindly, without knowing that the subject belongs to which group. Clinical parameters, including pocket depth (PD), clinical attachment level (CAL), and bleeding on probing (BOP), were measured at six sites per tooth.
(A) PD: Periodontal pocket depths were recorded using calibrated periodontal probe (William’s probe) by measuring distance from the gingival margin to the bottom of the gingival sulcus with gentle pressure (0.25 Ncm) around the cemento-enamel junctions (CEJ) at three points buccal and three points lingual having the probe placed parallel to the long axis of the tooth and 10 degrees tilted inward at the proximal points. All measurements were read to the nearest 0.5 mm.
(B) CAL: Clinical attachment levels were assessed using the same probe by measuring the distance from the CEJ to the bottom of the probable periodontal pocket. All measurements were read to the nearest 0.5 mm [21].
(C) BOP (+/-): Bleeding on probing was assessed by either the presence (+) or absence (-) of bleeding at the site of probing immediately after periodontal pocket depth measurement [22].
Clinical measurements were performed on six randomly selected subjects by the two examiners (A.Z. And M.K.). These measurements have been repeated after 10 days and Cohen’s Kappa Score was used to measure reliability level.
Whole unstimulated saliva samples were collected from all subjects to evaluate
relative amount of MMP-8 and MMP-9. Subjects were instructed not to eat or drink
for at least three hours prior to saliva collection. To minimize fluctuations
related to circadian rhythm in salivary secretion, all collections were performed
at a fixed time of day. They were also instructed to relax and swallow all saliva
present in their mouths five minutes before starting the saliva collection. While
seated and leaning forward, they were asked to spit all the saliva they produced
into a graduated test tube over a period of five minutes. Saliva samples
(approximately 0.2–5 mL) were transferred to a clean 1 mL microfuge tube and were
kept in ice and centrifuged for 20 min at 1000 rpm in a bench top-refrigerated
centrifuge. The samples were then kept at –80
Enzyme-linked immunosorbent assay (ELISA) was used to study the levels of MMP-8
and MMP-9 (Elabsciences®, Houston, Texas, USA) using saliva
samples. Results were read at 450 nm by using a microplate reader (Bio-Rad
Laboratories Inc., Hercules, CA) [24, 25]. Sandwich-ELISA as the method was
utilized as follows; micro-ELISA plate kit (Elabscience) has been pre-coated with
an antibody specific to Human MMP-8 and MMP-9. Standards or samples were added to
appropriate micro-ELISA plate wells and combined with the specific antibody.
Biotinylated detection antibodies specific for Human testes proteases and Avidin-
Horseradish Peroxidase (HRP) conjugate was added to each micro-plate followed by
incubation. Furthermore, free components were washed away. Afterwards, the
Substrate Reagent was added to each well, only those wells that contain Human
MMP-8 and MMP-9, biotinylated detection antibody, and Avidin-HRP conjugate
appeared blue in color. Enzyme-substrate reaction was terminated by adding Stop
Solution and appeared yellow in color. The optical density (OD) was then measured
by using Biotek Synergy HT microplate reader (Synergy HT, Biotek, Vermont, USA)
at a wavelength of 450 nm
Data were analyzed using SPSS 21.0 version statistical software (IBM Inc.,
Chicago, USA) and MedCalc® Statistical Software version 19.8
(MedCalc Software Ltd, Ostend, Belgium. Descriptive statistics (mean, standard
deviation, median, interquartile range, frequencies and percentages) were used to
describe the study and outcome variables. As the outcome variables are skewed,
non-parametric statistical tests (Kruskal Wallis test and Mann-Whitney U-test)
were used to compare the mean ranks of the outcome variables in relation to 6
study groups and between 2 groups. The Kruskal Wallis test was followed by
Conover test to observe the pair-wise comparison of mean ranks. Pearson
Chi-square test was used to compare the distribution of categorical responses
across the study groups. Kappa statistics was used to quantify the agreement
between the responses of categorical variables to report the intra and inter
reliability. A P-value of
A total of 180 subjects were included in this case-control study. Overall, out of the 180 subjects, 101 were females (56%) with mean age of 24.8 years. Descriptive statistics including mean, standard deviation and frequencies regarding age of study subjects including HC, GC, PC were 24.9 (5.4), 25.2 (7.8) and 26.2 (2.8), respectively. INN groups HINN, GINN, and PINN with values of 24.7 (3.5), 23.6 (3.8) and 24.1 (3) respectively, were shown in Table 1.
