Academic Editor: Anna Lewinska
Background: Abnormal Cu(II) ions levels may affect many biological
functions, and it is of great importance to detect Cu(II) ions in organisms.
Methods: Herein, we report a near-infrared (NIR) fluorescent probe
EtRh-N-NH
Graphical abstract

Highlights
(1) The Cu(II) response triggered 235 fold enhancement of emission intensity from
700 to 900 nm in 25 min.
(2) The response of the probe EtRh-N-NH
The copper element amount in the human body and other biological systems is only
trace but essential and indispensable [1, 2]. In organisms, copper, existing as
an oxidation state Cu(II), participates in many important redox reactions [3].
The Cu(II) concentration should maintain at a certain range of about 40
The traditional techniques based on inductively coupled plasma (ICP) method (including atomic absorption spectroscopy (AAS) [7], atomic emission spectroscopy (AES) [8], and mass spectrometry (MS) [9]) could detect Cu(II) ions concentration in high accuracy; however, it is difficult to monitor the Cu(II) compounds in real-time and in the biological system using these ICP methods [10]. There are lots of methods for the detection of copper ions, mainly including fluorescent hybrid nanomaterials [11] and fluorescent chemical sensors [12]. Emerging fluorescent hybrid nanomaterials includes quantum dots (QDs), metal nanoclusters (NCs), carbon dots (CDs), MXenes and polymer nanoparticles (NPs) [13, 14]. Fluorescence chemical sensors are mostly based on the following types: deoxyribonucleic acid [15], fluorophores [16, 17], metal-organic framework (MOF) [18], copolymers [19], Schiff bases [20], and silica nanoparticles [21]. Among them, chemical sensors containing fluorophores have attracted much attention. By introducing various ligands and fluorophores, such as fluorescein, rhodamine, naphthalimide and boron dipyrromethene (BODIPY), the selective detection of metal ions can be successfully achieved. The process involves different sensing mechanisms including photo-induced electron transfer (PET), fluorescence resonance energy transfer (FRET), intramolecular charge transfer (ICT), chelation enhanced fluorescence (CHEF), excited state intramolecular proton transfer (ESIPT) and Aggregation induced emission (AIE). Compared with nanomaterials, organic chemical probes are of superior biocompatibility, flexible structural modification and relatively low toxicity. In addition, fluorescence bioimaging technology allows us to detect a variety of metal ions including Cu(II) in vivo at a subcellular level [22, 23], thus they were receiving much more attention.
Cu(II) fluorescent chemical sensors are mostly designed and synthesized based on inorganic [rhenium (I), ruthenium (II), iridium (III), zinc (II), and gold (I)] [24] and organic materials [25]. And these probes can be classified into two categories based on their mechanism of the fluorescence production [26, 27]. The first ones produce fluorescence via the coordination with the Cu(II) ions; however, the fluorescence is generally weak due to the heavy atom effect and the low efficiency of photoinduced electron transfer (PET) process. Secondly, organic material chemical sensors are designed by the fluorescence technology of organic small molecules to sense metal ions. Through the complexation of ligands and metallic copper, it can produce spectral shifts, binding/association/dissociation constants, and color changes to achieve the sensing effect of Cu(II). The second category of Cu(II) detection probes produce fluorescence after the response with Cu(II) ions. Before the response, little fluorescence was emissive; thus, image with higher signal to background ratio can be achieved. Because of its instant response, high sensitivity and selectivity, easy measurement and real-time monitoring, fluorescent sensors based on organic materials have received extensive attention [28].
Among the NIR dyes, rhodamine derivatives [29, 30, 31, 32] are widely used as responsive
fluorescent probes by their characteristic spirolactam ring-opening and -closing
process (Supplementary Table 1). More importantly, the rhodamines are of high fluorescence
quantum yield, large molar extinction coefficient, good stability upon photo
irradiation, and easy structural modification. However, many reported rhodamines
and their derivatives used for Cu(II) detection exhibited short fluorescence
emission wavelength (mostly less than 600 nm), and probes with emission in the
NIR range (600–900 nm) are more advantageous because of their better tissue
penetration depth [33, 34, 35]. Besides, the Stokes shifts of the reported dyes are
usually small (less than 50 nm), leading to a severe autofluorescence issue in
fluorescence imaging [36]. Herein, we designed a new rhodamine-based
near-infrared probe EtRh-N-NH
 Scheme 1.
