Academic Editor: Elisa Belluzzi
Background: Obesity appears to significantly reduce physical activity, but it remains unclear whether this is related to obesity-induced damage to skeletal muscle (SM) and heart muscle (HM). Endurance exercise (EE) reduces obesity-induced defects in SM and HM, but its molecular mechanism is poorly understood. Methods: The UAS/GAL4 system was used to construct the regulation of SM-specific FOXO gene expression in Drosophila, and the transgenic drosophila was subjected to EE and high-fat diet (HFD) intervention. Results: The structure and function of SM and HM were impaired by a HFD and muscle-FOXO-specific RNAi (MFSR), including reduced climbing speed and climbing endurance, reduced fractional shortening of the heart, damaged myofibrils, and reduced mitochondria in HM. Besides, a HFD and MFSR increased triglyceride level and malondialdehyde level, decreased the Sirt1 and FOXO protein level, and reduced carnitine palmityl transferase I, superoxide dismutase, and catalase activity level, and they dow-regulated FOXO and bmm expression level in SM and HM. On the contrary, both muscle FOXO-specific overexpression (MFSO) and EE prevented abnormal changes of SM and HM in function, structure, or physiology caused by HFD and MFSR. Besides, EE also prevented defects of SM and HM induced by MFSR. Conclusions: Current findings confirmed MFSO and EE protected SM and heart from defects caused by a HFD via enhancing FOXO-realated antioxidant pathways and lipid catabolism. FOXO played a vital role in regulating HFD-induced defects in SM and HM, but FOXO was not a key regulatory gene of EE against damages in SM and HM. The mechanism was related to activity of Sirt1/FOXO/SOD (superoxide dismutase), CAT (catalase) pathways and lipid catabolism in SM and HM.
In both mammals and Drosophila, increasing evidence has shown that a high-fat diet (HFD) can not only induce obesity phenotype, but also lead to abnormal changes in many molecular signaling pathways in the body. For example, it has been reported that a HFD not only increases triglyceride (TG) levels in the body, but also is associated with an increased risk of oxidative damage, including increased malondialdehyde levels and reactive oxygen species (ROS) levels, and decreased catalase (CAT) and superoxide dismutase (SOD) activity levels [1, 2, 3]. Moreover, a HFD can down-regulated the triglyceride lipase gene (homologous to Drosophila brummer) and up-regulated the fatty acid synthase gene (homologous to Drosophila FAS) [4, 5, 6, 7, 8], which is an important molecular mechanism leading to the continuous accumulation of triglyceride in the body. What’s more, a HFD can inhibit Sirt1/FOXO pathway and induce mitochondrial synthesis defects and mitochondrial dysfunction in liver, heart, and muscle [9, 10, 11, 12, 13, 14, 15]. However, the role of FOXO gene in HSD-induced (High Salt Diet) skeletal muscle and myocardial damage is poorly understood.
Forkhead O transcription factor (FOXO) and FOXO-related pathways may play a
critical role in the regulation of lipid metabolism in heart [16]. For instance,
overexpression of FOXO-1 (in manimal) or FOXO (in Drosophila)gene in myocardial cells protects the heart from
lipid accumulation and heart dysfunction
induced by a HFD [17, 18]. Besides, brummer (bmm) gene encodes a
triglyceride lipase, which is involved in lipid metabolism, including regulation
of lipid accumulation and triglyceride (TG) homeostasis [19]. Overexpression of
bmm gene in heart prevents the heart from TG accumulation and heart
defects induced by HFD [17]. Moreover, Sirtuin 1 (Sirt1) in manimal and flies
encodes an NAD
In this study, to determine whether FOXO-mediated antioxidant pathways are involved in regulating EE against lipotoxicity induced by HFD in young muscle and heart, muscle or heart FOXO-specific overexpression (MFSO) flies and FOXO-specific RNAi(MFSR) flies were subjected to a HFD and EE intervention from day 3 and continued for 5 days, and then the function of skeletal muscle and heart, the lipid metabolic state, the antioxidant capacity, and the lipotoxicity level of them were measured by several methods.
The FOXO-UAS-overexpression (FOXO-UAS-OE)
flies (stock ID: 9575; FlyBase Genotype: y
Age-matched w
Normal food contained 1.6% soybean powder, 2.0% yeast, 6.7% corn meal, 0.7% agar, 4.8% sucrose, 4.8% maltose, and 0.3% propionic acid. Add 15% lard to normal food to make HFD [29, 30]. Flies were housed in a 25 °C incubator with 50% humidity and a 12-h light/dark cycle. Fresh food was provided every other day.
The melting point of lard was 28–48 °C. In our previous studies, we found that since coconut oil had a melting point of about 23 °C, and flies tended to stick to the melted coconut oil at 25 °C, which leaded to an unnatural death in large numbers. However, the melting point of lard was 28–48 °C. Flies could eat solid lard without sticking to it, which reduced unnecessary deaths.
When constructing the exercise device, the advantage of the flies’ natural negative geotaxis behavior was taken to induce upward walking [31]. Vials were rotated at the 60 rad/s. After vials each up-and-down turn, hold for 10 seconds for the flies to climb. Flies were exercised for 1.5 hours per day [32, 33]. All exercise groups flies started exercise from when they were 2-day old, and underwent a 6-day-long exercise program. All HFD flies started feeding high-diet food from when they were 2-day old, and underwent a 6-day-long high-fat diet program.