| Variables | HC | HINN | GC | GINN | PC | PINN | P-value |
|---|---|---|---|---|---|---|---|
| Age (years) mean (SD) | 24.9 (5.4) | 24.7 (3.5) | 25.2 (7.8) | 23.6 (3.8) | 26.2 (2.4) | 24.1 (3) | |
| Gender (Male: female) | 11 : 19 | 16 : 14 | 16 : 14 | 13 : 17 | 15 : 15 | 8 : 22 | |
| BOP % | 9% (2.9) | 6% (5) | 97% (0.06) | 78% (0.15) | 75% (0.18) | 58% (1.3) | |
| Mean (SD) | |||||||
| Enzyme-linked immunosorbent assay ELISA (ng/mL) | |||||||
| MMP-8 | 0.10 (0.19) | 0.06 (0.09) | 0.15 (0.30) | 0.12 (0.31) | 6.44 (4.2) | 0.18 (0.32) | |
| Mean (SD) | |||||||
| MMP-9 | 390.3 (512.3) | 144.9 (312.9) | 348.3 (575.1) | 167.8 (286.2) | 763.1 (621.6) | 87.6 (40.9) | |
| Mean (SD) | |||||||
| Kruskal Wallis test. *Statistically significant; BOP, bleeding on probing; MMP, matrix metalloproteinase; HC, Control subject with healthy periodontium; HINN, INN using subject with Healthy periodontium; GC, Control subject with Gingivitis; GINN, INN using subject with Gingivitis; PC, Control subject with Generalized Periodontitis Stage I; PINN, INN using subject with Generalized Periodontitis Stage I. | |||||||
Kappa statistics revealed that intra reliability of the first examiner (M.K) was statistically significant for 5 variables. On the contrary, for the second examiner (A.Z.), it was statistically significant for 4 variables. This indicates that the intra-examiner reliability was 0.90 for examiner 1 and 0.81 for examiner 2 which indicated a strong level of agreement [26]. In addition, Kappa statistics were used to measure the inter reliability of examination 1, which was assessed for 6 variables between the two examiners M.K. & A.Z., and the values were statistically significant for 5 variables. Afterward, the inter reliability test of examination 2 for 6 variables between the two examiners M.K, & A.Z., was statistically significant for 5 variables. The k value for inter-examiner agreement was 0.91 which indicates an “almost perfect” agreement [26].