Scheme 1.Mechanistic proposal of the response of
EtRh-N-NH
As described, EtRh-N-NH
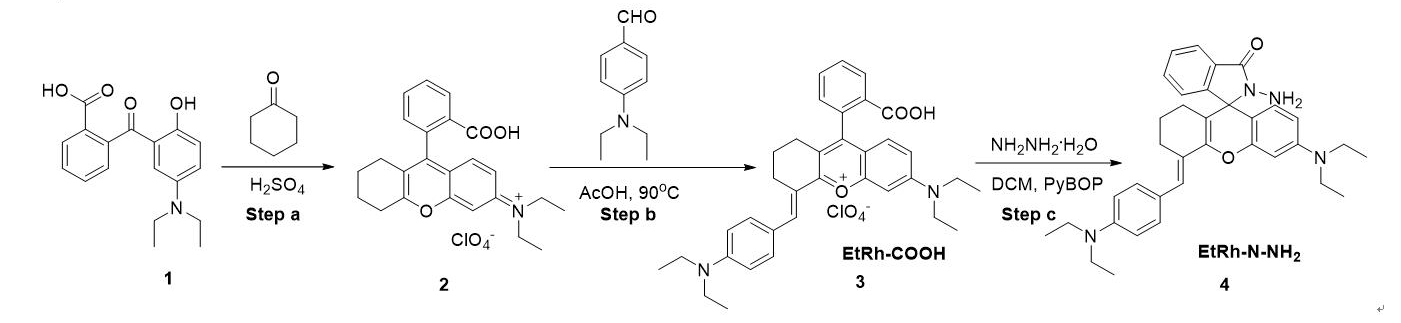 Scheme 2.
Scheme 2.Synthesis of EtRh-N-NH
As depicted in Scheme 3, we proposed the mechanism of the fluorescent turning-on
process of the designed probe EtRh-N-NH
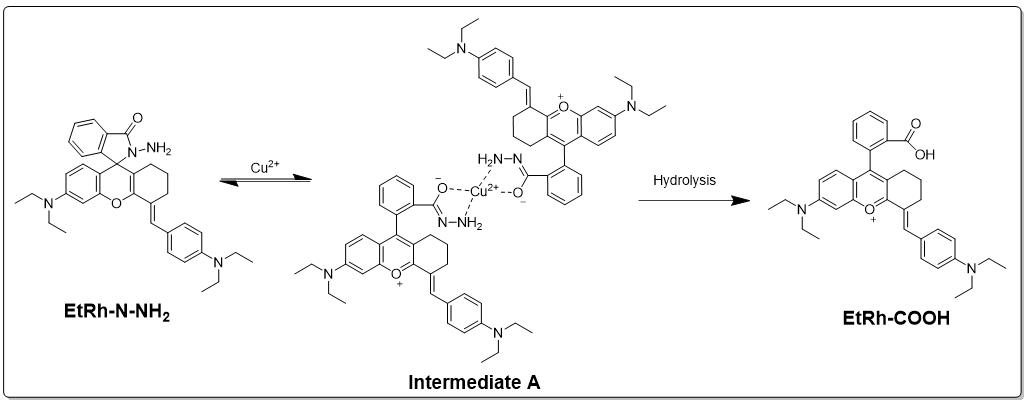 Scheme 3.
Scheme 3.Proposal of the mechanism for the fluorescent turning-on process.
