1 Department of Human Anatomy, Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University), 119991 Moscow, Russia
2 Department of Normal and Topographic Anatomy, M.V. Lomonosov Moscow State University, 119991 Moscow, Russia
Abstract
Locus coeruleus is a small bilateral nucleus in the brainstem. It is the main source of norepinephrine (noradrenaline) throughout the central nervous system (about 70% of all norepinephrine in the central nervous system), and, as shown in numerous studies, it is involved in regulating a significant number of functions. The detailed study of the functions of the Locus Coeruleus (LC) and its significance in human life became possible only after the development of histofluorescence methods for monoamines in the 1960s. The widespread locus coeruleus-norepinephrine (LC-NE) projection system regulates the entire central nervous system and modulates sensory processing, motor behavior, arousal, and cognitive processes. Damage to the LC and the associated decrease in norepinephrine levels are involved in a wide range of clinical conditions and pathological processes. Although much about the anatomy and physiology of the LC is currently known, its ultimate role in the regulation of behavior, control of the sleep-wake cycle, stress response, and the development of pathological conditions (such as Alzheimer’s disease, dementia, depression, suicidal behavior, chronic traumatic encephalopathy, and Parkinson’s disease) is not fully understood. Non-invasive visualization of the LC can be used for differential diagnosis, determining the stage of the disease, and predicting its course. Studying the dysfunction of the LC-norepinephrine system, involved in the pathogenesis of various neurological diseases, may ultimately form the basis for the development of new treatment methods based on the pharmacological elevation of norepinephrine levels. In this review, we will attempt to highlight the key points regarding the structure and function of the Locus Coeruleus, as well as outline the main directions and prospects for its study.
Keywords
- Locus Coeruleus
- norepinephrine
- projections
- neurodegenerative diseases
- magnetic resonance imaging
The Locus Coeruleus (LC) was first described in 1786 by French physician and anatomist Vicq-d’Azyr in his treatise “Traité d’Anatomie et de Physiologie” [1, 2]. According to other sources, the LC was discovered and presented in J.C. Reil’s work “Untersuchungen über den Bau des grossen Gehirns im Menschen” in 1809 [3]. The structure was named Locus Coeruleus, meaning “blue place” in Latin, by Karl Wenzel and Joseph Wenzel in their work “De penitiori structura cerebri hominis et Brutorum” in 1812 [3].
It was Karl Wenzel and Joseph Wenzel who first identified this structure as the Locus Coeruleus—the bluish or blue spot in literal translation [3]. In their work “De penitiori structura cerebri hominis et Brutorum” from 1812, the following description is given: “In the anterior base of that ventricle, in the middle on both sides, a certain oblong, narrow and blue spot immediately catches the eye, which in color resembles a vein, begins at the posterior end of the so-called aqueduct of the brain, and takes a direction almost parallel to the lower margin of the lower corpora quadrigemina, and ends towards the middle of the fifth ventricle” [4]. Approximate translation: “In the anterior part of the ventricle base, in the middle on both sides, a certain oblong, narrow and blue spot immediately catches the eye, which in color resembles a vein, begins at the posterior end of the aqueduct of the brain, takes a direction almost parallel to the lower margin of the lower corpora quadrigemina, and ends towards the middle of the ventricle”.
LC cells contain neuromelanin, which gives this structure its characteristic color. On sections, the nucleus appears bluish (precisely because of neuromelanin pigmentation—a by-product of catecholamine metabolism), which gave rise to the name Locus Coeruleus—the bluish spot [5]. However, researchers paid close attention to the LC after the development of the histo-fluorescence method, when it became possible to visualize tissue monoamines. Thanks to histo-fluorescence, it was established that LC is the site of norepinephrine synthesis and a wide network of LC neuron projections throughout the central nervous system (CNS) was also discovered [1, 6]. A significant contribution to the development of understanding of the monoamine projection system of LC was made by Loizou’s article published in 1969. As a result of histo-fluorescent fiber research, it was established that LC projections connect it with the motor nucleus of the trigeminal nerve, nucleus tractus solitarii, amygdala, hippocampus, and Edinger-Westphal nucleus [1].
Further research on the structure and projections of the LC involved selective staining methods using antibodies against dopamine-beta-hydroxylase (DBH), an enzyme responsible for the conversion of dopamine to norepinephrine [1]. Immunostaining of DBH revealed neurons containing norepinephrine and their numerous projections [7, 8]. Neurons in the LC can also be identified through immunoreactivity to tyrosine hydroxylase, a regulatory enzyme involved in the synthesis of norepinephrine [9].
While it was previously believed that the LC had a homogeneous structure and uniform effects on all its projections, recent studies using modern techniques such as optogenetics, chemogenetics, virus-mediated genetic tracing, and functional magnetic resonance imaging have demonstrated heterogeneity among LC neurons [10]. This raises questions about the varying responses of LC neurons to afferent stimuli and their different effects on CNS structures, which can have diverse and significant influences on brain function and behavior [7, 10].
Further investigation into the structure and function, as well as the development of new visualization techniques for the LC, will provide a better understanding of the mechanisms underlying various pathological conditions such as Alzheimer’s disease and Parkinson’s disease. Non-invasive methods of visualizing the LC may be used in the future as biomarkers for neurodegenerative diseases to assess the effectiveness of psychopharmacological research and clinical monitoring [11].