The test vials for flies’ climbing speed and climbing failure were the same as the vials for exercise training, and the test vials were left eight centimeters length for the flies to climb [18]. With a light box behind the vials, once flies were shaken to the bottom of the vials, a timed digital camera snapped a picture after 3 seconds. The height the fly climbs was clearly shown on the photographs. Each vial contained about 20 flies and was subjected to 5 trials.
A cohort of flies was observed during continuous stimulation by the exercise device. Flies were placed on the exercise device in vials of 20 each and made to climb until fatigued. A vial of flies was considered “fatigued” when 5 or fewer flies are able to climb higher than 2 inches for four consecutive drops. Times of removal could be plotted as a “time-to-failure” plot [31].
Flies were anesthetized with FlyNap (Carolina Biological Supply, Burlington, MA, USA) for 2–3 min. The head, ventral thorax, and ventral abdominal cuticle were removed by special glass needles to expose the heart and abdomen. Dissections were done under oxygenated artificial hemolymph [34]. Artificial hemolymph was allowed to equilibrate with oxygenation for 15–20 min before filming. To get a random sampling of heart function, a single 30-second recording (AVI format) was made for each fly by using high-speed camera. The heart physiology of the flies was assessed using a AVS Video Editor version 1.0 analysis program that quantifies diastolic interval (DI), systolic interval (SI), heart period (HP), diastolic diameter (DD), systolic diameter (SD), and fractional shortening (FS) [35]. The sample size was 17 flies for each group.
Twenty fly muscles were removed from the thorax and placed in 200 μL of Phosphate Buffered Saline (PBS) buffer (Sangon, Shanghai, China). After that, the sample is stored at –20 degrees Celsius and reserved. For the formal test, flies muscle were converted to homogenates in a homogenizer filled with 1 mL PBS (pH 7.2–7.4). The homogenates were centrifuged at 4 °C for 15 min with a speed of 2000 r/min. The supernatant was mixed with the reagents supplied in an MDA Assay Kit (MLBIO, Shanghai, China) and incubated at 95 °C for 40 min. The absorbance of the supernatant was measured at 530 nm. All operations were according to the manufacturer’s instructions. All assays were repeated three times.
For electron microscopic analysis, skeletal muscle and myocardium were dissected
in ice-cold fixative (2.5% glutaraldehyde in 0.1 M PIPES buffer at pH 7.4).
After 10 hours of fixation at 4 °C, samples were washed with 0.1 M PIPES,
post-fixed in 1% OsO
The FOXO level, Sirt1 level, CPT1 level, SOD activity level, and CAT activity level were measured by ELISA assay (Insect FOXO, Sirt1, CPT1, SOD, and CAT ELISA Kits, MLBIO, Shanghai, China). 10 flies’ muscles and 80 hearts were homogenized in PBS (pH 7.2–7.4). Samples were rapidly frozen with liquid nitrogen and then maintained at 2 °C –8 °C after melting. Homogenize the samples with grinders, and centrifugation was conducted for 20 min at 2000–3000 rpm. Then we removed the supernatant. Assay: take the blank well as zero, and read absorbance at 450 nm within 15 min of adding Stop Solution. All assays were repeated 3 times.
About 20 flies’ muscles and 80 hearts were
homogenized in Trizol (Invitrogen, California, USA). 10
The 1-way analysis of variance (ANOVA) with least significant difference (LSD)
tests was used to identify differences among the groups at the same age and the
same gene type. Independent-sample t tests were used to assess
differences between the 2-day old flies and 8-day old flies at the same
group. p-values for lifespan curves and climbing endurance curves were
calculated by the log-rank test. Analyses were performed using the Statistical
Package for the Social Sciences (SPSS) version 16.0 for Windows (SPSS Inc.,
Chicago, IL, USA), with statistical significance set at p
Increasing evidence conforms that the climbing speed and climbing endurance of flies can reflect their climbing performance [35], and it has been comformed that a 5-day HFD with 30% coconut oil contributes to climbing performance decline of flies [23, 36]. Since coconut oil had a melting point of about 23 °C, flies tended to stick to the melted coconut oil at 25 °C, which leaded to an unnatural climbing impairement and death in large numbers. To prevent these from occurring, we make high-fat food by adding 15% lard to normal food in this study.
The results showed that during the period from 3 to 8 days of age, the climbing
height (CH) of w
 Fig. 1.