The comparison of mean ranks of BOP % among the six study groups showed highly
statistically significant difference (P
 Fig. 1.
Fig. 1.Comparison between control groups and INN groups in the mean ranks of bleeding on probing percentage (BOP %) a mong the six study groups.
When comparing the mean ranks of BOP % between each two groups with the same
periodontal diagnosis, a highly statistically significant difference was found
between each of Gingivitis groups and Periodontitis groups (P
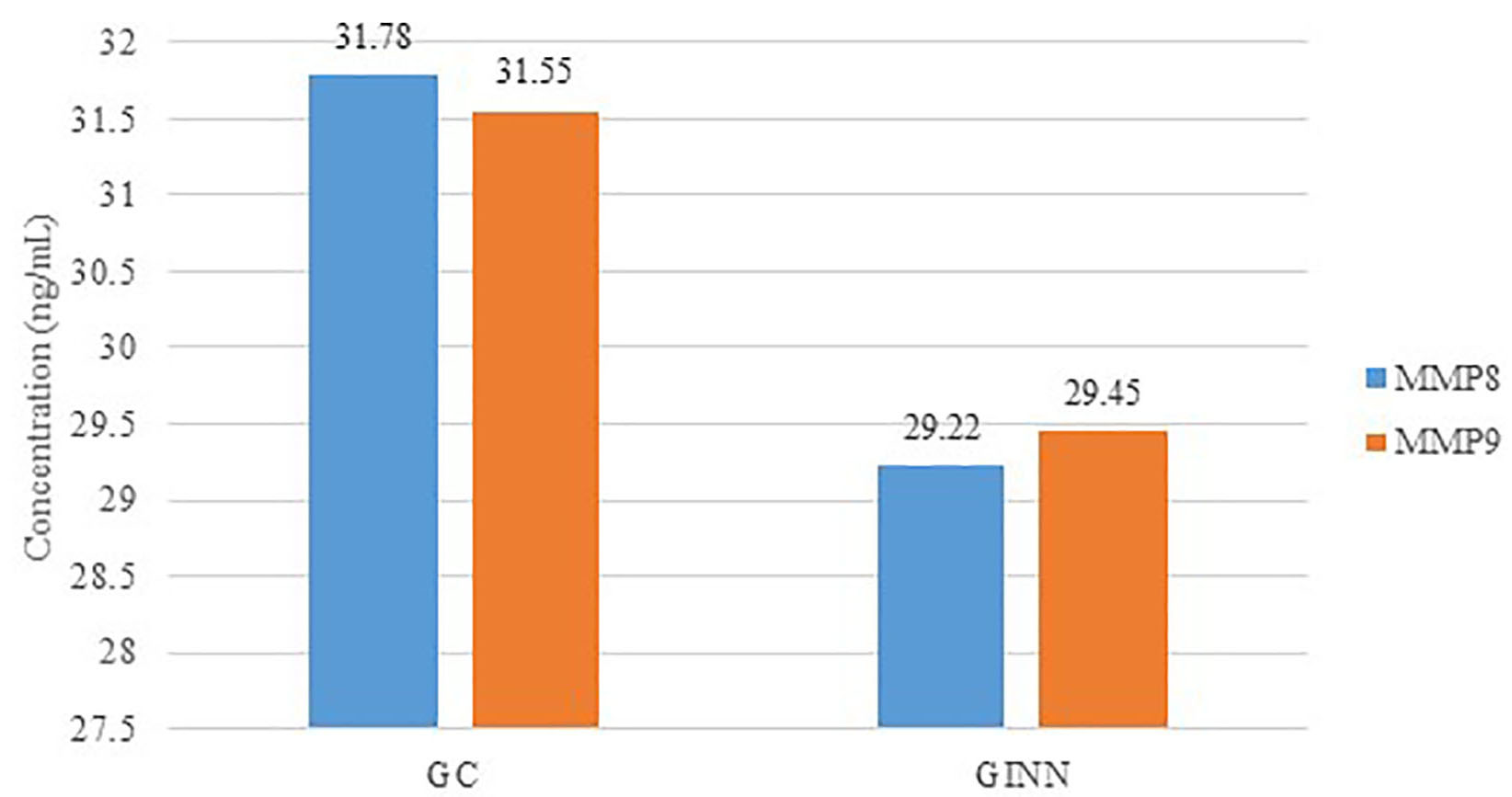 Fig. 2.
Fig. 2.Comparison between control subjects with Gingivitis (GC) and INN taking subjects with Gingivitis (GINN) in the levels of MMPs.
| Variables | GINN | GC | P-value | |||
| Median | Mean | Median | Mean | |||
| (IQR) | Ranks | (IQR) | Ranks | |||
| BOP % | 0.20 (0.1) | 78% (0.15) | 0.13 (0.06) | 97% (0.06) | ||
| Enzyme-linked immunosorbent assay ELISA (ng/mL) | ||||||
| MMP-8 | 0.03 (0.1) | 29.22 | 0.01 (0.2) | 31.78 | 0.552 | |
| MMP-9 | 49.7 (134) | 29.45 | 65.0 (491) | 31.55 | 0.632 | |
| Mann-Whitney U-test. *Statistically significant; BOP, bleeding on probing; MMP, matrix metalloproteinase; GC, Control subject with Gingivitis; GINN, INN using subject with Gingivitis. | ||||||
| Variables | PINN | PC | P-value | |||
| Median (IQR) | Mean ranks | Median (IQR) | Mean ranks | |||
| BOP % | 0.3 (0.3) | 58% (1.3) | 0.8 (0.3) | 75% (0.18) | ||
| Enzyme-linked immunosorbent assay ELISA (ng/mL) | ||||||
| MMP-8 | 0.02 (0.2) | 15.57 | 45.00 (7.2) | 45.43 | ||