To understand how the LC influences various functions of the body, a detailed study of its anatomical organization is necessary [10]. Significant difficulties in studying the structure of the LC are due to its small size and shape, as well as its deep location in the brainstem, which makes it difficult to record the activity of LC neurons and determine the relationship between activity and functional manifestations [7, 10].
The Locus Coeruleus is a cluster of relatively large neurons located bilaterally in the brainstem. It is a tubular, symmetrical nucleus with a central ventral expansion located on both sides of the fourth ventricle [5, 10, 12, 13]. The nucleus is located laterally to the wall of the fourth ventricle and medially to the nucleus mesencephalicus nervi trigemini [14]. The LC is situated approximately 1 mm below the floor of the fourth ventricle, 3 mm lateral to the midline, and 14–21 mm above the ponto-medullary junction [15].
According to the classification by Dahlström and Fuxe, the LC (also known as nucleus A6) together with the nucleus epi-coeruleus (A6e—rostral expansion) and nucleus sub-coeruleus (A6sc—ventral expansion) form the NA-neuronal complex. For simplicity, the entire NA-neuronal complex is often referred to as Locus Coeruleus [12].
The body of the LC can be divided into three main components: the anterior pole, the compact nucleus that expands dorsoventrally, and the posterior pole [14]. The posterior pole of the LC lies at the level of the inferior colliculi, and from there, the LC extends along the floor of the fourth ventricle [16].
Among the LC neurons, two populations are distinguished: large multipolar
(
The length of the LC is 12–17 mm, the width is 2.5 mm, and the height is 2.0 mm
[12, 15, 16]. The volume of the LC averages 9.53 mm
Information about the number of cells varies significantly in different studies. Depending on the hemisphere, the LC contains between 22,000 and 52,000 norepinephrine cells (NA cells) (on average, between 19,000 and 35,000) [16]. These differences can be attributed to different markers used for neuron identification [12, 16]. The left and right halves of the LC are symmetrical, with no gender differences in the total number of cells or average neuron volume [18].
The cell bodies of LC neurons have a round or oval shape [16]. Dendrites, which are long and thin, usually branch relatively infrequently. In contrast, axons have extensive bifurcations, potentially indicating innervation of multiple areas [9].
LC projections are unmyelinated and, therefore, conduct impulses slowly (usually less than 1 m/s). The synthesized norepinephrine in LC neurons, released from the presynaptic terminal, interacts with postsynaptic adrenoceptors. Then, most of the norepinephrine is taken up and transported back by the norepinephrine transporter, and in the cytoplasm of neurons, norepinephrine undergoes oxidative deamination by monoamine oxidase (MAO). In effector cells, where a smaller portion of norepinephrine enters, norepinephrine is inactivated by catechol-O-methyltransferase (COMT) [19, 20]. In addition to the typical exocytosis of norepinephrine, LC terminals have non-synaptic release sites for neurotransmitters, allowing for paracrine regulation by influencing other neurons, glial cells, and microvessels [19, 21, 22]. It is the LC that innervates most of the microvascular bed of the CNS. It has been estimated (based on the data that LC contains an average of 32,000 neurons and the total length of all capillaries is 640 kilometers) that one LC neuron innervates about 20 meters of capillaries [19]. Two or more capillaries wrap around the body of each neuron, which is also unique to brain structures [23]. Due to abundant vascularization, there is an increased risk of LC damage by circulating toxic substances, even at low concentrations [24].
Among LC neurons, there is a division between neurons that project to the primary motor cortex and neurons that project to prefrontal cortex regions (cingulate, orbitofrontal, and medial prefrontal gyri). The difference lies in different excitability thresholds and protein expression, suggesting the involvement of individual LC neurons in the regulation of different functions [1].
The distribution of NE-containing fibers in cortical and subcortical structures shows significant regional specificity [1]. Extensive projections from the LC are directed to the forebrain, cerebellum, brainstem, and spinal cord [7]. Recent research has shown that neurons in the LC are topographically arranged depending on the specific targets of their projections. Neurons projecting to cortical and subcortical structures (such as the limbic system and neocortex) are mainly located in the rostral part of the nucleus, while neurons projecting to the cerebellum and spinal cord are predominantly located in the ventral and caudal parts of the LC [7, 12].
LC neurons also differ in the expression of neuropeptides depending on the area
of the LC. Neurons expressing galanin (about 80% of all neurons) are
predominantly localized in the dorsal and central areas of the LC. Neurons
expressing neuropeptide Y (about 20%) are located in the dorsal part [7]. Other
neuropeptides have also been found in LC neurons, such as vasopressin,
somatostatin, enkephalin, neurotensin, corticotropin-releasing factor, and
neurotrophic growth factor. Their expression is also uneven depending on the
anatomical area of the LC, but their role is not well understood at the moment
[7, 10]. LC neurons also differ in the expression of neurotransmitter receptors,
such as adrenergic receptors and nicotinic acetylcholine receptors. For example,
Currently, the topographic organization of LC neurons is being studied using viral reagents and optogenetic methods [7]. In recent years, LC magnetic resonance imaging has been increasingly considered as a potential biomarker for neurodegenerative diseases such as Parkinson’s disease and Alzheimer’s disease [25], as LC degeneration occurs in the early stages of these diseases [12, 26].