Fig. 1.The climbing height in 3 seconds of flies. (A) Curve
of climbing height (CH) in w
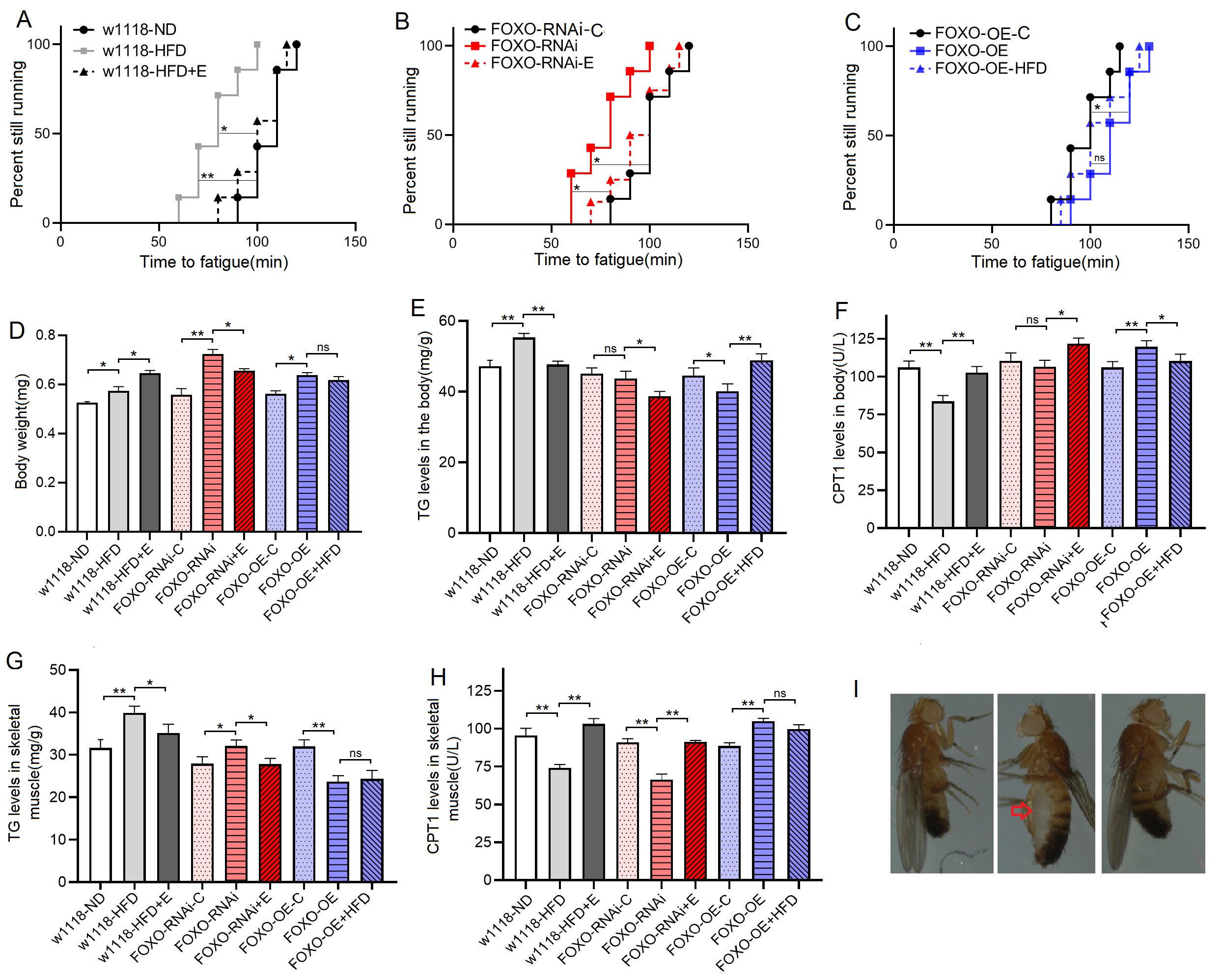 Fig. 2.
Fig. 2.The climbing endurance and lipid metabolism of 8-day old flies.
(A) Time to fatigue (TTF) in w
To further investigate the changes in physiology induced by this HFD in SM,
