1 Hainan Provincial Key Laboratory for Human Reproductive Medicine and Genetic Research & Key Laboratory of Reproductive Health Diseases Research and Translation, Ministry of Education & Hainan Provincial Clinical Research Center for Thalassemia, Department of Reproductive Medicine, National Center for International Research “China-Myanmar Joint Research Center for Prevention and Treatment of Regional Major Disease” by the Ministry of Science and Technology of China, Haikou Key Laboratory for Preservation of Human Genetic Resource, The First Affiliated Hospital of Hainan Medical University, Hainan Medical University, 571101 Haikou, Hainan, China
2 School of Management, Hainan Medical University, 571199 Haikou, Hainan, China
†These authors contributed equally.
Abstract
Transcription factors (TFs) are essential proteins regulating gene expression by binding to specific nucleotide sequences upstream of genes. Among TF families, the forkhead box (FOX) proteins, characterized by a conserved DNA-binding domain, play vital roles in various cellular processes, including cancer. The FOXA subfamily, encompassing FOXA1, FOXA2, and FOXA3, stands out for its pivotal role in mammalian development. FOXA1, initially identified in the liver, exhibits diverse expression across multiple organ tissues and plays a critical role in cell proliferation, differentiation, and tumor development. Its structural composition includes transactivation domains and a DNA-binding domain, facilitating its function as a pioneer factor, which is crucial for chromatin interaction and the recruitment of other transcriptional regulators. The involvement of FOXA1 in sex hormone-related tumors underscores its significance in cancer biology. This review provides an overview of multifaceted roles of FOXA1 in normal development and its implications in the pathogenesis of hormone-related cancers, particularly breast cancer and prostate cancer.
Keywords
- FOXA1
- transcription factor
- organogenesis
- development
- breast cancer
- prostate cancer
A transcription factor (TF) refers to a protein capable of specifically binding to the upstream specific nucleotide sequences of genes and regulating their transcription [1, 2, 3]. It can recognize the promoter of eukaryotes and form a transcription initiation complex with RNA polymerase II, thereby binding together in the promoter region of a gene to initiate and regulate gene expression [1, 2, 3]. The forkhead box (FOX) TF family, also known as FOX proteins, belongs to a subgroup of ‘helix-turn-helix’ proteins [1, 2, 3, 4, 5, 6, 7]. It has a conserved DNA-binding domain protein consisting of approximately 100 amino acids [8, 9]. This domain’s structure is similar to a winged helix domain or a forkhead domain, making it evolutionarily conserved, hence named FOX proteins [10].
The FOX family has 19 subfamilies (FOXA to FOXS), with subfamilies such as FOXP, FOXM, FOXC and FOXA receiving significant attention and research [11, 12, 13]. Several key members of these subfamilies are closely associated with cancer and are involved in the occurrence, maintenance, and progression of malignant tumors [14]. Among the FOX family of proteins, the transcriptional regulatory factor FOXA family (including FOXA1, also named as hepatocyte nuclear factor 3A [HNF3A]; FOXA2, also named as HNF3B; and FOXA3, also known as HNF3G) is currently the most well-studied, playing a crucial role in the development of mammals [15, 16, 17, 18, 19].
FOXA1 was initially isolated from the liver [20, 21, 22, 23, 24]. Foxa1 gene is located on chromosome 14q21.1, with a size of 6508 base pairs (bp) and an mRNA length of 3509 bp. The protein-coding sequence is in the region from 278 to 1696 bp in the mRNA, with a size of 1419 nucleotides. FOXA1 is a protein-coding gene, and its encoded protein consists of 472 amino acids, with a molecular weight of 49,148 Da [25, 26, 27]. The functional structure of FOXA1 includes the N-terminal transactivation domain, centrally shared DNA-binding domain common to the family, and C-terminal transactivation domain associated with histone H3/H4 [14]. The FOXA1 TF is expressed in various organ tissues such as the pancreas, breast, prostate, liver, lungs, brain, gastrointestinal tract, and kidneys [7]. FOXA1 plays a crucial regulatory role in cell proliferation, migration, normal cell growth, differentiation, organ and embryonic development, and tumors [20].
In addition to its role as a classic TF, FOX proteins function as a pioneer factor, closely interacting with chromatin, promoting the binding of other transcription regulatory factors [7, 14, 28, 29, 30, 31]. The following overview will discuss the various aspects of FOXA1’s role in normal development and its functions in sex hormone-related tumors.