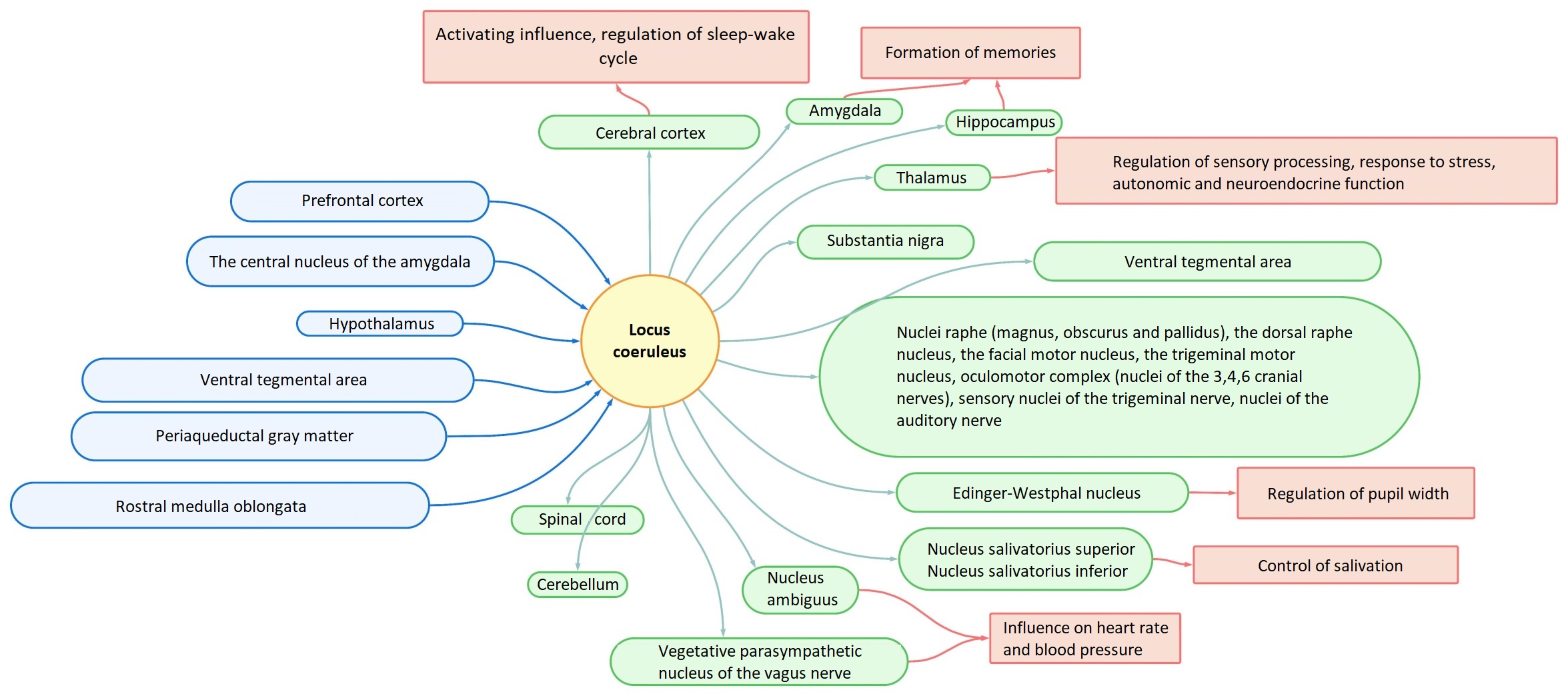
A large number of afferent projections converge on LC neurons, and in turn, diffuse projections from LC neurons spread throughout the brain (Fig. 1) [3, 12]. LC receives afferent information from many regulatory centers (prefrontal cortex, hypothalamic nuclei, rafe nucleus, amygdala, solitary nucleus, paragigantocellularis nucleus, and prepositus hypoglossus nucleus). LC integrates information from the autonomic nervous system, neuroendocrine nuclei, limbic system, and cognitive centers. Efferent projections from LC spread to many layers and areas of the cerebral cortex, intermediate, middle, medulla, and spinal cord, as well as the cerebellum [14].
