1. Introduction
One of the most predominant disorders of the central nervous system that poses
significant problems within the realm of global public health is Alzheimer’s
disease (AD), which is a neuro-degenerative condition that is associated with
cognitive and memory loss [1]. Amyloid plaques and intracellular neurofibrillary
tangles (NFT), two significant neuropathological signs, are its defining
characteristics [2, 3]. As the number of AD patients rises, their families
experience considerable misery, and society as a whole is heavily burdened
socioeconomically [4]. Currently, the fight against AD is neurology’s most
pressing unmet medical need. For a long time, there has been no specific cure for
AD, only a comprehensive approach to controlling the progression of the disease
[5]. Therefore, it is critical in the realms of public health to develop
effective treatment interventions for AD.
The pathophysiology of AD is heavily influenced by oxidative stress [6]. It
contributes to A deposition, tau hyperphosphorylation, and subsequent
synapses and neuronal death in the onset of AD [7]. Illustratively, elevated
levels of A1-40 and A1-42 have been demonstrated to connect to
increased amounts of oxidative byproducts of lipids, nucleic acids and proteins
in the cortex and hippocampus of AD [8]. Moreover, a decrease in superoxide
dismutase (SOD)1 (cytoplasmic isoform) was decreased or a deficiency in SOD2
(mitochondrial isoform) resulted in an increase in tau phosphorylation in Tg2576
AD transgenic mice [9]. Oxidative stress has been associated with AD, which
suggests that the former is significantly involved in the pathological process of
the latter. Thus, the reduction of oxidative stress can be very crucial in the
treatment of AD.
The cellular antioxidative response is thought to be mainly regulated by the
nuclear factor erythroid-2-related factor-2 (Nrf2) [10]. Normally, Nrf2 is
localized in cytoplasm, but it is translocated to the nucleus after exposure to
oxidatively stress conditions, where it activates genes that protect against
oxidation [11]. Due to Nrf2’s critical role in neuroprotection in AD, its
deletion or mutation worsens memory loss, cognitive decline, and A
pathology [12, 13]. It has been observed that when the Nrf2 gene was
knocked out in amyloid precursor protein (APP) transgenic mice of AD animal model, deficits of cognitive,
memory and spatial learning of model mice were significantly aggravated [14]. In
comparison with brains in healthy individuals, it is well known that Nrf2
signaling was diminished in the brains of AD patients, with a particular
reduction in Nrf2 expression in the nuclear compartment of neurons in the
hippocampus [15]. As a result of the above assertion, Nrf2 is crucial in AD
treatment. Besides, Nrf2 is the target of the Akt/GSK3 pathway, one of
the numerous upstream signaling pathways [16]. Phosphorylation of Akt activates
GSK3, promotes the transfer of Nrf2 from Keap1-binding sites to the
nucleus, and then inhibits oxidative stress by transactivating downstream target
genes via AREs [17]. Several studies have shown that the Akt signaling pathway
plays a key role in AD. For example, Lee et al. [18] have shown that
fucoxanthin exerts resistance to amyloid-beta peptide-induced oxidative damage
through the Akt/GSK-3 signaling pathway. Xiong et al. [19]
showed that BMSCS-exosomes containing GDF-15 alleviated the SH-SY5Y cell damage
model of AD through Akt/GSK-3. Together, the Akt/GSK3 signaling
pathway may be crucial in AD treatment via Nrf2.
In traditional Chinese medicine, the roots of the Amaranthaceae plant
Achyranthes bidentata Blume are frequently used to treat dementia [20].
Ecdysterone (ECR) is one of the main active ingredients of Achyranthes
bidentata Blume and its discovery has increased medicinal value while its
antioxidant activity has been reported [21]. Additionally, earlier research has
demonstrated that ECR can enhance rat C-fos expression, alleviate cognitive
impairment brought on by oral administration of the -amyloid peptide
fragment 25-35 (A25-35), and facilitate learning and memory. The gene,
which measures neuronal activity, is directly linked to memory and learning in
the cerebral cortex and hippocampus [22]. Furthermore, the complimentary pathways
linked with c-Jun N-terminal kinase and Akt have also been demonstrated by Xu
et al. [23] to be the mechanism by which ECR shields SH-SY5Y cells from
-amyloid-induced apoptosis. However, it is not clear whether ECR
regulates Nrf2 in an Akt/GSK3-dependent manner to inhibit oxidative
stress and thus improve cognitive impairment.
Based on available literature, it was postulated that ECR may regulate Nrf2 in
an Akt/GSK3-dependent manner to inhibit oxidative stress and thus
improve cognitive impairment. Therefore, we sought to explore the neuroprotective
activity of ECR using the AD model of A25-35 treated PC12 cells and
senescence-accelerated mouse prone 8 (SAMP8). Besides, elucidation of the
potential mechanism of ECR in AD treatment was carried out by investigating its
effect on the Akt/GSK3 and Nrf2 antioxidant systems.
2. Materials and Methods
2.1 Chemicals and Antibodies
Abcam (Cambridge, UK) provided antibodies for A-1 (cat. no. ab201060),
BCL-2 (cat. no. ab182858), Bax (cat. no. ab32503), HO1 (cat. no. ab1346) and Nrf2
(cat. no. ab62352), while cell signaling tech., (Danvers, MA, USA) supplied
antibodies for p-tau (cat. no. 12885), Akt (cat. no. 9272), P-Akt (cat. no.