| MMP-9 | 81 (38) | 15.7 | 640 (512) | 45.3 | ||
| Mann-Whitney U-test. | ||||||
With respect to comparison of mean ranks of deep pocket depth (PD
There was no statistically significant difference in the mean ranks of the MMP-8
and MMP-9 levels between GINN and GC (Gingivitis groups) (P = 0.552,
P = 0.632), respectively. These differences between the two groups are
illustrated in Table 2 and Fig. 2. The comparison of the mean ranks of MMP-8 and
MMP-9 between PINN and PC (Periodontitis groups) showed highly statistically
significant difference (P
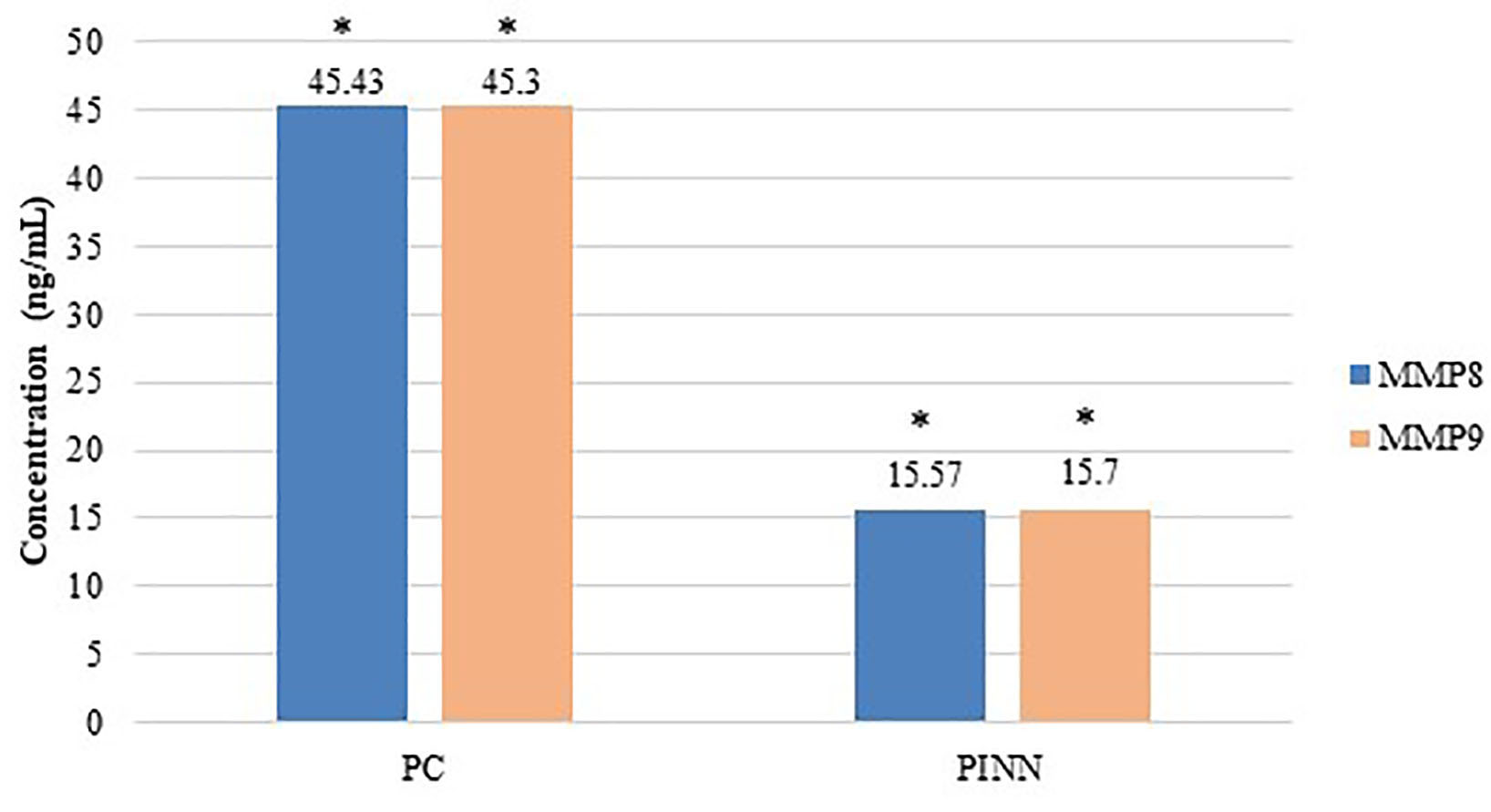 Fig. 3.
Fig. 3.Comparison between control subjects with Generalized Periodontitis Stage I (PC) and INN using subjects with Generalized Periodontitis Stage I (PINN) in the levels of MMPs.
This study illustrates the relationship between clinical periodontal parameters including: (PD), (CAL), and (BOP); as well as biological periodontal parameters which includes the salivary levels MMP-8 and MMP-9 in relation to the use of INN. A significant association was shown to be significant between INN use and lowering the levels of MMP-8 and MMP-9 as well as BOP %. These differences were shown among different status of periodontal health or disease but was pronounced clinically and statistically among patients diagnosed with active Generalized Periodontitis Stage I. This can be logically explained by the presence of more pronounced inflammation associated with periodontitis cases due to periodontal tissue destruction which is known to express higher levels of pro-inflammatory biomarkers including MMP-8 and MMP-9 [27, 28]. Therefore, having this medication targeting thisproteases specifically with its anti-inflammatory effect, it actions was more observed in such cases.