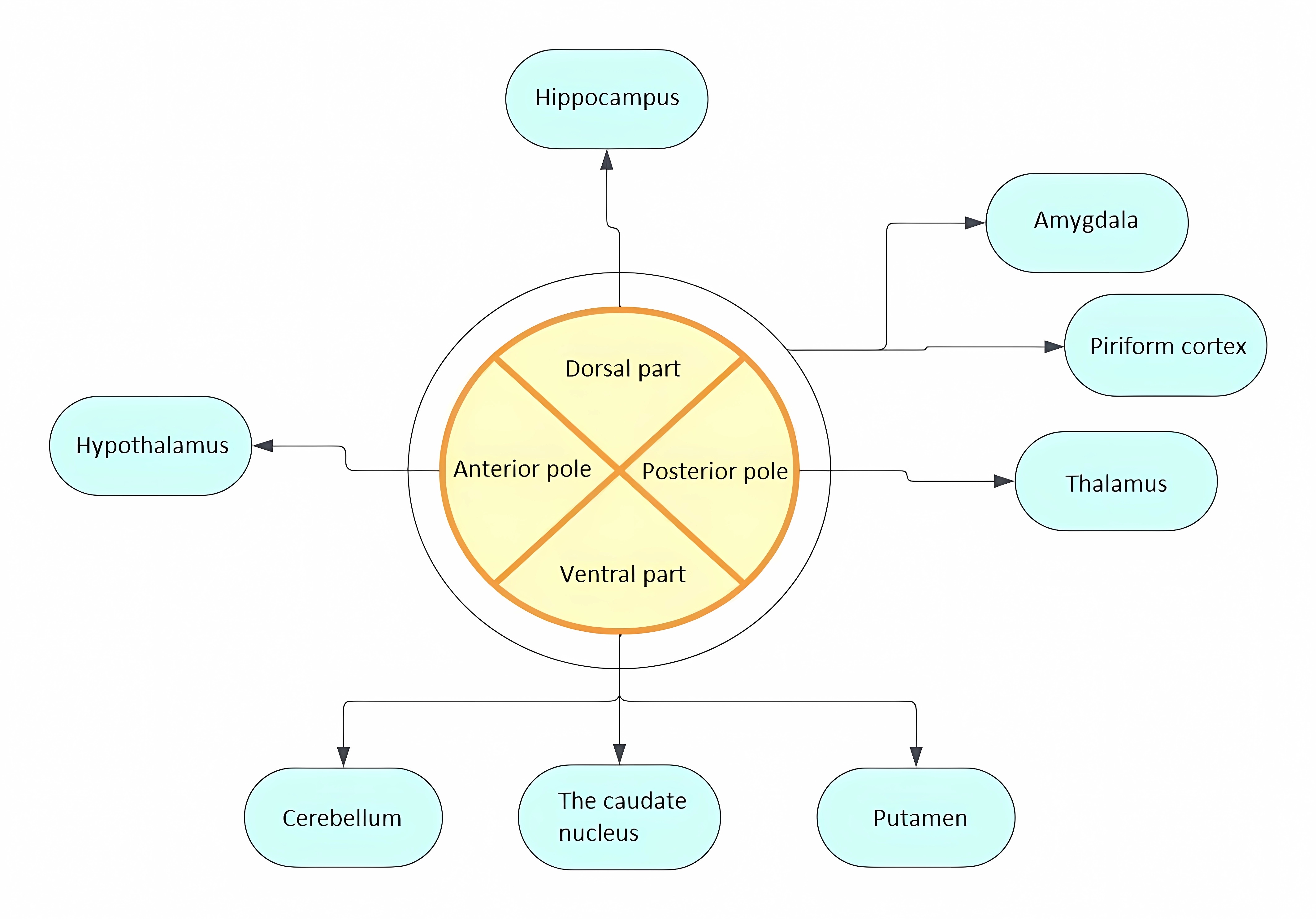
 Fig. 1.
Fig. 1.Scheme of afferent and efferent projections of LC. LC, Locus Coeruleus.
It is believed that diffuse efferent projections from LC can explain the diversity of functions performed [27]. Initially, the efferent topography of LC was determined by studying retrograde transport of HRP (horseradish peroxidase) from areas innervated by norepinephrine: cerebral cortex, cerebellum, hippocampus, hypothalamus, thalamus, amygdala, pear-shaped cortex, septum. The results obtained not only demonstrated efferent projections to all these areas but also allowed conclusions to be drawn about the heterogeneity of neurons within the LC. It was shown that individual groups of neurons send projections to different regions of the brain (Fig. 2). Thus, dorsal neurons predominantly project to the hippocampus, ventral neurons to the cerebellum, caudate nucleus, and putamen, while posterior pole neurons project to the thalamus and anterior pole neurons to the hypothalamus. Neurons projecting to the amygdala and pear-shaped cortex are scattered throughout the LC [28]. Thus, the division of LC neurons into separate groups along the dorsoventral and rostrocaudal axes depending on efferent targets was demonstrated [14, 28]. In subsequent studies, it was found that subcortical structures receive bilateral innervation, while the cerebral cortex receives ipsilateral projections [14]. Currently, viral vector labeling methods with subsequent visualization using tomography and mapping of projections by sequencing barcode RNA (MAPseq—Multiplexed Analysis of Projections by Sequencing) are used to study projections [29, 30]. Viral vectors selectively expressing chemogenetic activators of LC neurons are also used to determine functionally specific subpopulations of LC neurons, allowing behavioral responses to stimulation of individual groups of neurons to be tracked [31].