4060), GSK3 (cat. no. 12456), P-GSK3 (cat. no. 5558), LaminB1
(cat. no. 17416), caspase-3 (cat. no. 9662S), cleave caspase-3 (cat. no. 9664S),
GAPDH (cat. no. 5174), and goat anti-rabbit (cat. no. 14708) and mouse (cat. no.
14709) IgG (H+L) HRP. Affinity (Melbourne, FL, USA) provided ECL reagent (cat.
no. KF8003), while Sigma-Aldrich (St. Louis, MO, USA) supplied Dulbecco’s
modified-Eagle medium (DMEM) and fetal bovine serum (FBS). Also, we obtained
trypsin, multicolor protein marker, sodium-dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) kit and 3-(4,5-dimethyl-thiazol-2-yl)-2,
5-diphenyl-2H-tetrazolium bromide (MTT) dye from Solarbio (Beijing, China).
Millipore (Millipore, Bedford, MA, USA) supplied polyvinylidene fluoride
membranes (PVDF). The BCA protein concentration assay (Enhanced) (cat. no. P0010)
and One-step TUNEL cell apoptosis detection kit (cat. no. C1089) were provided by
Beyotime (Shanghai, China). Malondialdehyde (MDA) (cat. no. ml094962), Superoxide
dismutase (SOD) (cat. no. ml092620), and reduced glutathione (GSH) (cat. no.
ml092952) content assay kits were supplied by Elisa (Shanghai, China). Macklin
Biochemical Co., Ltd. (Shanghai, China) provided ECR (purity 98%) (cat. no.
H811108) and Donepezil (cat. no. D849374).
2.2 Experimental Animals
Changzhou Cavins Laboratory Animal Co., Ltd. (Changzhou, China) provided the
male mice, namely senescence accelerated-resistant mouse (SAMR1) and
Senescence-accelerated mouse prone 8 (SAMP8), which were without any specific
pathogen, 4 months old and weight 30–35 g. All the animals were fed in cages in
the same quiet environment with a light (12 L)/dark (12 D) cycle, respectively
fed and drank sterilized feed and water. The experiment was carried out after 7
days of adaptation. The Jiangsu University (UJS IACUC) institutional committee
for the care and use of laboratory animals reviewed and approved (approval
number: UJS-IACUC-2022031401) for studies involving animals.
2.3 Animal Grouping and Treatment
One week later, 48 qualified animals were selected, and 40 SAMP8 mice except for
8 SAMR1 mice in the control group were randomly divided into 5 groups. All the
animals were divided into six groups: SAMR1 blank group (Control), SAMP8 Model
group (Model), SAMP8 model + ecdysterone Low dose group (Low), SAMP8 model +
ecdysterone Medium dose group (Medium), SAMP8 model + ecdysterone High dose group
(High), SAMP8 model + Donepezil group (Positive). Mice in the administration
group were given ECR intragastric administration (high, medium and low doses were
5, 10, and 20 mg/kg/day, respectively) [24]. The control group and model group
were intragastrically given 0.9% sodium chloride solution of the same volume,
and the positive group was intragastrically given Donepezil (1 mg/kg/day). The
drug treatment group and the control group were fed the same way in different
cages. And continued administration for 4 weeks. Animals were sacrificed after
the treatment, and tissues were collected and kept in a –80 °C freezer
until further analysis.
2.4 Cell Culture and Treatment
One of the most popular cell lines for neuroscience research is PC12, which is
employed in investigations on synaptogenesis, neurotoxicity, neuroprotection,
neurosecretion, and neuroinflammation [25]. STR profiling was used to confirm the
PC12 Cell Line, and mycoplasma testing came out negative. Every cell was
cultivated at 37 °C and 5% CO in a humidified incubator. In order
to cultivate PC12 cells, DMEM with 10% FBS, 100 U/mL penicillin, and 0.1 mg/mL
streptomycin (C0222, Beyotime) was used. Later on, we categorized the cells into
five groups, viz., control, model, low-dose ECR (25 µM), medium-dose ECR
(50 µM) and high-dose ECR (100 µM). Only normal media of equal volume
was used to cultivate the cells in the control category. The model group was
treated with A25-35 in oligomer form at 25 µM (A4559, Sigma) for
24 h to establish an in vitro AD model. Likewise, we employed
A25-35 (25 µM) to treat the cells in low, medium and high dose
groups in normal medium for 24 h, before replacement with medium containing ECR
(25, 50 and 100 µM) for another 24 h.
2.5 Testing of Mice Behavior
2.5.1 Navigation in Morris Water Maze (MWM)
In brief, we placed the platform in the middle of the fourth quadrant, while an
automatic camera system with a computer connection was put in place above the
pool to monitor and record the swimming activities of the mice. The mice’s
swimming distance and travel time to the platform (to avoid the incubation
period) were automatically estimated. Mice were thrown into the water with their
backs to the pool wall from one of the four starting places. We looked at the
movement patterns within 90 s before timing the duration within which the mice
found the platform. The experimenter took the mice to the platform, where they
rested for 15 s before the next training when they could not find the platform
inside 90 s (90 s was recorded as the escape incubation period). We trained the
mice 4 times a day at a fixed time period. Within 4 times of training, the mice
were put into water from 4 different quadrants, while the interval of each
training was 1 min. After three days of continuous training, the formal
experiment began. Eventually, we recorded the time taken by the mice from the
time they were placed into the pool to the time they found the platform. Once a
day for 5 consecutive days, we recorded the time to be 90 s when the time for the
mice to find the platform exceeded 90 s.