SM-related genes and proteins were detected by high-throughput
sequencing, RT-PCR, and ELISA in young w
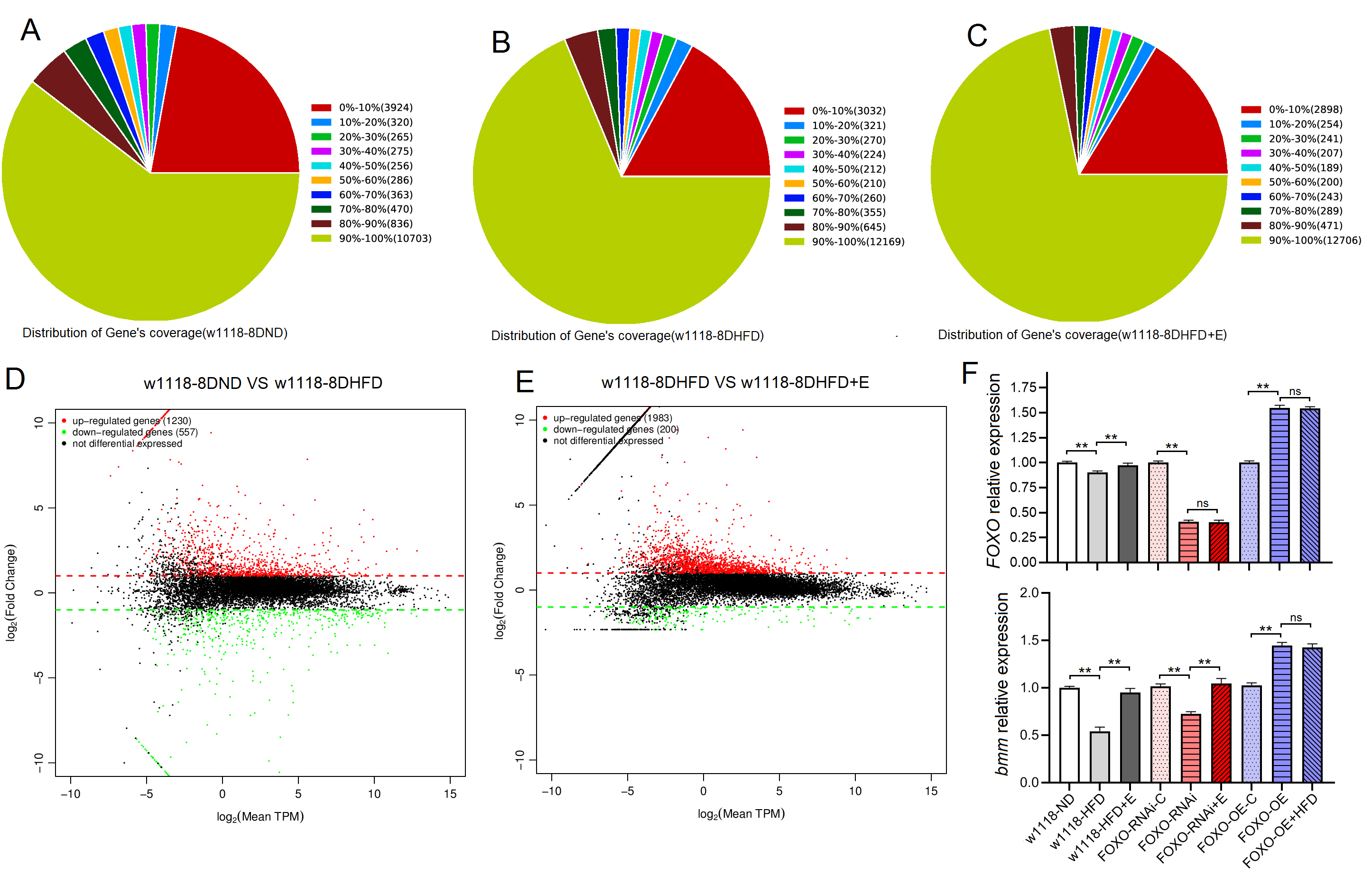 Fig. 3.
Fig. 3.High-throughput sequencing and RT-PCR results of skeletal muscle
of Drosophila w
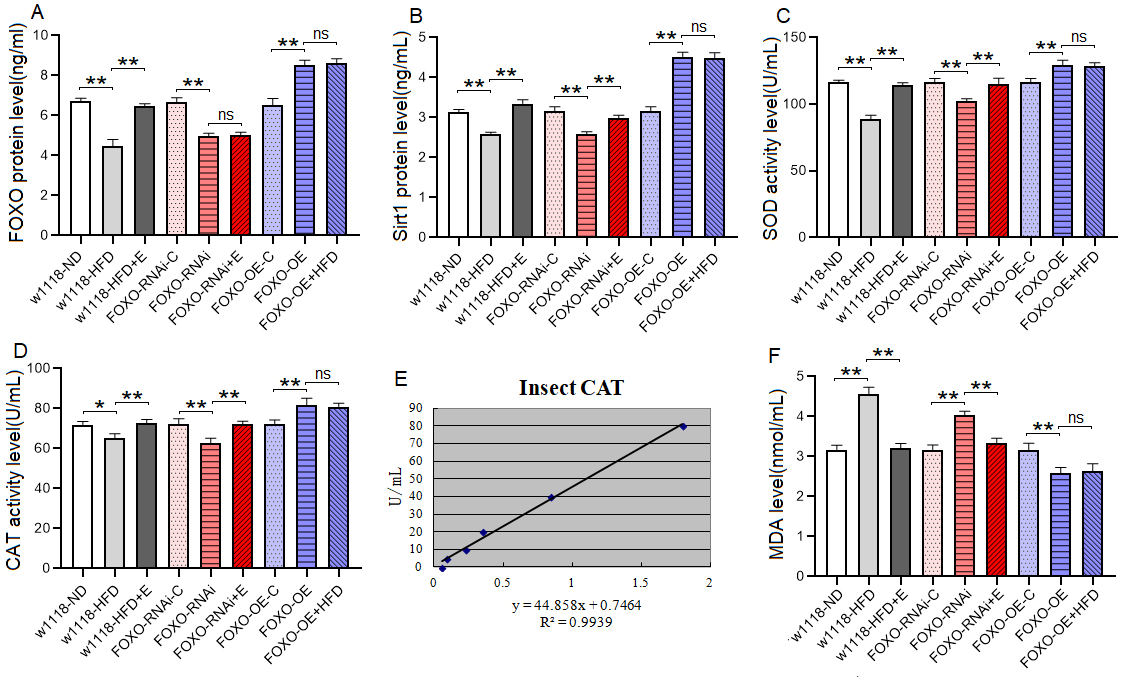 Fig. 4.