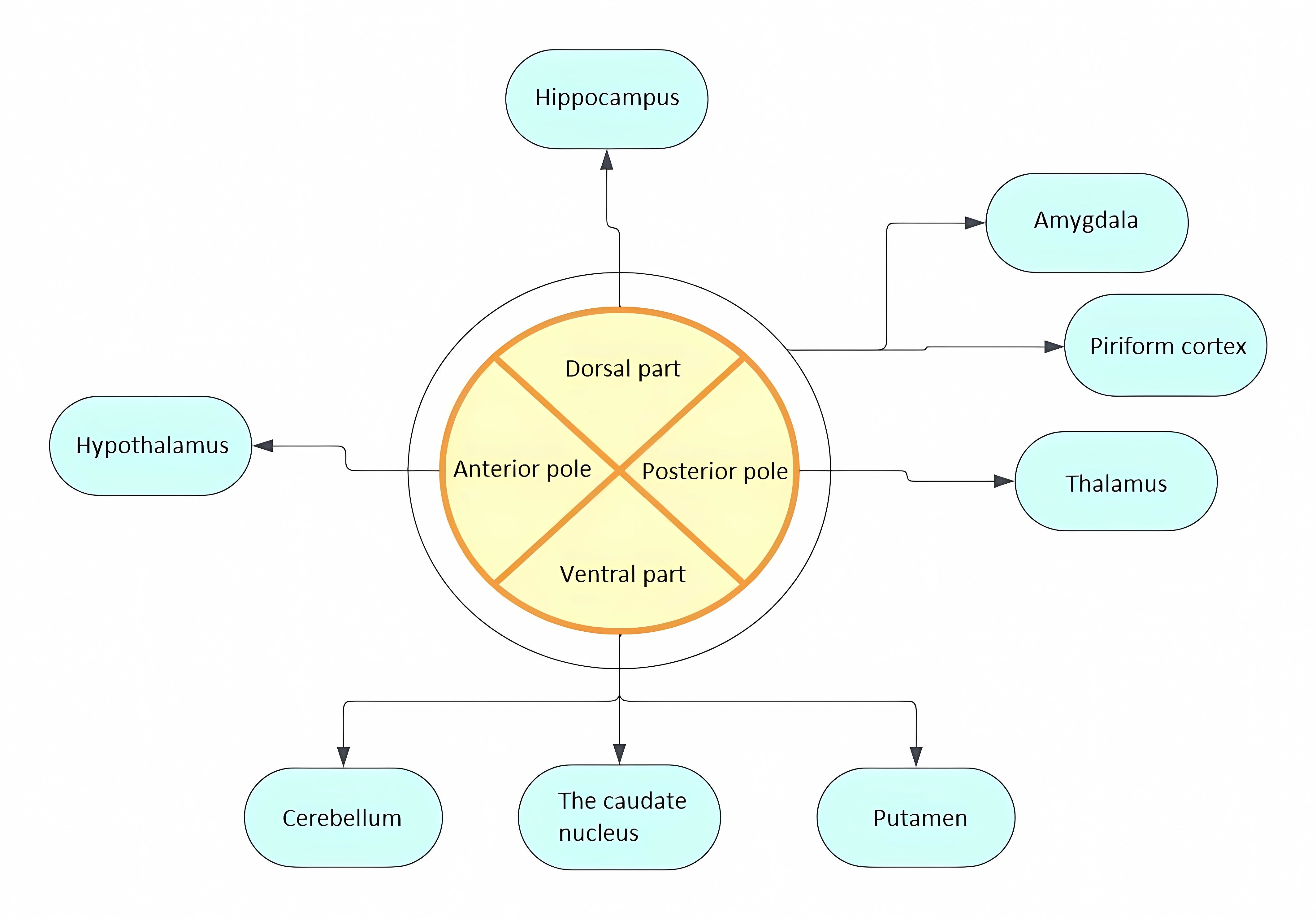 Fig. 2.
Fig. 2.Distribution of LC projections in relation to the dorsoventral and rostrocaudal axes depending on efferent targets.
It is worth noting that noradrenergic stimulation, due to receptor
heterogeneity, can lead to both activation and inhibition of target cells
[27, 32]. Postsynaptically, norepinephrine can interact with 3 types of
adrenoceptors: alpha-1 (
Let’s take a closer look at individual efferent pathways originating from the LC
and the effects of noradrenaline (Fig. 3). The projections of the LC innervate
all lobes of the cerebral cortex. It is noted that by acting on
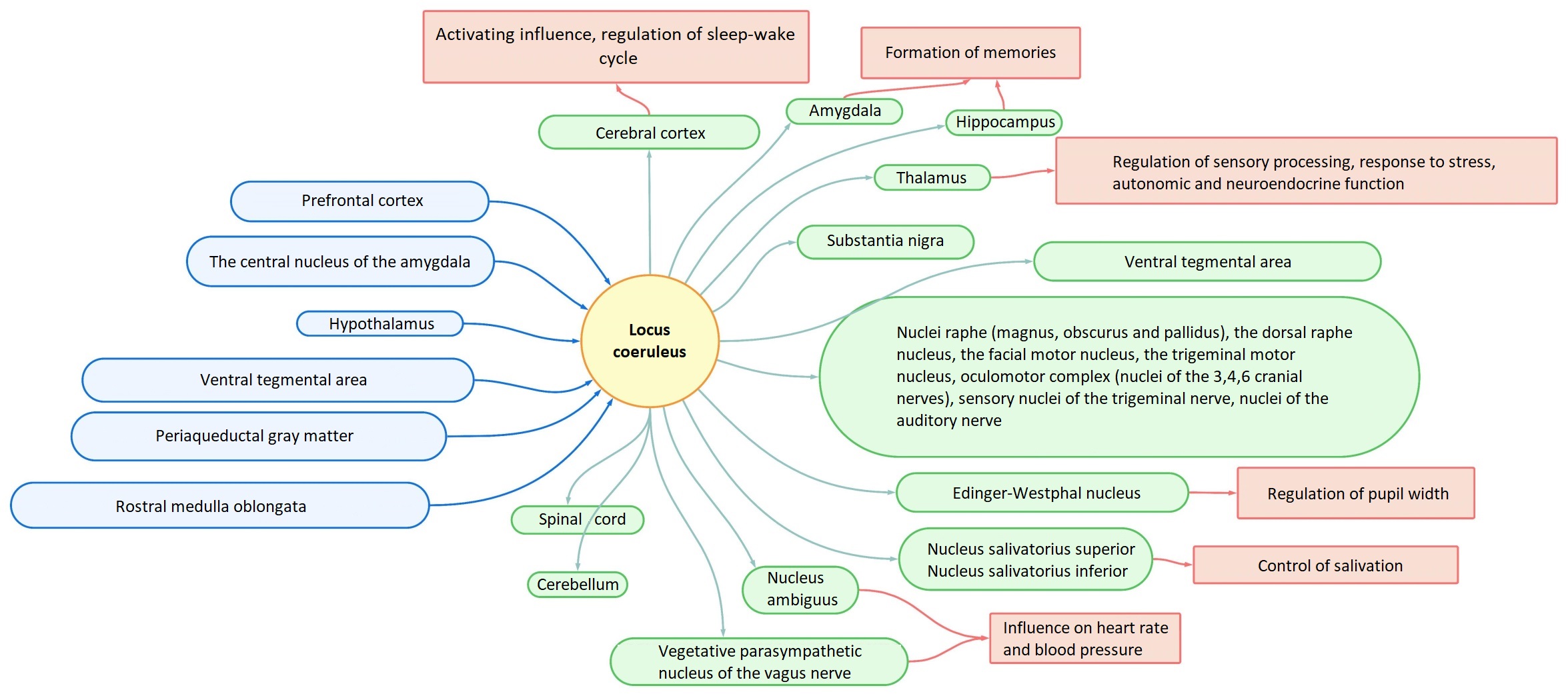 Fig. 3.
Fig. 3.Scheme of afferent and efferent projections of LC with some induced effects.
The effect of dorsally located LC nuclei on
Speaking of brainstem structures, the LC mainly has an inhibitory effect on
parasympathetic nuclei as a result of action on
The projections from LC reach the spinal cord: they act on sensory neurons in
the dorsal horn (which indirectly indicates the possible influence of LC on
sensory information processing), motor neurons in the ventral horn (which
suggests possible involvement in maintaining muscle tone), and preganglionic
neurons in the lateral horns [27, 32, 33]. In the sensory neurons of the dorsal
horns, inhibitory
Efferent projections from LC are generally grouped into three categories: ascending pathway to the cerebral cortex, cerebellar pathway, and descending pathway to the spinal cord. The ascending pathway includes projections to the entire neocortex, midbrain, thalamus, limbic system, and basal forebrain. Projections of the ascending pathway play a role in maintaining cognitive functions, memory formation, stress response, pain modulation, and some sympathetic and parasympathetic functions [27].
Extensive projections from various areas of the brain are directed to the LC. Modern studies using viral labeling confirm a wide range of brain areas from which information is received by the LC through afferent pathways [7, 9, 27]. Among the areas of the neocortex, special attention is drawn to the prefrontal cortex, from which glutamatergic projections are sent to the LC [9, 27, 32].