2.5.2 Morris Water Maze Space Exploration Experiment
After the navigation experiment, the mice in each group rested for one day, and
then tested their memory ability through the space exploration experiment. Mice
were added to the pool from the third quadrant after the platform in the fourth
quadrant was removed. Each mouse group was observed and counted as they passed
the platform in the fourth quadrant over the course of 90 s.
2.6 Nissl Staining
Paraffin sections of mouse hippocampal tissue were dewaxed and immersed in
water, dyed with 1% toluidine blue for 10 min, and rinsed with distilled water.
The colour is then separated in 70% alcohol for seconds to minutes. Then in
anhydrous ethanol dehydration, xylene is transparent. Finally, seal it with a
neutral glue and observe the cornu ammonis 1 (CA1) and CA3 regions of the hippocampus under a
microscope.
2.7 Immumohistochemical Staining
After administration, hippocampal tissue was taken from mice and placed in 4%
(w/v) paraformaldehyde (PFA) solution overnight. Before cutting the tissue into
slices (4 µm), we embedded them in paraffin. Dewaxing of paraffin sections
to water was carried out, before antigen repairing, blocking and sealing as well
as overnight incubation at 4 °C with primary antibodies of A and P-Tau
and afterwards with secondary antibodies. Later, we visualized the tissue with 3,
3-diamino-benzidine tetra-hydrochloride before counterstaining with
hematoxylin, dehydration, mounting and imaging using a high-power microscope.
Image J software (V1.8.0; LOCI, University of Wisconsin, Madison, WI, USA) was
used for quantitative analysis of staining results.
2.8 Quantification of Reactive Oxygen Species (ROS) Levels in
Tissues and Cells
Measurement of ROS levels in tissues: At the end of the last administration, the
hippocampus tissues of mice were extracted and cut into pieces, and then
completely soaked in 2 mL digestive fluid (DMEM containing 1 mg/mL collagenase Ⅳ
and 1 mg/mL DNA enzyme Ⅰ). The above mixture was then placed in a 37 °C water bath
for digestion. Ice was added after 45 min to stop digestion. The digested brain
tissue solution was run through a 40 µm cell filter in order to
exclude cell masses and tissue masses that had not been adequately digested. To
obtain a single-cell suspension, the filtrate was centrifuged at 1000 rpm for 5
min while the cell precipitation was resuspended in PBS. Wash with PBS twice,
centrifuge at 1000 r/min for 5 min, remove the supernatant, add an appropriate
volume of diluted DCFH-DA working liquid, and incubate at 37 °C for 30 min in the
dark. Following incubation, the cells underwent two PBS washes in order to
eliminate any remaining DCFH-DA from the cells. In 500 µL of PBS, the cells
were suspended. The ROS positive rate was found using flow cytometry.
Measurement of ROS levels in cells: PC12 cells were placed in a 6-well plate,
3.5 10 cells/well, and adherent cultured for 24 h. 24 h after
the preparation of the model in vitro, the medium containing different
concentrations of ecdysterone was replaced. Following a 24-hour period, the
original supernatant was disposed of, the cells in the 6-well plate underwent two
PBS washes, the diluted DCFH-DA probe was applied, and the mixture was incubated
for 15–30 min in a dark environment. After incubation, the supernatant was
discarded and washed twice with PBS to remove the DCFH-DA probe that did not
enter the cell. Add 1 mL of 0.02% pancreatic enzyme without EDTA, and when the
cells become round, add PBS to terminate digestion, and gently blow the cells
with a pipette to suspend the cells. Collect in EP tube, centrifuge at 3500 rpm
for 5 min, and discard supernatant. After adding 1 mL of pre-cooled PBS at 4
°C to the fully suspended cells, they were centrifuged for 5 min at 3500
rpm, and the supernatant was disposed of. In 500 µL of PBS, the cells were
suspended. Using flow cytometry, the ROS positivity rate was discovered.
2.9 Determination of Malondialdehyde (MDA), Superoxide Dismutase
(SOD) and Reduced Glutathione (GSH) Levels
The isolated hippocampal tissue was homogenized in cold phosphate buffer (pH
7.4) and centrifuged at 4 °C at 10,000 rpm for 15 min. The centrifuged
supernatant (serum, cell culture supernatant) was collected and 100
µL supernatant was added to the plate. The levels of MDA, SOD, and
GSH in supernatant, serum, and cell culture supernatant were measured using
specific kits according to the manufacturer’s instructions.
2.10 Western Blotting
Extraction of total protein from hippocampus and determination of protein
content were carried out respectively with RIPA lysis and BCA protein assay kit.
Later on, we performed electrophoresis of protein (40 µg) for 1 h on
SDS-PAGE (10%) to PVDF membrane with 120 V of transmembrane step. After being
blocked with 5% BSA, membranes were incubated with the primary antibodies
(BCL-2, Bax, HO-1, P-Akt, Akt, GSK3, P-GSK3, LaminB1,
Cleave-caspase 3, caspase 3 and Nrf2) for a whole night at 4 °C. Before
using goat anti-mouse antibody or goat anti-rabbit antibody that had been
HRP-labeled for 1 h at room temperature, the membrane was subjected to three Tris
Buffered Saline with Tween (TBST) washes. After three washes of the membrane with
TBST, we quantified peroxidase-labelled protein bands with an ECL kit before
assessment of protein intensity with Image J.