Fig. 4.The Sirt1/FOXO pathways activity and lipotoxicity in skeletal
muscle. (A) FOXO protein level. (B) Sirt1 protein level. (C) SOD
protein activity level. (D) CAT protein activity level. (E) Standard curve of CAT
test. (F) MDA level. For protein and MDA measurement, the sample size was about
20 flies’ skeletal muscle for each group, and the 1-way analysis of variance
(ANOVA) with least significant difference (LSD) tests was used to identify
differences among the groups with similar genetic backgrounds. Measurements were
taken 3 times. Data are represented as means
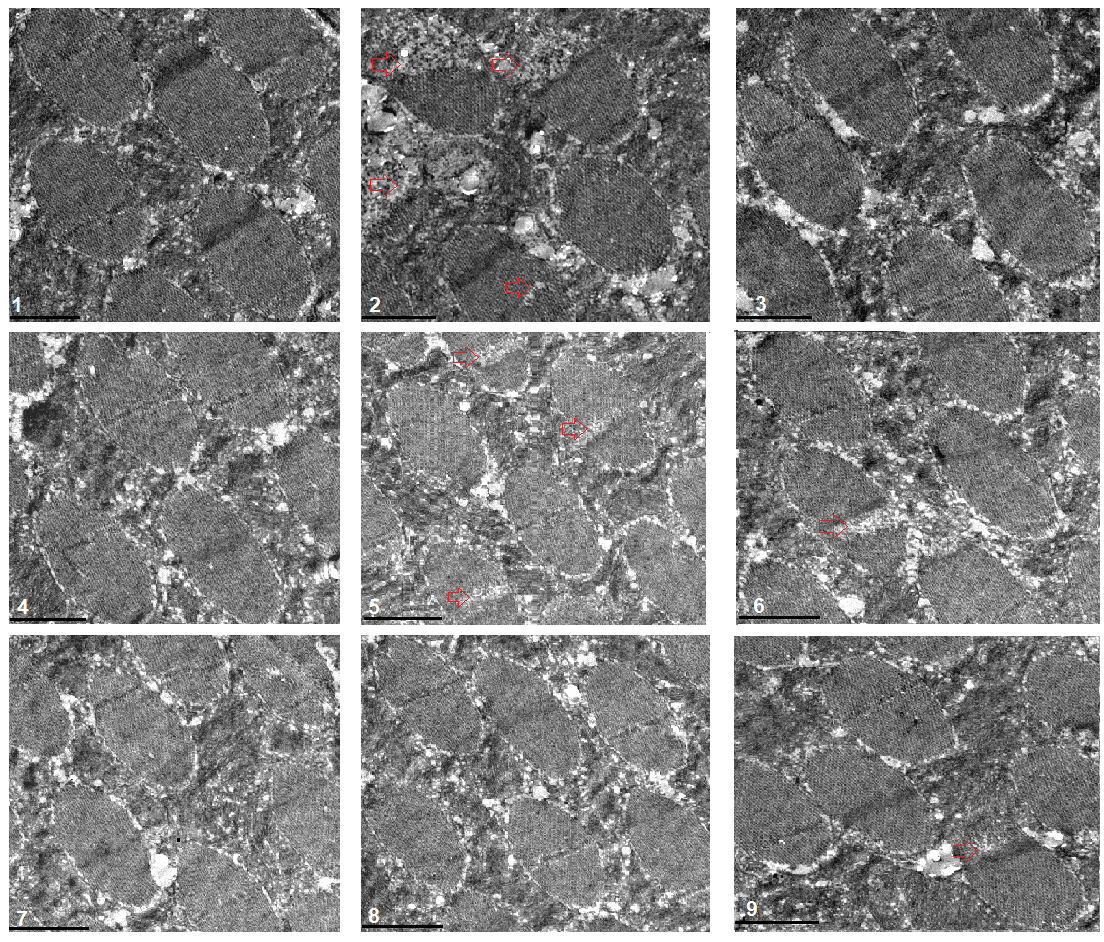 Fig. 5.
Fig. 5.Transmission electron microscopy of muscle. 1:
w
These results suggeseted that a 6-day HFD with 15% lard induced abnormal changes in lipid metabolism and antioxidant capacity, including the inhibition of CPT1 activity and bmm expression and dow-regulation of Sirt1/FOXO/SOD, CAT pathways, thus increasing the lipid accumulation in SM.
Drosophila has emerged as an important model to study the effects of HFD on metabolism, heart function, behavior, and ageing [39, 41, 42, 43]. For example, a 5-day HFD increases TG levels and disrupts insulin/glucose homeostasis in flies, which is similar to mammalian responses [44, 45], and this is consistent with our findings. It has been reported that the cardiac FOXO gene plays a key role in the regulation of lipid metabolism in the adult Drosophila heart [12], but few have reported the role of FOXO gene in the regulation of lipid metabolism in SM. The above experimental results indicate that FOXO gene and its related pathways may also be involved in the regulation of lipid metabolism in SM, but the role of SM-FOXO gene in regulating skeletal muscle lipid metabolism still needs to be further confirmed.
To comfirmed that the role of FOXO gene in regulating ipid metabolism in SM,
muscle FOXO-specific overexpreesion (MFSO) and RNAi (MFSR) were built by
UAS/MHC-Gal4 system. The results showed that during the period from 3 to 8 days
of age, the climbing height (CH) of FOXO-RNAi-C flies increased
significantly with the increase of age (p
These results suggested that although FOXO knockdown in SM did not induce dysregulation of systemic lipid metabolism, muscle FOXO-specific knockdown in SM can lead to decrease in its function and abnormal lipid metabolism, including impaired climbing ability, increased lipid accumulation, attenuated lipid catabolism and antioxidant, which is similar to the effect of a HFD on SM. These results also suggested that FOXO gene played an important role in regulating lipid metabolism and antioxidant in SM, but whether this role is critical remains to be confirmed.