Afferent projections from the central nucleus of the amygdala are believed to be involved in regulating the processing of emotional stimuli and the response to stress [27, 32]. In the context of the involvement of the LC in the stress response, let’s focus more on CRF-containing afferent projections to the LC (CRF—corticotropin-releasing factor) [9, 32]. Projections from the hypothalamic area to the LC send: the ventrolateral preoptic nucleus, paraventricular nucleus, Lateral Hypothalamic/Perifornical Area (LH/PF), tuberomammillary nucleus. Inhibitory projections from GABAergic neurons of the ventrolateral preoptic nucleus to the LC, together with inhibitory projections from the LC to the ventrolateral preoptic nucleus, form a feedback system involved in regulating the sleep-wake cycle [27, 32]. Excitatory projections from the paraventricular nucleus contain CRF as a neurotransmitter (along with projections from the central nucleus of the hypothalamus). Excitatory projections from LH/PF, containing orexin, influence wakefulness and muscle tone [32].
Dopamine-releasing neurons in the ventral tegmental area provide dopaminergic innervation to the LC. Cholinergic projections are sent to the LC from the pedunculopontine (PPT) and laterodorsal (LDT) tegmental nuclei of the pons. These dopaminergic and cholinergic projections, apparently, also participate in stimulating wakefulness [9, 32]. Dopaminergic influence may play a role in reward-based learning processes [27].
Mutual projections from LC to the periaqueductal gray matter and back may underlie the phase switching of sleep, namely the transition to the rapid eye movement (REM) phase [32]. GABAergic and glutamatergic projections are directed from the rostral brainstem to the LC [32]. The nucleus prepositus hypoglossi sends GABAergic inhibitory projections. Glutamatergic projections from the rostroventrolateral medulla are believed to be involved in controlling cardiovascular functions. Additionally, projections containing the endogenous opioid enkephalin, which activates opioid receptors and suppresses cell activity, originate from the nucleus paragigantocellularis lateralis and nucleus prepositus hypoglossi. Thus, the LC is one of the structures mediating the effects of opioids [32, 35].