2.11 Immunofluorescence
Tissue or cell samples that have been fixed in PFA (4%) were subjected to
drying, paraffin embedding, slicing, dewaxing, hydrating, antigen extraction,
blocking, and all-night incubation at 4 °C with primary antibody.
Afterwards, sections were subjected to three PBS washes before the FITC conjugate
secondary antibody was used for their incubation for 1 h at 37 °C. Later
on, an anti-fade mounting medium with 4, 6-diamidino-2-phenyl-indole (DAPI)
was used to mount the slices after washing. Ultimately, we evaluated the sections
with a fluorescent microscope.
2.12 TUNEL Staining
Following the manufacturer’s instructions, TUNEL labeling was used to identify
neuronal cell death in the hippocampal regions of mice. Under a fluorescent
microscope, the sections were taken in pictures. To count the cells that were
TUNEL positive, Image J was employed. Data was expressed as a ratio of the number
of TUNEL positive cells to the square millimeter.
2.13 Cell Viability Assay
Assessment of the effects of various dosage forms on cell viability was
accomplished with the MTT assay. Growing of PC12 cells was carried out in 96-well
plates for 24 h with 8000 cells/well. Twenty-four (24) h after an in
vitro cell model preparation, we exposed the cells to various amounts of
drug-containing serum. Following a 24 h treatment period, we applied MTT (5
mg/mL, 20 µL) to each well, before the removal of supernatant after
4 h. Later on, we added DMSO (150 µL) to each well, while complete
dissolution of dirty crystals was accomplished with 10 min of oscillations at
low-speed. At a wavelength of 570 nm, we used an enzymatic-labeled meter to
determine the absorbance OD values.
2.14 Flow Cytometry for Apoptosis Analysis
According to the manufacturer’s instructions, we detected cell apoptosis with
Annexin V-FITC/propyl iodide (PI) apoptotic kit. To put it simply, we inoculated
PC12 cells into 6-well plates with 3.5 10 cells/well density,
before culturing for 24 h. After 24 h of preparation, the cultured medium
containing different concentrations of ECR was changed and treated for 24 h.
Afterwards, we used a binding buffer to digest, collect, centrifuge and resuspend
the cells. At ambient temperature without any light exposure, we incubated the
mixture after the addition of Annexin V-FITC (5 µL) and PI (5 µL).
Later on, we examined the apoptotic cells using flow cytometry and the program
FlowJo (V7.1.0; TreeStar, Ashland, OR, USA).
2.15 Statistical Analysis
To do the statistical analysis, GraphPad Prism 8.0 (GraphPad Software, Inc., San
Diego, CA, USA) was used. We expressed the data with mean SD. One-way or
two-way analyses of variance (ANOVA) were used to assess all of the data.
Statistically, the accepted significant level was p 0.05, while
acceptance at p 0.01 or p 0.001 was regarded as being
very significant.
3. Results
3.1 ECR Improves Cognitive Deficits in SAMP8 Mice
The aging process of SAMP8 mice is accompanied by complex physiological changes
related to cognitive dysfunction, such as brain A deposition, increased
oxidative stress, Tau hyperphosphorylation and neuroinflammation, which is
currently recognized as a natural senescence dementia model [26]. In this regard,
we assessed the potential of ECR to improve cognitive impairment in vivo
using SAMP8 and SAMR1 as the respective mice models and controls. Usually, MWM
which includes experiments such as navigation and exploration of space
exploration by laboratory animals, is a well-known approach for testing spatial
learning and memory in trials of these animals [27]. We first employed MWM to
assess the learning and memory capacity of mice to determine the impact of ECR on
cognitive deficits in SAMP8 mice. According to Fig. 1A, the escape latency of the
model group was much higher during the first 5 days compared to that of the
control group, and the learning impairment of SAMP8 mice was improved after ECR
or positive drug administration, and ECR was dose-dependent. On the last day of
the space exploration experiment, mice in the ECR and positive groups increased
the number of crossings in the platform region (Fig. 1B) and the time spent in
the target quadrant region (Fig. 1C) compared to the model group. Additionally,
we tested SAMR1 mice using MWM but found no significant effect on their behavior
due to ECR treatment (Supplementary Fig. 1). Nissl staining was
performed to evaluate histopathological abnormalities in the hippocampus, which
are frequently linked to the course of the disease. As shown in Fig. 2A–C, SMAP8
mice displayed considerably fewer intact neurons in hippocampal regions (CA1 and
CA3) compared with SAMR1 mice. Nonetheless, we observed a reversal of the above
alteration after ECR and positive drug treatment, wherein amid ECR effect was in
dose dependent fashion. In addition, immunohistochemical staining showed that
A and P-tau expressions in the hippocampus of model mice were increased
markedly compared to control mice, but we observed a reversal of this increase
after ECR and positive drug treatments (Fig. 2D–F). These data indicate that ECR
effectively alleviated memory loss and learning disabilities in SAMP8 mice.