To validate the mechanism, we performed the HPLC experiments. As shown in Fig. 1,
the retention times for EtRh-COOH and EtRh-N-NH
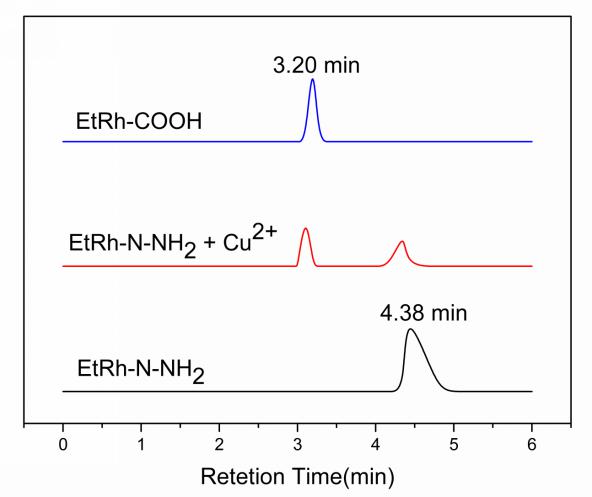 Fig. 1.
Fig. 1.HPLC chromatograms of EtRh-COOH (Blue), probe EtRh-N-NH
The absorbance spectra of EtRh-N-NH
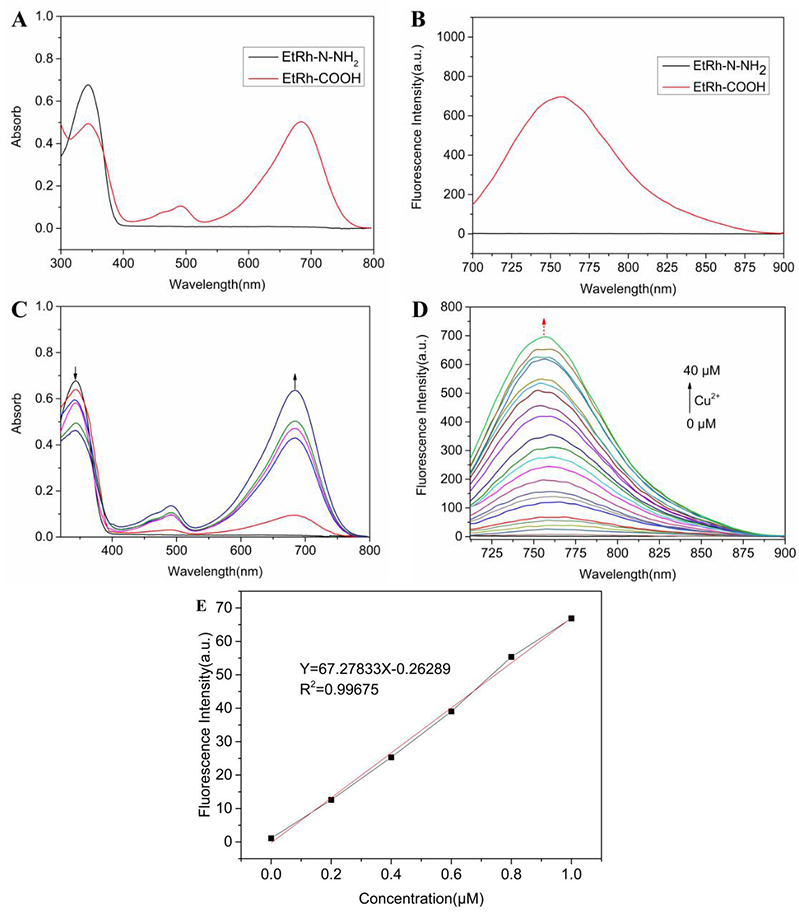 Fig. 2.