The extensive dendrites of LC neurons form a dense cluster outside the LC in the pericoerulear areas. Afferent projections from various structures are directed to these areas: the prefrontal cortex, amygdala, lateral hypothalamus, and nucleus raphe dorsalis [1, 10].
The diffuse network of LC projections throughout the brain suggests involvement in many regulatory systems of the central nervous system [36]. Numerous studies demonstrate that the LC is involved in a wide range of functions: regulation of the sleep-wake cycle, participation in cognitive processes (such as attention, memory, learning, and decision-making), modulation of visual attention, pain modulation, regulation of neuroglial homeostasis, functioning of the blood-brain barrier and neurovascular nodes, formation of fear, regulation of the stress response [12, 25, 36]. Additionally, the LC is involved in regulating muscle tone, breathing, and sensory processing [5]. Differences in the effects elicited by noradrenaline (activation or inhibition) and specific response reactions are apparently explained by different postsynaptic receptors, dynamics of noradrenaline reuptake, and limited projection targets of individual groups of LC neurons [36].
The locus coeruleus-norepinephrine (LC-NE) system has two modes of functioning: tonic and phasic. Short-term phasic reactions alternate with tonic reactions [37]. The phasic mode involves filtering out insignificant stimuli, selective attention, and focused task performance, while the tonic mode contributes to shifting away from the current task in favor of exploratory behavior, less focused on performing a specific task [21, 38]. Switching between these modes affects the state of behavioral reactions, transitioning from exploratory behavior to performing a specific task [38]. Since experiments on humans and animals have demonstrated a connection between the dynamics of pupil dilation and the functioning modes of the LC-NE system, one of the non-invasive indirect methods of studying LC activity is measuring pupil dilation (pupillometry) [26, 37, 39].
The LC is part of the reticular ascending activating system, which is involved
in regulating the sleep-wake cycle and may play a role in sleep disorders
[5, 16, 25, 26]. Studies of LC activity during sleep in humans are conducted using
functional magnetic resonance imaging, which evaluates hemodynamic reactions in
response to changes in brain activity, positron emission tomography to assess
norepinephrine transporter density and pharmacological interventions. During
sleep, the slow wave sleep phase (NREM—non-rapid eye movement) and the rapid
eye movement phase (REM) alternate. It is believed that the LC-NA system is
involved in the transition between NREM and REM sleep. During REM sleep, the
level of NA in the prefrontal cortex and thalamus becomes lower compared to the
wakefulness level. In the REM sleep phase, compared to NREM sleep, indicators of
heart rate, respiratory rate, and blood pressure increase, but as a result of
inhibiting motor neurons in the spinal cord, skeletal muscles are in a state of
atonia. During wakefulness, spinal motor neurons receive signals from the motor
cortex and from LC neurons. Norepinephrine acts on
Pharmacological studies of the influence of the LC-NE system on the sleep-wake
cycle in rats have demonstrated that injection of
LC neurons are activated in response to changes in the environment as well as
visceral stress signals, such as bladder filling or changes in blood volume. This
activation occurs through afferent projections from the nucleus tractus solitarii
of the vagus nerve and from the central nucleus of the amygdala [40, 41, 42]. The
norepinephrine released in response to stimulation affects the forebrain,
modulating the stress response [42]. Additionally, the LC plays a role in
maintaining the normal functioning of the blood-brain barrier and regulates
cerebral blood flow in response to stress [19]. Norepinephrine released from LC
neurons also acts on microglial cells, oligodendrocytes, and astrocytes,
influencing inflammation, myelin production, and the functioning of astrocytes
[19]. As mentioned earlier, norepinephrine can be released into the extracellular
space, providing paracrine regulation [19, 21, 43, 44]. With this release, NE acts
on astrocytes and microglia, affecting metabolic activity, inflammation, and
other indicators [44]. Astrocytes express
Studies have shown that the density of LC neurons is associated with the rate of decline in cognitive processes. Examination of the density of LC neurons, conducted on postmortem samples of participants who underwent annual cognitive tests, demonstrated a link between decreased cognitive function and neuron density in the LC: higher density was associated with a slower decline in cognitive abilities [45]. Disorders in the functioning of the LC-NE system are observed in various nervous system pathologies. Let’s take a closer look at some of them.
Alzheimer’s disease—a leading neurodegenerative brain disease, leading to
deterioration of cognitive functions and the development of dementia [46]. In alzheimer’s disease (AD),
insoluble
The reduction in the number of LC neurons correlates with the severity of AD and life expectancy, while the morphology of the neurons also changes: the size of the soma of the neurons, the number of dendrites, and the volume of the nuclei change [16, 51, 52]. According to research, the number of LC neurons decreases by approximately 30% as the disease progresses from the absence of cognitive symptoms to mild symptoms and by 25% when transitioning from mild to moderate AD [47, 50]. Neurons in the rostral part of the LC, projecting to cortical structures and the hippocampus, are more susceptible to degeneration [47, 53]. It is assumed that the increased vulnerability of LC neurons to tau pathology is due to incomplete myelination of their axons, extensive interaction with the capillary bed, and proximity to the circulating cerebrospinal fluid in the fourth ventricle, which enhances the action of environmental toxins. Increased bioenergetic demand may also play a role, leading to increased oxidative stress [20, 47]. The accumulation of neuromelanin in LC neurons may have both neuroprotective and neurotoxic effects depending on the level of intracellular accumulation [54]. Neuromelanin chelates and reduces the toxicity of heavy metals. However, simultaneous accumulation of heavy metals can lead to neuronal death and release of toxins into the extracellular space [47].