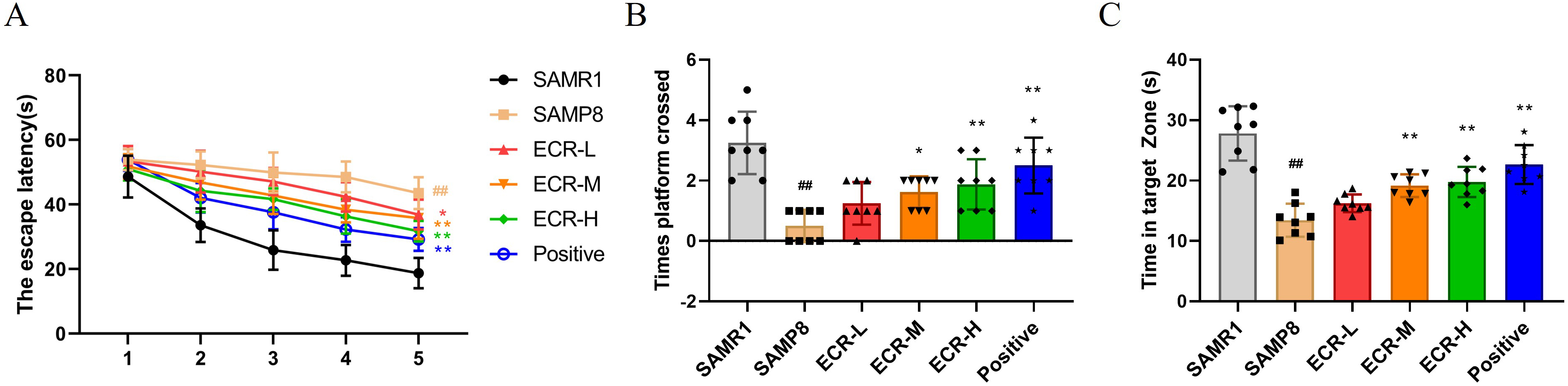 Fig. 1.
Fig. 1.
ECR improves cognitively deficient behavior in SAMP8 mice.
Effects of ECR treatment on (A) escape latency, (B) number of times crossed the
target platform position and (C) time spent in the target quadrant in Morris
water maze (MWM). (A) Data were analyzed using two-way ANOVA and a Bonferroni
test or (B,C) using a one-way ANOVA and Tukey’s post hoc test and presented as
the mean standard deviation (SD), n = 8 in each group. The groups were
compared as follows; p 0.05 and p 0.01
compared to model mice; p 0.01 compared to control mice.
ECR, ecdysterone; SAMP8, senescence-accelerated mouse prone 8; ANOVA, analyses of
variance.
 Fig. 2.
Fig. 2.
ECR improves cognitive deficits in SAMP8 mice. (A) Illustration
of images of Nissl staining of hippocampal regions (CA1 and CA3). Surviving cells
per 1 mm of (B) CA1 and (C) CA3 were analyzed quantitatively. In each group, n =
3, while the scale bar = 100 µm. (D) By using immunohistochemical staining,
A and P-Tau expressions in the hippocampus were discovered. (E,F)
Quantitative analysis of immunohistochemical staining. In each group, n = 3,
while the scale bar = 100 µm. The data were presented as the mean
SD after being subjected to one-way ANOVA and Tukey’s post hoc test analysis. The
groups were compared as follows; p 0.05 and p 0.01 compared to model mice; p 0.01 compared to control
mice.
3.2 Impacts of ECR on Oxidative Stress in SAMP8 Mice
Scientists have shown that oxidative stress has a significant influence on the
development of AD [7]. Based on this assertion, we evaluated oxidative
stress-linked biomarkers to ascertain the involvement of this process in the
ability of ECR to ameliorate cognitive deficits in SAMP8 mice. As shown in Fig. 3A,B, ROS levels considerably increased in the hippocampus of SAMP8 mice compared
to the control group but decreased significantly after treatment with ECR or
Donepezil, and ECR showed a dose-dependent manner. Important indexes that reflect
oxidative system imbalance are GSH, MDA and SOD. As can be seen from Fig. 3C–E,
SOD (Fig. 3D) and GSH (Fig. 3E) activities in the hippocampus of the model group
were significantly decreased, while MDA (Fig. 3C) content was increased. These
changes were significantly reversed by ECR or Donepezil, with a stronger reversal
effect observed at higher doses of ECR. Similarly, the detection of MDA, SOD and
GSH levels in the serum of mice was consistent with that in the hippocampus (Fig. 3F–H). Thus, inhibition of oxidative stress by ECR may facilitate its
ameliorating effect on cognitive deficits in SAMP8.
 Fig. 3.
Fig. 3.
Antioxidative effect of ECR against oxidative stress in SAMP8
mouse model. (A,B) Determination of levels of ROS in the hippocampus with flow
cytometric technique after administration of different dosage forms (n = 3). (C)
MDA, (D) SOD, and (E) GSH in the hippocampus were detected by biochemical kits (n
= 6). (F) MDA, (G) SOD, and (H) GSH in serum were detected by biochemical kits (n
= 6). The data were presented as the mean SD after being subjected to
one-way ANOVA and Tukey’s post hoc test analysis. The groups were compared as
follows; p 0.05 and p 0.01 compared to
model mice; p 0.01 compared to control mice. ROS, reactive
oxygen species; MDA, Malondialdehyde; SOD, superoxide dismutase; GSH,
glutathione; DCF, 2,7-dichlorofluorescein.
3.3 ECR Inhibits Neuron Apoptosis in SAMP8 Mice
Much evidence suggests that peroxidation of lipids and proteins is the
consequence of oxidative system imbalance, which ultimately culminates in
apoptosis in cells [28]. To investigate whether inhibition of oxidative stress by
ECR may further result in reduced cell apoptosis, we evaluated cell apoptosis and
expression levels of apoptotic-linked protein in the hippocampus of the mice. To
achieve this, we employed TUNEL staining to observe the ECR effect on the
apoptosis of neurons in the hippocampus. In terms of results, we discovered a
substantially increased number of apoptostic-positive cells in model mice
compared to control, with the cells displaying distinctive morphological features
of cellular apoptosis. Meanwhile, we observed a significantly decreased number of
TUNEL positive cells in the hippocampus of mice that received ECR compared to
model mice (Fig. 4A,B). Following that, we ascertained the alterations in
proteins that have been linked to apoptosis with the western blotting technique.