Fig. 2.Spectrum studies of the probe EtRh-N-NH
The effect of pH on the fluorescent change of probe EtRh-N-NH
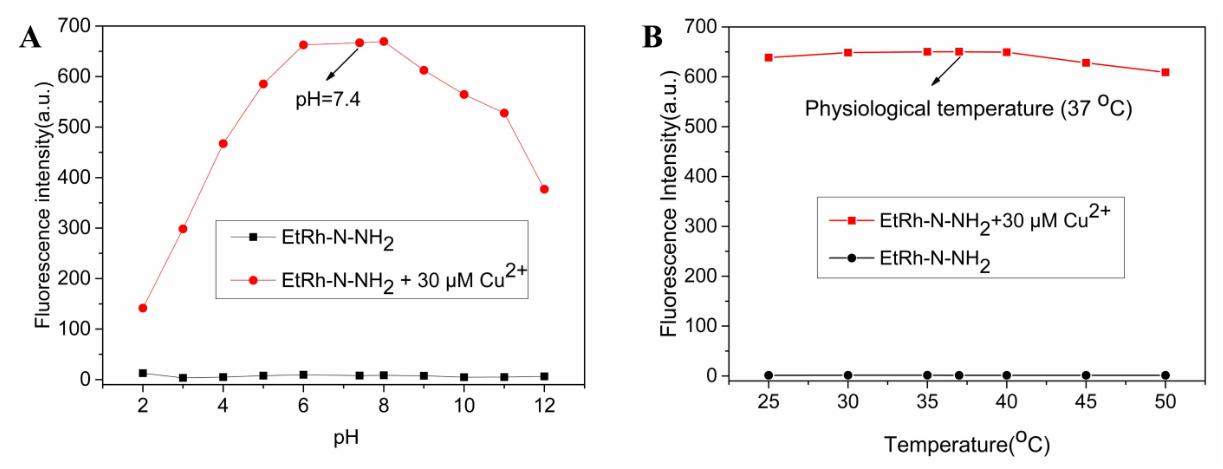 Fig. 3.
Fig. 3.The investigation of the influence of pH and temperature to the
response. (A) Fluorescence response of 10
The selectivity of the probe over Cu(II) was examined to check if the probe can
be employed in complex biochemical environment. Firstly, the solution of the
probe EtRh-N-NH
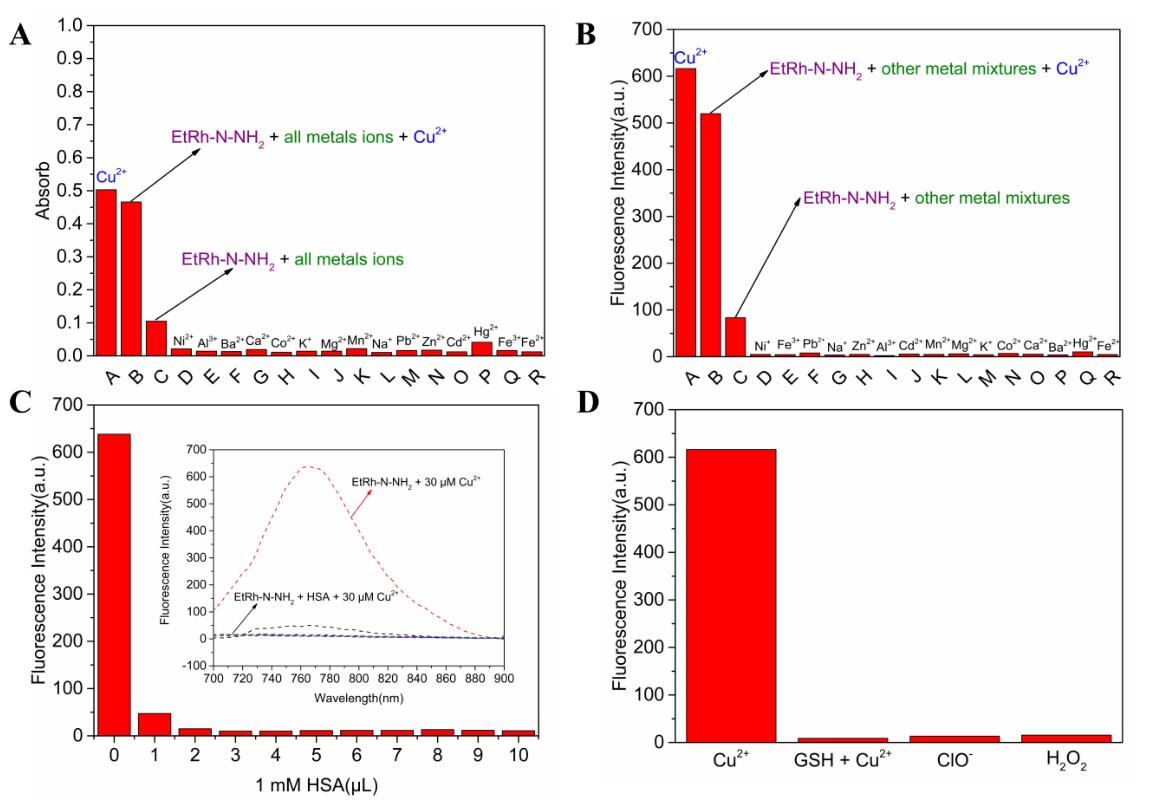 Fig. 4.