There is a theory about transsynaptic spread of tau protein, which, considering the wide projections of LC, may lead to axonal spread of tau throughout the brain, although there is no definitive evidence for this theory [20, 51]. Presumably, accumulating in LC neurons, tau may affect their functioning and disrupt the release of norepinephrine.
The degeneration of LC neurons leads to a decrease in the concentration of norepinephrine, which contributes to the disruption of the normal functioning of the sympathetic nervous system and cognitive impairments. Early manifestations of AD, such as sleep disturbances and mood disorders, may also be explained by dysfunction of the LC-NE system [47]. In addition, due to the decrease in the concentration of norepinephrine, which normally suppresses the production of pro-inflammatory cytokines, the anti-inflammatory effect is reduced [16]. Also, changes in the action of norepinephrine on blood vessels can disrupt cerebral perfusion [46, 47, 51]. Infiltration of microcirculatory vessels and thickening of capillary walls leads to microangiopathy and disrupts the normal functioning of the blood-brain barrier [47]. As a result, dysfunction of the LC-NE system leads to a decrease in the neuroprotective and anti-inflammatory effects of norepinephrine [47]. To compensate for the loss of LC neurons, the noradrenergic system undergoes restructuring: reuptake of NE decreases, axons and dendrites sprout in projection areas, and early stages of the disease show noradrenergic hyperactivity [11, 47].
The paramagnetic properties of neuromelanin, which accumulates in LC neurons, allow for in vivo identification of LC using magnetic resonance imaging (MRI) [55]. Currently, thanks to the use of neuromelanin-sensitive MRI, new data is emerging on the dynamics of changes in LC as AD progresses and their correlation with clinical symptoms [16]. Visualization of LC in vivo can be used to study structural changes in LC as the disease progresses [11].
The integrity of the LC, which can be studied using neuromelanin-sensitive MRI, may serve as a potential early marker of AD [48]. Studying early accumulation of tau in the LC before cognitive decline is a promising direction in early detection and treatment of norepinephrine deficiency in AD patients, and the use of noradrenergic drugs may contribute to improving cognitive and behavioral disorders [47, 52]. Research is also being conducted on the use of drugs that compensate for the deficiency of the noradrenergic system: l-3,4-dihydroxyphenylserine (l-DOPS)—a synthetic precursor of NE, an inhibitor of norepinephrine transport [47, 56].
The second most common neurodegenerative disease after AD is Parkinson’s disease [54]. The basis of the motor disorders, the main symptoms of Parkinson’s disease, is a deficiency of the neurotransmitter dopamine in the substantia nigra and basal ganglia, which is normally synthesized in the neurons of the substantia nigra. It is assumed that a combination of genetic, age-related, and environmental factors plays a role in the etiology of Parkinson’s disease. The main pathomorphological feature of Parkinson’s disease is intracellular inclusions—Lewy bodies, consisting mainly of the pathological protein alpha-synuclein. It is also characteristic of Parkinson’s disease to decrease the level of neuromelanin in the substantia nigra [54]. Clinically, Parkinson’s disease manifests with motor symptoms such as hypokinesia, tremor, and muscle rigidity. Non-motor manifestations include apathy, orthostatic hypotension, hypersalivation, pain, urinary retention, constipation, insomnia, bradyphrenia, depression, and cognitive impairments [54, 57, 58]. Moreover, the manifestation of non-motor symptoms may be related to the spatial disintegration of LC neurons [57]. In Parkinson’s disease, there is a loss of neurons throughout the length of the LC. It is assumed that damage to the LC in Parkinson’s disease precedes damage to the substantia nigra, which allows considering the state of the LC as a potential early marker for preclinical diagnosis [11].
Research shows that degeneration of LC neurons and the accumulation of Lewy bodies in them occurs before such processes in the substantia nigra [55]. Non-invasive neuroimaging of the LC using MRI allows for the assessment of LC integrity and its correlation with the functioning of the noradrenergic system and cognitive and behavioral symptoms, which could potentially lead to a better diagnosis of neurodegeneration before the onset of symptoms [11]. In addition, targeting the noradrenergic system represents an additional therapeutic strategy in the treatment of Parkinson’s disease, especially for compensating cognitive impairments, as the main dopaminergic therapy used in the treatment of Parkinson’s disease does not lead to improvement in cognitive function [59, 60]. It is believed that restoring norepinephrine levels may lead to an improvement in patients’ condition [59].
Further study of the anatomy and physiology of the LC is necessary for a more complete understanding of the involvement of the LC-NE system in the normal functioning of many functions and systems in the body, particularly in the processing of sensory information, behavior, and regulation of visceral functions. Understanding the mechanisms of NE involvement in the decline of cognitive functions and the development of neurodegenerative diseases may be the basis for the development of new therapy methods that can slow the pace of neurodegeneration and thus improve patients’ condition. In the future, neuroimaging of the LC could become a biomarker for neurodegenerative diseases for early diagnosis and evaluation of pharmacotherapy effectiveness.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by IDB, ATN, MSG, MVO, NAR, VNN. The manuscript was written and approved by all authors. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.