Regarding the findings, we saw that model mice expressed less Bcl-2 than control
mice did, while the former group expressed more Bax and cleaved caspase-3 (Fig. 4D,E) . Nevertheless, ECR treatment could significantly reverse these changes,
wherein the reversal was more obvious with an increase in ECR dose (Fig. 4C,F).
These data suggest that, in the hippocampus of SAMP8 mice, ECR has a protective
impact against cell apoptosis, and that this protective effect increased with
increasing ECR concentration within a specific range.
 Fig. 4.
Fig. 4.
ECR inhibits neuron apoptosis in SAMP8 mice. (A) Representative
images of TUNEL staining in each group. (B) Apoptotic cells were quantitatively
analyzed. In each group, n = 3, while the scale bar = 50 µm. (C) Detection
of apoptosis-linked proteins, namely Bax, Bcl-2, cleave caspase-3, and caspase-3
in the hippocampus with western blotting technique. (D,E) Normalization of
quantified levels of protein to GAPDH (n = 3). (F) Normalization of the
quantified level of protein to caspase-3 (n = 3). The data were presented as the
mean SD after being subjected to one-way ANOVA and Tukey’s post hoc test
analysis. The groups were compared as follows; p 0.05 and
p 0.01 compared to model mice; p 0.01
compared to control mice. TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
3.4 ECR Improves Cognitive Deficits in SAMP8 Mice by Activating
Akt/GSK3 to Regulate Nrf2
Immunofluorescent staining and western blotting techniques were utilized to
detect the expression of proteins linked to the Akt/GSK3 signaling
pathway and Nrf2 to confirm that the potentiality of ECR to improve cognitive
deficits in SAMP8 involves inhibition of oxidative stress and neuronal apoptosis.
The immunofluorescence results (Fig. 5A–C) imply that Akt and P-GSK3
expression levels in model mice reduced considerably compared to control mice,
but ECR treatment could partially reverse this effect. Additionally, there was no
discernible change in the level of Nrf2 expression between the model group and
the control group, however, ECR therapy may dramatically raise the Nrf2
expression level. As shown in Fig. 5D–H, western blot analysis revealed that ECR
treatment could not only significantly increase P-Akt and P-GSK3
expression levels in the hippocampus of SAMP8 mice, but also markedly increased
the hippocampal expression levels of Nucleus-Nrf2 and the Nrf2-related protein
HO1. However, compared with the model group, ECR treatment significantly reduced
the expression level of Cytosolic-Nrf2 (Fig. 5I). These findings suggest that ECR
may improve cognitive impairments in SAMP8 mice via activating Akt/GSK3to regulate Nrf2 and prevent oxidative damage.
 Fig. 5.
Fig. 5.
ECR improves cognitive deficits in SAMP8 mice by activating
Akt/GSK3 to regulate Nrf2. Detection of (A) Akt, (B) Nrf2 and (C)
P-GSK3 expressions in the hippocampus with immunofluorescence staining.
In each group, n = 3, while scale bar = 50 µm. (D) Analysis of Akt, P-Akt,
P-GSK3, GSK3, nuclear-Nrf2, Cytosolic-Nrf2 and HO1 in the
hippocampus with western blotting. (E) Normalization of P-Akt protein level to
Akt (n = 3). (F) Normalization of P-GSK3 protein level to GSK3
(n = 3). (G) Normalize HO1 protein level to GAPDH (n = 3). (H) Normalize
Cytosolic-Nrf2 protein level to GAPDH (n = 3). (I) Normalize Nuclear-Nrf2 protein
level to LaminB (n = 3). The data were presented as the mean SD after
being subjected to one-way ANOVA and Tukey’s post hoc test analysis. The groups
were compared as follows; p 0.05 and p
0.01 compared to model mice; p 0.01 compared to control
mice. Akt, protein kinase B; Nrf2, nuclear factor erythroid-2-related factor-2; GSK3, glycogen synthase kinase 3;
HO-1, heme oxygenase-1.
3.5 Protective Effect of ECR on Oxidative Stress of Neurons in
A25-35-induced PC12 Cells
After demonstrating the potential of ECR to alleviate cognitive impairment via
prevention of oxidative stress and death of neurons in vivo, we
conducted several in vitro experiments to investigate the probable
mechanistic action of ECR in cognitive impairment improvement. First, we screened
the cell use concentrations of A25-35 and ECR by MTT assay
(Supplementary Fig. 2). We cultured PC12 cells and established an AD
model treated with 25 µM A25-35. Then we observed the effect of
ECR on cell vitality by MTT, and the results showed that compared with the
control group, the cell vitality decreased significantly after A25-35
stimulation, but the cell vitality was reversed after ECR administration (Fig. 6C). Excessive ROS mediated antioxidant stress system imbalance is thought to be
related to the AD process. Therefore, we investigated the ECR effect on oxidative
stress in the A25-35-induced AD model in PC12 cells with flow
cytometric technique. Findings (Fig. 6A,B) of the above experiment showed that
levels of ROS in model mice increased markedly compared to control, but their
levels dose dependently decreased in mice that received ECR. Additionally, PC12
cells stimulated with A25-35 showed alterations in SOD and GSH levels as
well as an increase in MDA levels, with ECR reversing these modifications in the
same manner as stated above (Fig. 6D–F). The expression levels of proteins
associated with the Nrf2 antioxidant system and the levels of Cytosolic-Nrf2 and
Nucleus-Nrf2 were detected using western blot to determine if the suppression of
ECR on oxidative stress in A25-35-induced PC12 cells in vitro
is connected to the Nrf2 antioxidant system. Fig. 6G–M showed the results of
western blot and cellular immunofluorescence analysis. We discovered that ECR
activated the Nrf2 system, which substantially increased the expression of Nrf2
at the protein level in the nucleus.