Members of FOX TF FOXA subfamily, including FOXA1, FOXA2, and FOXA3, play crucial roles in various stages of mammalian life, starting from embryonic development, continuing through organogenesis, then extending into adulthood, contributing significantly to metabolic and internal environmental stability [31]. Foxa2 mRNA is first gene expressed in mouse embryonic development. Its expression is observed in anterior primitive streak and node during foregut formation, followed by expression in gut, floor plate and notochord [32]. Foxa1 can be detected in late primitive streak of mouse embryo, subsequently in floor plate, notochord, and gut [32]. Foxa3 is last activated gene, expressing in hindgut differentiation [33]. Expression of Foxa1 and Foxa2 is observed in tissues derived from the endoderm, mesoderm, and ectoderm of adult mice [34]. While Foxa1 and Foxa2 are mainly restricted to tissues of endodermal origin, such as the lungs, liver, stomach, and small intestine, Foxa3 is more widely expressed, not only in endoderm-derived tissues, such as the liver and gastrointestinal tract but also in the ovaries, testes, heart, and adipose tissue, with the exception of the lungs [35]. Numerous studies have been conducted over decades to explore their respective roles.
Researchers targeted the Foxa1 gene in mice using a vector knockout
approach to examine FOXA1 in tissue development [36]. Subsequent findings
revealed that the descendants of mice with Foxa1 gene deletion were born
in Mendelian proportions, with homozygous mutants having the same birth weight as
their wild-type littermates. However, between 8 and 10 days after birth, there
was a rapid decrease in the body weight of Foxa1 mutant mice, ultimately leading
to severe hypoglycemia. Further research indicated that animals lacking the
Foxa1 gene experienced impaired pancreatic development, resulting in
sustained hypoglycemia, lower plasma insulin levels, and elevated glucagon
levels. Despite the presence of hypoglycemia, plasma glucagon levels were
significantly reduced, associated with pancreatic glucagon precursor gene
down-regulation. This suggests that FOXA1 can regulate transcription of the
pancreatic glucagon precursor gene and activate its initiation [36]. In 1999, a
study using homologous recombination in embryonic stem cells generated mice
lacking Foxa1. Homozygous mutant mice exhibited a complex phenotype characterized
by abnormal feeding behavior, progressive starvation, persistent hypoglycemia,
and emaciation, with a higher mortality rate between days 2 and 14 after birth.
These mice display physiological counterregulatory responses to corticosteroid
and growth hormone production, inhibiting insulin secretion but failing to
stimulate glucagon secretion. These results indicate that FOXA1 plays a crucial
role in regulating glucose homeostasis and pancreatic function [36]. In a study
conducted in 2004 on isolated pancreatic islet beta cells, the lack of FOXA2 was
found to result in excessive insulin release in response to amino acid
stimulation but a complete loss of insulin secretion in response to glucose
stimulation. This suggests that FOXA2 controls insulin secretion through the
activation of multiple pathways [37]. A study in 2010 found that individually
knocking out the Foxa1 gene in pancreatic beta cells had no effect on
normal pancreatic function. However, a double knockout of both Foxa1 and Foxa2 in
mice with a mixed 129SvEv/C57BL/6 background led to the impairment of glucose
homeostasis and insulin secretion. In addition, cells with a double knockout of
Foxa1 and Foxa2 showed more pronounced defects in glucose-stimulated insulin
secretion of the isolated mice islet compared to cells lacking only Foxa2 [38].
These data suggest that actions of systemic FOXA1 loss on pancreatic phenotype is
not only achieved by affecting the development of pancreatic beta cells but is
also compensated for by the specific effects of FOXA2 on pancreatic beta cells.
The compensatory effect for FOXA2 and FOXA1 is not limited to pancreatic islet
beta cells but also exists in organs such as the liver and lungs. Knocking out
Foxa1 or introducing a Foxa2 mutation specifically in developing respiratory
cells had almost no impact on overall lung structure in Foxa2Delta/Delta and
Foxa1
Although the regulatory functions of FOXA1 and FOXA2 seem compensatory in various systems, in tissues primarily dependent on hormone signaling such as prostate and breast, research indicates that FOXA1 itself is a key regulatory factor for tissue-specific differentiation and functional regulation [40, 41, 42]. Breast cancer in women is affected by their reproductive history. Hormonal environment also affects the progression of the disease. Therefore, experiments using mouse mammary glands as a model have been conducted to study breast cancer development mechanisms.