Fig. 4.Selectivity studies. (A) The UV-vis absorption and (B)
fluorescent intensity (
Human serum albumin (HSA) is the most abundant serum protein in the blood and
can be used as a carrier for a variety of cargo molecules. Its apparent affinity
for divalent copper has always been a concern, and it is considered to be the
main Cu(II) binding ligand in blood and cerebrospinal fluid. We tested the
binding ability of the probe to Cu(II) in the presence of different
concentrations of HAS (Fig. 4C). The results proved that the affinity between HSA
and Cu(II) was much high than the affinity between EtRh-N-NH
In view of the above HSA and GSH responsive results, and considering the high
content of HSA in the organism and GSH in the cells, the imaging of Cu(II) in
living cells with the probe EtRh-N-NH
The chemical reagents used are commercially available and purchased from J&K or
Innochem chemical reagents. Commonly used solvents and chemical raw materials
were all at analytically pure standard, and they were used without purification.
The
Compound 2 was synthesized according to the method previously reported
by our group [39]. Under ice bath conditions, 3 g of raw material
2-(4-diethylamino-2-hydro-xybenzoyl) benzoic acid (9.6 mmol) and 1.98 mL
cyclohexanone (19.2 mmol) were placed in a 15 mL round bottle, and the resulting
mixture was heated at 90
4-Diethylaminobenzaldehyde (0.13 g, 0.76 mmol) was added to a solution of
Compound 2 (0.3 g, 0.63 mmol) in 10 mL of glacial acetic acid, and the
resulting mixture was heated at 100
Hydrazine hydrate (1.9 mmol) and PyBOP (0.09 g, 0.19 mmol) were added to a
solution of EtRh-COOH (0.1 g, 0.19 mmol) in dry dichloromethane. The
mixture was vigorously stirred at ambient temperature (~20
In conclusion, we developed a rhodamine derivative EtRh-N-NH
NIR, near-infrared; ICP, inductively coupled plasma; AAS, atomic absorption spectroscopy; AES, atomic emission spectroscopy; MS, mass spectrometry; PET, photoinduced electron transfer; MeOH, methanol; EtCN, acetonitrile; PBS, phosphate buffer saline; UV, ultravioletradiation; LOD, limit of detection; EtOH, ethanol; LSCM, laser scanning confocal microscope; NMR, nuclear magnetic resonance; TMS, tetramethylsilane; TLC, thin layer chromatography; IR, infrared radiation; DMSO, Dimethyl sulfoxide.
LH, YL, HZ and SM designed the study. YL, PW and ML designed and performed the experiments. LH, YL, PW, ML and SM analyzed the data. YL and SM wrote the paper.
Not applicable.
Thanks to all the peer reviewers for their opinions and suggestions.
The authors would like to acknowledge financial support from Beijing Key Laboratory of Environmental & Viral Oncology, Beijing Key Laboratory for Green Catalysis and Separation, Beijing Postdoctoral Research Foundation (No. 2020-ZZ-028), Postdoctoral Research Foundation of Beijing Chaoyang district (No. 2020-ZZ-027).
The authors declare no conflict of interest.