In addition, we observed that PC12 cells treated with A25-35 had an
increased apoptotic rate and an increased expression of apoptosis-related
proteins. In light of the flow cytometry result, the proportion of apoptotic
cells in PC12 increased under the intervention of A25-35 compared to
control, while different doses of ECR significantly decreased apoptotic cells
proportion in the above-mentioned cells (Fig. 6N,O). Furthermore, we evaluated
the potential of ECR to prevent apoptosis in PC12 cells with western blot.
Protein expression of Bcl-2 was found to be downregulated in PC12 cells
stimulated with A25-35, whereas the levels of caspase-3 and Bax were
upregulated. ECR treatment ably upregulated Bcl-2 expression at protein level but
downregulated Bax and caspase-3 protein expressions compared to PC12 cells in the
model group (Fig. 6P–S).
 Fig. 6.
Fig. 6.
Protective effect of ECR on oxidative stress of neurons in
A25-35-induced PC12 cells. (A) Determination of ROS levels in PC12
cells treatment with different dosage forms with flow cytometry. (B) Levels of
ROS quantifications (n = 3). (C) Assessment of cell viability with MTT assay and
treatment of A25-35 induced PC12 cells with various doses of ECR (n =
6). Detection of biomarkers of oxidative stress (D) MDA, (E) SOD and (F) GSH in
PC12 cells after treatment with different dosage forms (n = 6). (G) The
expression levels of Nrf2 antioxidant system-related proteins HO1, NQO1,
Cytosolic-Nrf2 and Nuclear-Nrf2 were analyzed by western blotting. (H) Normalize
Nuclear- Nrf2 protein level to LaminB (n = 3). (I–K) Normalize HO1, NQO1 and
Cytosolic-Nrf2 protein levels to GAPDH (n = 3). (L,M) Immunofluorescence staining
analysis of Nrf2 expression in PC12 cells after treatment with different dosage
forms. In each group, n = 3, while scale bar = 50 µm. (N,O) Determination
of ECR effect on the percentage of apoptotic cells in PC12 cells induced by
A25-35 with flow cytometric technique (n = 3). (P) Western blotting
analysis of Bax, Bcl-2, cleave caspase-3 and caspase-3 protein expressions in
PC12 cells after different treatments. (Q,R) Normalization of levels of Bax and
Bcl-2 protein to GAPDH (n = 3). (S) Normalization of the level of cleaved
caspase-3 to caspase-3 (n = 3). The data were presented as the mean SD
after being subjected to one-way ANOVA and Tukey’s post hoc test analysis. The
groups were compared as follows; p 0.05 and p 0.01 compared to model mice; p 0.01 compared to
control mice. MTT, 3-(4,5-dimethyl-thiazol-2-yl)-2, 5-diphenyl-2H-tetrazolium
bromide; PI, propidium iodide; NQO1, NADH dehydrogenase (quinone 1).
Collectively, ECR demonstrated a beneficial effect on oxidative stress and cell
apoptosis accordingly induced in PC12 cells by A25-35.
3.6 ECR Regulates Nrf2 by Activating the Akt/GSK3 Pathway
to Protect Cell Damage of A25-35-induced PC12 Cells
During this experiment, we employed Akt inhibitor MK2206 to further clarify the
possibility that ECR protected PC12 against oxidative stress and apoptosis
induced by A25-35 via regulation of Nrf2 by the Akt/GSK3
pathway. On the basis of the finding (Fig. 7A), we identified that the declined
cell viability in A25-35-induced cells was ameliorated by ECR, but
inhibition of Akt caused this effect to disappear. Similarly, ECR treatment
alleviated intracellular ROS levels in A25-35-induced PC12 cells, but
this phenomenon could also be eliminated by inhibiting Akt (Fig. 7B).
Additionally, as depicted in the western blot results presented in Fig. 7C, ECR
treatment increased the expression levels of P-GSK3 and Nucleus-Nrf2,
while decreasing the expression level of Cytosolic-Nrf2 in
A25-35-induced PC12 cells. However, these changes would disappear due to
the inhibition of Akt. Collectively, these findings provide further evidence that
ECR controls Nrf2 by activating the Akt/GSK3 pathway to protect PC12
cells from damage induced by A25-35.
 Fig. 7.
Fig. 7.