To further confirm whether FOXO gene played a key role in the regulation of
lipid metabolism in SM, SM- FOXO gene was specifically overexpressed and the
transgenic flies were subjected to a HFD intervention. The results showed that
during the period from 3 to 8 days of age, the CH of FOXO-OE-C flies and
FOXO-OE flies increased significantly with the increase of age
(p
However, in MFSO transgenic flies, the CH of FOXO-OE+HFD flies
increased significantly with the increase of age during the period from 3 to 8
days of age (p
These results suggested that FOXO-specific overexpression in SM enhanced function and lipid metabolism of SM, including enhanced climbing ability and lipid catabolism, decreased lipid accumulation, and activated Sirt1/FOXO/SOD, CAT pathways related to antioxidant. In addition, the results showed that in MFSO transgenic flies, a 6-day HFD could not significantly reduce climbing ability, attenuate lipid catabolism, and inhibit Sirt1/FOXO/SOD, CAT pathways in SM. Therefore, the present results confirmed that in skeletal muscle, FOXO gene played a key role in preventing damages induced by a HFD, and its mechanism may be related to regulation of lipid catabolism and Sirt1/FOXO/SOD, CAT pathways related to antioxidant.
Exercise training improves the climbing performance in adult flies, and it delays age-related climbing performance decline in aging flies [37, 38, 39, 40]. Increasing evidence confirmes that a 5-day EE reduces lipid accumulation in SM and HM, and it increases their antioxidant capacity in flies [28, 29, 30, 31, 32], but the molecular pathways through which EE regulates skeletal muscle lipid metabolism are poorly understood.
In this study, the results displayed that the CH of
w
To confirm whether the FOXO gene plays a critical role in EE resistance to
damages caused by lipid accumulation in SM, MSFR flies were subjected to EE
intervention. The results showed that during the period from 3 to 8 days of age,
the climbing height (CH) of FOXO-RNAi+E flies decreased significantly
with the increase of age (p
These results suggested that EE could prevent defects induced by MSFR through activating muscle lipid catabolism and Sirt1/FOXO/SOD, CAT pathways, and FOXO gene was not a key gene for EE against damages induced by lipid accumulation in SM.
It has been confirmed that cardiac FOXO gene play a vital role in regulating cardiac lipid metabolism and heart function, cardiac FOXO overexpression protects heart function from a HFD (with 30% coconut oil)-induced damage in adult drosophila flies, and cardiac FOXO RNAi can induce cardiac dysfunction, and this is similar to HFD (with 30% coconut oil)-induced cardiac dysfunction [12]. However, it remains unclear whether HFD (with 15% lard) could also cause cardiac dysfunction, and the role of FOXO gene in EE against lipid metabolism abnormalities and cardiac dysfunction in heart still remains unclear.
The results showed that at 8 days of age, the diastolic interval (DI) and heart
period (HP) of w
 Fig. 6.
Fig. 6.Heart function in 8-day old flies. (A) Heart diastolic period.
(B) Heart systolic period. (C) Heart period. (D) Diastolic diameter. (E) Systolic