Mouse mammary gland development can be divided into two main stages:
pre-pubertal hormone-independent mammary development and hormone-dependent
mammary development post-puberty. Studies have found that the post-pubertal
hormone-dependent development of the mouse mammary gland is mediated by
ER
Most mammary development occurs during adolescence after birth. Therefore,
researchers have investigated Foxa1’s impact on postnatal mammary morphogenesis
in Foxa1
An experiment conducted in the mouse prostate revealed that FOXA1 is detected
during development and adulthood of urogenital sinus epithelium, while sonic
hedgehog (Shh) and FOXA2 are limited to basal cells of budding prostate. FOXA2
level decreased to nearly undetectable levels shortly after mouse birth. In the
absence of FOXA1 in epithelial cells, Nkx3.1 (prostate-specific homeobox protein)
is downregulated, and several markers regulated by androgens, specific to the
prostate, and novel Foxa1 targets are missing. These data imply a crucial role
for FOXA1 in controlling prostate morphology and differentiation [45]. The
phenotype of Foxa1
FOXA1 was first purified from the liver [47]. FOXA1 is a specific regulatory
factor in liver development. However, studies using HNF3
The liver develops from the foregut endoderm, which forms a hepatic bud invading the septum transversum, giving rise to the liver and intrahepatic bile duct tree. Experiments in mice lacking both Foxa1 and Foxa2 genes showed no apparent hepatic bud in the embryos and loss of expression of alpha-fetoprotein, a liver cell marker. Thus, Foxa1 and Foxa2 play a crucial role in foregut endoderm development and initiating liver development in mice [48]. A study in 2010 used conditional gene ablation in the late stages of liver development in the mice, showing that simultaneous loss of Foxa2 and Foxa1 caused bile duct fibrosis and proliferation [49]. Further research revealed that abnormal bile duct formation due to the simultaneous loss of Foxa1 and Foxa2 is at least partially attributed to the induction of interleukin 6 (IL-6) expression. IL-6 expression serves as a proliferative signal for bile duct cells in mice [49]. Glucocorticoid receptor is a negative IL-6 regulator. In the absence of both FOXA1 and FOXA2, glucocorticoid receptor cannot bind to IL-6 promoter, leading to enhanced IL-6 expression in liver. Therefore, after liver-specific differentiation, normal bile duct development requires FOXA1 and FOXA2 to prevent excessive proliferation of bile duct cells. FOXA1 and FOXA2 act as terminators of bile duct expansion in adult liver [49].
A study observed FOXA1 expression in lung buds at 10.5 days in mouse embryos
[34]. In adult mouse nasal passages, FOXA1 had strong expression in respiratory
epithelium, while FOXA2 had weaker expression in mucous gland epithelium, and no
expression of FOXA1 and FOXA2 was detected in olfactory gland epithelium of the
mice. In adult mouse trachea and bronchi, FOXA1 and FOXA2 were detected in Clara
cells, epithelial cells, cilia, and basal cells. In peripheral lung,
co-expression of FOXA2 and FOXA1 was detected in type II alveolar cells and
epithelial cells of the mice [34]. While Foxa2 was selectively knocked out from
mouse respiratory epithelial cells during lung bud stages, strict dependence on
Foxa2 was found during lung alveolarization in transgenic mice, indicating its
key role in alveolar formation process and regulating epithelial cell
differentiation after birth [50]. Foxa1
Immunohistochemical analysis using mice revealed that in the early embryonic
stages, FOXA2 is expressed in notochord, node and floor plate, while FOXA1 is
expressed only in notochord and floor plate. In adult mice, FOXA2 and FOXA1 have
overlapping expression in the ventral midbrain, with distinct spatial
distributions. Both are significantly expressed in the cerebellum (Purkinje
cells) and olfactory bulb of mice [34]. Mutation in the Foxa2 gene in
mice (Foxa2
Immunohistochemical analysis in mice revealed that from embryonic origin to adulthood, FOXA2 and FOXA1 are co-expressed in gastrointestinal epithelium [34]. FOXA2/FOXA1 can activate promoters of mucin 2 (Muc2) expressed in goblet cells and preproglucagon expressed in enteroendocrine cells. Functional experiments using the Cre-loxP system in mice with simultaneous loss of Foxa2 and Foxa1 in intestine showed a lack of cells expressing pancreatic polypeptide and a reduction in peptide YY (L cells) and somatostatin (D cells) [62]. The mRNA levels of preproglucagon, somatostatin, and peptide YY in Foxa1/Foxa2 mutant mice were decreased in small intestines. TFs islet-1 and paired box 6 levels in small intestine were also reduced, suggesting that FOXA2 and FOXA1 affect TF network in enteroendocrine lineage. Loss of FOXA1 and FOXA2 also resulted in a reduced number of goblet cells and altered expression of secretory Muc (Muc2, Muc5b, Muc5ac, and Muc6). These results indicate that FOXA2 and FOXA1 are key players of the endocrine lineage in gastrointestinal tract, playing a crucial role in controlling the differentiation of secretory cells of the mice. A study further demonstrated that FOXA2 and FOXA1 control Muc2 expression in intestinal epithelial cells of mice [63]. The critical roles of FOXA1 protein in goblet cells generation and development and function of enteroendocrine cells are crucial for maintaining normal intestinal absorption, and their loss may lead to nutritional deficiencies, potentially contributing to growth retardation in Foxa1/Foxa2-deficient mice during early development [63].