ECR regulates Nrf2 by activating the Akt/GSK3 pathway
to protect cell damage of A25-35-induced PC12 cells. (A) Determination
of the effect of high concentration of ECR on cell viability of PC12 cells
induced by A25-35 before and after Akt inhibitor treatment with MTT
assay (n = 6). (B) Effect of high concentration of ECR on ROS levels in PC12
cells induced by A25-35 before and after Akt inhibitor treatment was
analyzed by flow cytometry (n = 3). (C) Western blotting analysis of the effects
of high ECR concentration on levels of Cytosolic-Nrf2, Nuclear-Nrf2,
P-GSK3 and GSK3 expressions in PC12 cells before and after Akt
inhibitor treatment. Normalization of the level of nuclear-Nrf2 protein to LaminB
(n = 3). Normalization of the level of P-GSK3 protein to GSK3
(n = 3). Normalize Cytosolic-Nrf2 protein level to GAPDH (n = 3). The data were
presented as the mean SD after being subjected to one-way ANOVA and
Tukey’s post hoc test analysis. p 0.01, means significant
difference.
4. Discussion
Herein, we attempted to understand the mechanism underlying the potential of ECR
to ameliorate cognitive impairment by conducting a series of experiments
(in vitro and in vivo). Results obtained from SAMP8 animal
models in vivo suggest that ECR may regulate Nrf2 to prevent oxidative
stress in an Akt/GSK3-dependent manner, thereby reducing cognitive
impairment. Moreover, in vitro findings demonstrated that ECR regulates
Nrf2 via the Akt/GSK3 pathway, protecting PC12 cells from
A25-35-induced cell damage. These findings collectively provide initial
evidence that ECR could reduce cognitive impairment via the prevention of
oxidative stress, amid regulation of Nrf2 via the Akt/GSK3 pathway.
The leading cause of dementia and a growing global health concern, AD has
significant effects on both individuals and society [29]. Despite significant
research efforts to find a therapeutic approach to stop the course of AD or to
cure it, currently available medications are only effective in treating its
symptoms and their efficacy is still insufficient [5]. Hence, efficacious
treatment options for AD are urgently needed. ECR is a natural substance and the
main steroid hormone. It has anti-oxidative and neuroprotective properties [22, 30]. However, the therapeutic impact of ECR on AD and its molecular mechanism
have not been sufficiently investigated. The SAMP8 mouse model’s cognitive
deficiencies were found to be improved by ECR and positive treatment in this
study, and ECR was demonstrated in a dose-dependent manner.
Oxidative stress, which is caused by an excess of ROS, has been associated with
various disorders. Due to the high oxygen consumption in the brain, these free
radicals may cause more visible damage than what cell molecules can scavenge
[31]. Oxidative stress has emerged as a crucial approach in the prevention and
treatment of AD since it plays a significant role in the disease progress and may
be regarded as a major factor in its development [32, 33, 34]. Therefore, we evaluated
the ECR effect on oxidative stress in the hippocampal of SAMP8 mice or PC12
celles induced by A25-35. Our findings demonstrated that oxidative
stress is activated in A25-35-induced PC12 cells or SAMP8 model mice,
but that ECR treatment increases SOD and GSH levels while decreasing ROS and MDA
levels. This suggests that ECR may improve cognitive deficiencies by preventing
oxidative stress. Additionally, AD patients frequently experience neuronal death
in the brain, particularly in the hippocampus, which is largely brought on by
apoptosis, with oxidative stress serving as the primary trigger [35]. As a
result, we investigated how ECR affected apoptosis in PC12 cells induced by
A25-35 or in the hippocampus of SAMP8 mice. Our findings suggested that
ECR, particularly in the high-dose group, attenuates the decline in Bcl-2 and the
rise in Bax and Cleave-caspase 3 protein expression in the hippocampus of SAMP8
mice. Similar results were observed for A25-35-induced PC12 cells. Based
on the above in vivo and in vitro results, we suggested that
ECR has an anti-apoptotic potential in AD, probably through inhibition of
oxidative stress.
Apart from crucially regulating the antioxidative system, Nrf2 has been found to
additionally control the response of inflammation, homeostasis of intracellular
redox and other processes of biological importance [36]. Besides, research has
demonstrated that one of the body’s most crucial antioxidant defense mechanisms
is the Nrf2/HO1 pathway [37]. It has been posited that the antioxidative system
of Nrf2 (for example) might reduce the severity of several diseases by preventing
oxidative stress when downstream genes like NQO1 and HO1 are controlled [38].
Additionally, one of the several upstream signaling pathways that target Nrf2 is
the Akt/GSK3 pathway [16]. GSK3 regulates cell survival
activity as a critical factor downstream of Akt, and Akt-mediated phosphorylation
of GSK3 inhibits its expression [39]. Of note, drugs that possess
inhibitory action against GSK3 have demonstrated potential
pharmacological treatments for AD, since they could lessen CI and
neuropathological symptoms in vivo [40]. Therefore, we verified the ECR
effect on Nrf2 and Akt/GSK3 pathway. Research conducted both in
vitro and in vivo has demonstrated that ECR can powerfully enhance Akt
and GSK3 phosphorylation levels, as well as boost Nucleus-Nrf2 protein
expression and enhance its function within the nucleus. The increased
HO-1 and NQO1 expression levels found in this investigation offered more proof.
Moreover, the current work evidenced through Akt inhibitor the potentiality of
ECR to activate the antioxidative system of Nrf2 via the Akt/GSK3
pathway. The results showed that Akt inhibitors eliminated changes in cell
activity, oxidative stress, and the expression of P-GSK3 and
Nucleus-Nrf2 after ECR treatment. Collectively, these findings suggest that ECR
can regulate Nrf2 via the Akt/GSK3 pathway to attenuate oxidative
stress-induced apoptosis, and thereby improve cognitive impairment.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5. Fig. 6.
Fig. 6. Fig. 7.
Fig. 7.