diameter. (F) Fractional shortening. (G) Systolic image and diastolic image of
flies. SD, systolic diameter; DD, diastolic diameter. For heart function, sample
size was 17 hearts for each group, and the 1-way analysis of variance (ANOVA)
with least significant difference (LSD) tests was used to identify differences
among the groups with similar genetic backgrounds. Data are represented as means
The results showed that at 8 days of age, the diastolic diameter (DD) of
w
The results showed that at 8 days of age, the systolic diameter (SD) of
w
The results showed that at 8 days of age, the fractional shortening (FS) of
w
The results showed that at 8 days of age, the heart TG level of
w
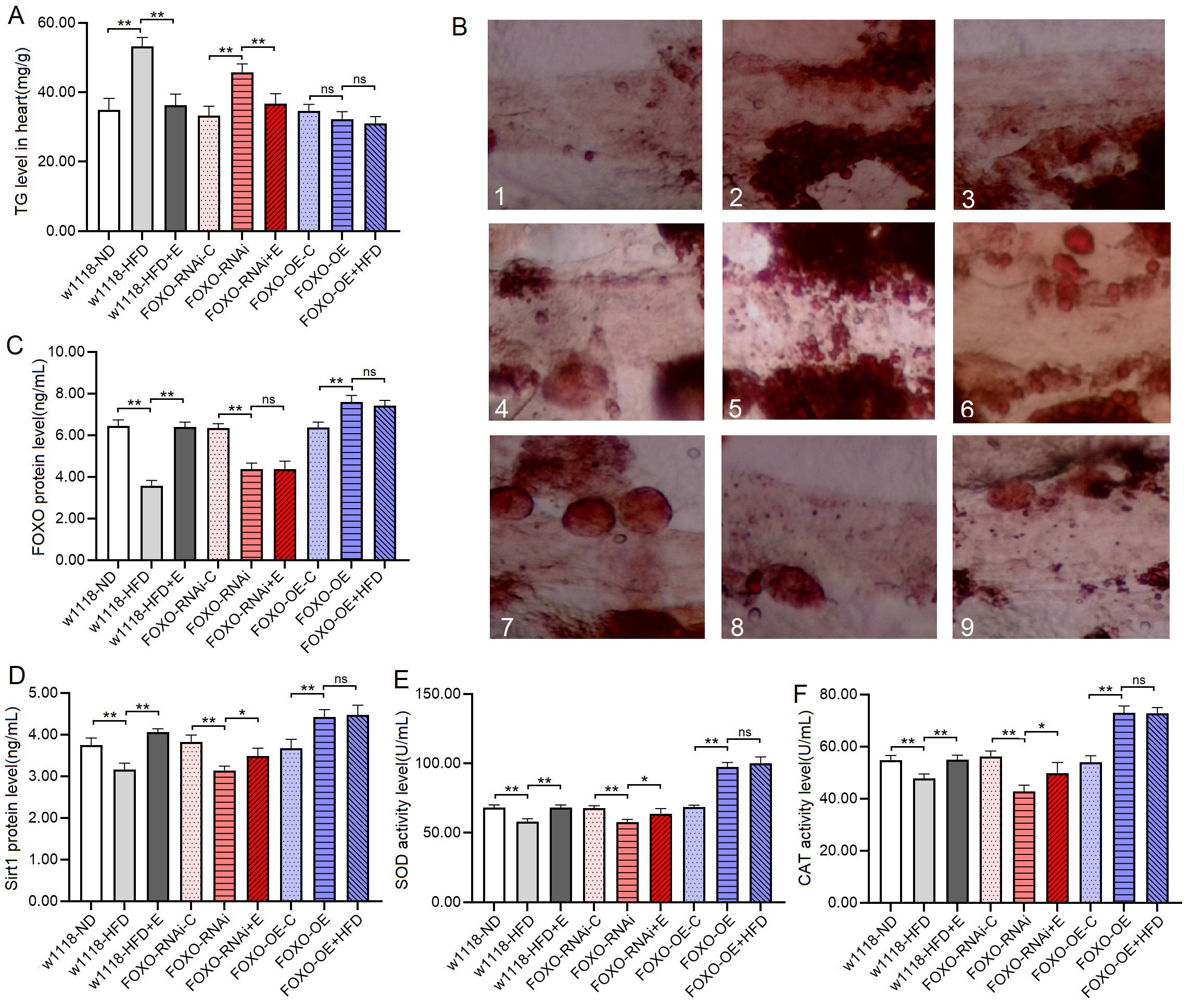 Fig. 7.
Fig. 7.Heart TG level and heart Sirt1/FOXO pathways activity in 8-day
old flies. (A) Heart TG level. (B) Oil red O staining of the heart of
Drosophila. 1: w
The proteins and activity of proteins showed that at 8 days of age, the heart
FOXO protein level, Sirt1 protein level, SOD and CAT activity level of
w
The transmission electron microscope images displayed that a HFD and FSR disrupted the alignment of myofibrils and reduced the number of mitochondria, but EE protected them from damage induced by induced by HFD and FSR. FSO protected the alignment of myofibrils and the number of mitochondria from damage induced by HFD (Fig. 8).
 Fig. 8.
Fig. 8.Transmission electron microscopy of heart. 1: w
These results suggested that a HFD (with 15% lard) and MFSR induced cardiac defects, including weakening cardiac ability to contractility, increasing lipid accumulation and oxidative damage, inhibiting antioxidant capacity, and damaging myofibrils and mitochondria. However, EE and MFSR could prevent heart defects caused by a HFD (with 15% lard), and EE could prevent heart defects caused by MFSR. Therefore, cardiac FOXO gene played a key role in regulating HFD-induced cardiac dysfunction, which was consistent with the results of previous study [12], but FOXO was not a key gene for EE against heart defects caused a HFD in young flies, and its mechanism may be related to regulation of lipid catabolism and Sirt1/FOXO/SOD, CAT pathways related to antioxidant.
Drosophila has emerged as an important model to study the effects of a HFD on metabolism, heart function, behavior, and ageing [41, 42, 43, 44]. For example, a HFD (with 30% coconut oil) increases TG levels and disrupts insulin/glucose homeostasis, and it also causes cardiac TG accumulation, reduced heart contractility, conduction blocks, and severe structural pathologies, reminiscent of diabetic cardiomyopathies in flies, which is similar to mammalian responses [45, 46]. In this study, young male fruit flies were given a HFD (with 15% lard) intervention starting at 3 days of age for 6 consecutive days. After feeding a HFD, the body TG level and the TG level of SM and HM were increased in flies, and this HFD also impaired the exercise performance and heart function in young male flies. These metabolic, cardiotoxic, SM Lipid toxicity phenotypes induced by a HFD were similar to those reported in previous studies, and this also suggested that the establishment of the Drosophila obesity model was successful in this study.