Furthermore, it is well-documented that among all MMPs, MMP-8 and MMP-9 are the proteases associated with periodontal disease, and their salivary and GCF levels correlate positively with the severity of the disease [27, 28]. Larivee et al. [29], reported that collagenase activity increases with the severity of the inflammation. This is also true in terms of clinical inflammatory signs having BOP percentage as a strong positive indicator.
Findings of present clinical study can be strongly supported by previous in-vitro investigation that led to patent award proposing the use of vitamin A. Which was focusing on the effect of different forms of vitamin A on health and diseased human gingival fibroblast. Ozick et al. [30] concluded that vitamin A increased gingival fibroblast population and growth collagen production and strengthen connective tissue.
Only one previous study was done to investigate salivary MMP-9 levels in subjects being treated with INN. Their results showed a higher activity of MMP-9 in the stimulated whole saliva of 13 subjects taking oral INN for acne treatment compared with controls [31]. These results can be different from this study’s findings due to the difference in periodontal status of subjects participated, sample size, and methodological use to collect salivary sample as well as MMP-9 detection.
While other study reported no direct correlation between periodontal disease and use of INN when observed in Papillon Lefevre Syndrome patients. The different in results can be explained by having different form of periodontal disease studied, as present study focused only on plaque induced gingivitis and chronic periodontitis, which differs from aggressive periodontitis in terms of pathogenicity and rate of destruction as well host immune response in affected individuals [32].
Although it should be noticed despite the positive effect observed by INN especially in periodontal disease affected individuals, INN has been associated with numerous side effects due to its usual long-term use. The most common adverse effects involving the skin and mucous membranes include cheilitis and drying of oral and nasal mucosa. Xeropthalmia is common, with subsequent contact lens intolerance and possible conjunctivitis [33]. Another adverse effect is the impact of isotretinoin on liver function, which can lead to alterations in serum aminotransferase levels and lipid levels [34]. Various data linking isotretinoin to depression or suicide have appeared in the medical and psychological literature since 1983 [35].
Several limitations had been noted in the present study. This includes being a case-control study, one time data collection with no further follow-up was done. It might be of importance since medication is used for prolonged time frame thus, INN response can differ from individual to another and also potential long-term use side-effects can be evaluated properly, long term studies can be recommended to confirm observations. Another limitation was calculating amount of MMPs only from saliva sample, therefore, future investigations using gingival crevicular fluids, which is considered as a more reliable and accurate body fluids in relation to periodontal related cytokines, is highly recommended to confirm the present findings. Finally, it is important to highlight the effect of this drug based on patient oral hygiene performance and compliance as the was not highlighted in the present study.
In conclusion, although Isotretinoin is well-known to cause xerostomia and dryness of the oral cavity, findings of the present study showed anti-inflammatory effect on periodontal parameters in Plaque Induced Gingivitis and Chronic Periodontitis patients, which can promote its possible future use in periodontal treatment. These findings and speculations need to be confirmed by future well-designed longitudinal and clinical trials. In the search for a clinically relevant biomarker, further directions are needed for identifying whether the MMP is in its active or inactive state for such assays to be more significant.
Conceptualization done by RNA and MAH; methodology design and implementation by RNA, SA, MAK and AA; software admission and validation as well data collection and analysis by MAK; validation RSA, SAQ and MAK; formal analysis revision by RNA. All authors contributed in writing manuscript, reviewing and editing, and final approval.
This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013. The protocol was approved by the Institutional Committee of Research Ethics at the King Saud University, Riyadh, Saudi Arabia Research Project No. (E-19-3856). Each participating patient signed an informed consent form after the nature of the study had been explained to them. Each participating patient was informed that they could withdraw from the study at any time without jeopardizing their rights to proceed with dental care at the Dental College.
Authors’ are very grateful to Miss Rhodanne Nicole A. Lambarte for her assistance in the laboratory part, and Prof. Shaik S. Ahamed for the statistical analysis.
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. RG-1441-498.
The authors declare no conflict of interest.
INN, Individuals Taking Isotretinoin (INN); PD, Pocket Depth; CAL, Clinical Attachment Level; BOP, Bleeding on Probing; ELISA, Enzyme-Linked Immunosorbent Assay; MMP-9, Matrix Metalloproteinase-9; GCF, Gingival Crevicular Fluid; TIMP, Tissue Inhibitor of Metalloproteinase; HINN, Healthy Periodontium; GINN, Generalized Plaque induced Gingivitis; HC, Healthy Periodontium; GC, Generalized Plaque Induced Gingivitis; PC, Generalized Periodontitis Stage I; CEJ, Cemento-Enamel Junctions; HRP, Horseradish Peroxidase; OD, Optical Density.