It has been reported that Sirt1/FOXO-related pathways play an important role in
lipid metabolism. For example, in both mammal and flies, overexpression of
FOXO or bmm in myocardium alleviates cardiac TG accumulation
and heart dysfunction induced by a HFD [5, 17, 18, 23]. Cardiac dysfunction
induced by a HFD persists for two subsequent generations in Drosophila,
and this is associated with reduced expression of bmm and
PGC-1
FOXO is a highly conserved transcription factor that plays an important role in cellular homeostasis. FOXO protein is also involved in the regulation of cell cycle, apoptosis and muscle regeneration [59, 60]. Silent information regulator 1 (Sirt1), a homolog of Sir2 in Drosophila, appears to control the cellular response to stress by modulating FOXO, which acts as a sensor of insulin signaling pathways and a regulator of biological lifespan [61, 62, 63]. Sirt1 is an NAD+-dependent deacetylase and a commonly expressed protein that regulates gene expression through histone deacetylation, and it plays a complex role in the pathology, progression and treatment of a variety of diseases [64]. Sirt1 plays a key role in FOXO function through NADH-dependent deacetylation in oxidative stress response, which may contribute to stress resistance and longevity of cells [65]. In oxidative stress response, FOXO protein is translocated from the cytoplasm to the nucleus, promoting the expression of its target genes encoding antioxidant enzymes such as MnSOD, CAT, and glutathione peroxidase (GPX), and thus enhancing the antioxidant capacity of cells [66].
Excess lipids cannot be packaged into lipid droplets, resulting in a chronic rise in circulating fatty acids, which can reach toxic levels in non-adipose tissue. The deleterious effects of lipid accumulation in non-adipose tissue such as heart and skeletal muscle are known as lipotoxicity [67, 68, 69, 70, 71]. Malondialdehyde (MDA) is the best product of lipid peroxidation, and it is produced from polyunsaturated fatty acids through chemical reactions and enzyme-catalyzed reactions. MDA is the most commonly measured biomarker of oxidative stress and lipid peroxidation, and a HFD increased the MDA level in muscle and heart [27, 72, 73, 74]. Moreover, the brummer (bmm) gene encodes the lipid storage droplet associated TG lipase Brummer, which is a homolog of human adipocyte triglyceride lipase (ATGL) [19, 75]. Lack of food or chronic overexpression of bmm depletes organismal fat stores in vivo, and loss of bmm activity causes obesity in flies [76]. FOXO gene overexpression limited the accumulation of lipids and increased bmm expression in heart [17]. Therefore, the regulation of FOXO on lipid metabolism is also related to the upstream regulation of bmm-related and CPT1-related lipid catabolic pathway.
Our findings suggeseted that a HFD and MFSR reduced lipid catabolism, mitochondrial function, and down-regulated Sirt1/FOXO/SOD, CAT pathways in skeletal muscle and heart of young male Drosophila, and it increased MDA level and damaged myofibrils in skeletal muscle and heart. Besides, MFSR and EE effectively protected SM and HM from damage caused by a HFD, and EE effectively protected SM and HM from damage caused by MFSR. Therefore, the existing research evidence and our results strongly confirm that Sirt1/FOXO-related pathways play a key role in the regulation of lipid metabolism.
Exercise is one of the best strategies to treat and prevent obesity [77, 78, 79, 80]. There are many differences in long-term metabolic adaptations between endurance trained athletes and obese insulin-resistant volunteers, and examining the effects of chronic exercise training in diabetes-prone populations may reveal more interesting information about the role of lipotoxicity in the development of insulin resistance [67]. There is evidence that exercise can effectively counter lipotoxicity in skeletal and cardiac muscles [5, 70, 71]. On the one hand, exercise can increase fat consumption as energy, which can reduce fat accumulation and prevent obesity. Exercise, on the other hand, increases the antioxidant capacity of skeletal muscles and the heart, which reduces oxidative stress damage in skeletal muscle and heart [30, 81]. Therefore, these findings strongly support our results.
Exercise is also closely related to the activity of the Sirt1/FOXO-related
pathways. Exercise improves lipotoxic cardiomyopathy induced by cardiac
dSir2 knockdown, and it prevents further deterioration of lipotoxic
cardiomyopathy induced by a HFD in cardiac dSir2 knockdown flies [23]. Endurance
exercise activates the cardiac Nmnat/NAD+/SIR2/FOXO pathway and the
Nmnat/NAD+/SIR2/PGC-1
Current findings confirmed that MFSO and EE protected SM and heart from defects caused by a HFD via enhancing FOXO-realated antioxidant pathways and lipid catabolism. FOXO played a vital role in regulating HFD-induced defects in SM and HM, but FOXO was not a key regulatory gene of EE against damages in SM and HM. The mechanism was related to activity of Sirt1/FOXO/SOD, CAT pathways and lipid catabolism in SM and HM.
FOXO, Forkhead transcription factor O; EE, Endurance exercise; HFD, High-fat
diet; HM, Heart muscle; SM, skeletal muscle; MFSR, Muscle-FOXO-specific-RNAi;
MFSO, Muscle-FOXO-specific-overexpression; PGC-1
All the generated data and the analysis developed in this study are included in this article.
Research idea and study design—DtW; data acquisition—DtW, JhJ; data analysis/interpretation—DtW, YlC; statistical analysis—DtW; supervision—WqH. Each author contributed during manuscript drafting or revision and approved the final version of the manuscript.
All experimental designs and protocols involving animals were approved by the Animal Ethics Committee of the Ludong University, Yantai, People’s Republic of China (approval LDU-IRB2022030801) and complied with the recommendations of the academy’s animal research guidelines.
We thank the Core Facility of Drosophila Resource and Technology, Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences for providing fly stocks and reagengts.
This work is supported by the Shandong Province Natural Science Foundation (No. ZR2020QC096) and the National Natural Science Foundation of China (No. 32000832).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