FOXA1 is overexpressed in breast, prostate, lung, thyroid, and esophageal cancers [64, 65, 66, 67, 68]. However, FOXA1 is underexpressed in advanced-stage bladder cancer, leading to the increased proliferation of tumor cells [69]. Therefore, the function of the FOXA1 TF is complex, exhibiting both oncogenic and tumor-suppressive effects. The following sections will provide a review of FOXA1’s roles in breast and prostate cancers.
In breast cancer, estrogen receptors (ERs) and androgen receptors (ARs) jointly modulate cell differentiation and proliferation. They are often co-expressed, but AR also expressed in ER-negative breast cancer. Research suggests that AR activation in ER-negative breast cancer cells is acted through ERs as an intermediate pathway to regulate gene transcription [70]. In ER-negative breast cancer cells, androgens potentiate proliferation, whereas in ER-positive breast cancer cells, androgens hinder proliferation [71, 72]. ER is a key characteristic in most breast cancers, and binding of ER to genome is associated with FOXA1 expression. FOXA1 is an essential modulator of ER-DNA binding and its target genes transcription [73].
FOXA1 is highly expressed in ER-positive breast cancer, with an expression rate of up to 97%. Additionally, FOXA1 promotes AR DNA binding in ER-positive/-negative breast cancer cells. Ratios of FOXA1, ER, and AR may affect the proliferation and invasion of cancer cells. Despite this, FOXA1 is considered the best predictor of breast cancer recurrence [74].
A study in 2007, involving immunohistochemical analysis of tumor tissues from
404 breast cancers, found that 300 exhibited FOXA1 expression with a score
Hormone therapy is a crucial method for treating ER-positive breast cancer
patients. ER, a TF of nuclear receptor family with two isoforms, ER
Multiple studies have demonstrated the statistically significant correlation between FOXA1 and ERs. However, the high expression of FOXA1 is still found in some ER-negative breast cancers [79]. Using microarray data, researchers categorized breast tumor cells via steroid receptor activity into three types: basal (ER- AR-), luminal (ER+ AR+) and molecular apocrine (ER- AR+). Estrogen signaling is most active in luminal type, whereas androgen signaling is most active in molecular apocrine type. Molecular apocrine type exhibits high AR activity and expression, with another characteristic being the expression of FOXA1 [80].
Clinically, apocrine cancer, a rare type of breast cancer, is characterized by
ER negativity and AR positivity (ER- AR+), accounting for approximately 1–4% of
all breast cancer cases [81]. For ER-negative breast cancer, chemotherapy is the
primary treatment method, with fewer alternative treatment options. Therefore,
targeting AR has become a promising therapeutic strategy for this subtype of
breast cancer. Apocrine cancer features ER negativity and AR positivity, but
lacks profile similar to ER+ luminal type. In a study conducted in 2011, Robinson
et al. [70] used the ER-AR+ molecular apocrine cell model of tumors
(referred to as MDA-MB-453 cells) to analyze how AR binds. The authors ultimately
found a binding characteristic similar to ER-binding breast cancer cells.
Simultaneously, they discovered that AR binding was mediated by FOXA1, and
silencing Foxa1 inhibited AR binding, leading to the proliferation of most cells
expressing apocrine cancer gene characteristics. These findings suggest that AR
can only act with ER cis-regulatory elements in the presence of FOXA1, initiating
transcription similar to ER-modulated transcription in luminal breast cancer. AR
occupies 37% of FOXA1-binding sites, confirming that FOXA1 is a crucial
intermediary for AR transcription in this cell type. Hirata and colleagues [82]
demonstrated that FOXA1 confers resistance to apoptosis induced by transforming
growth factor-
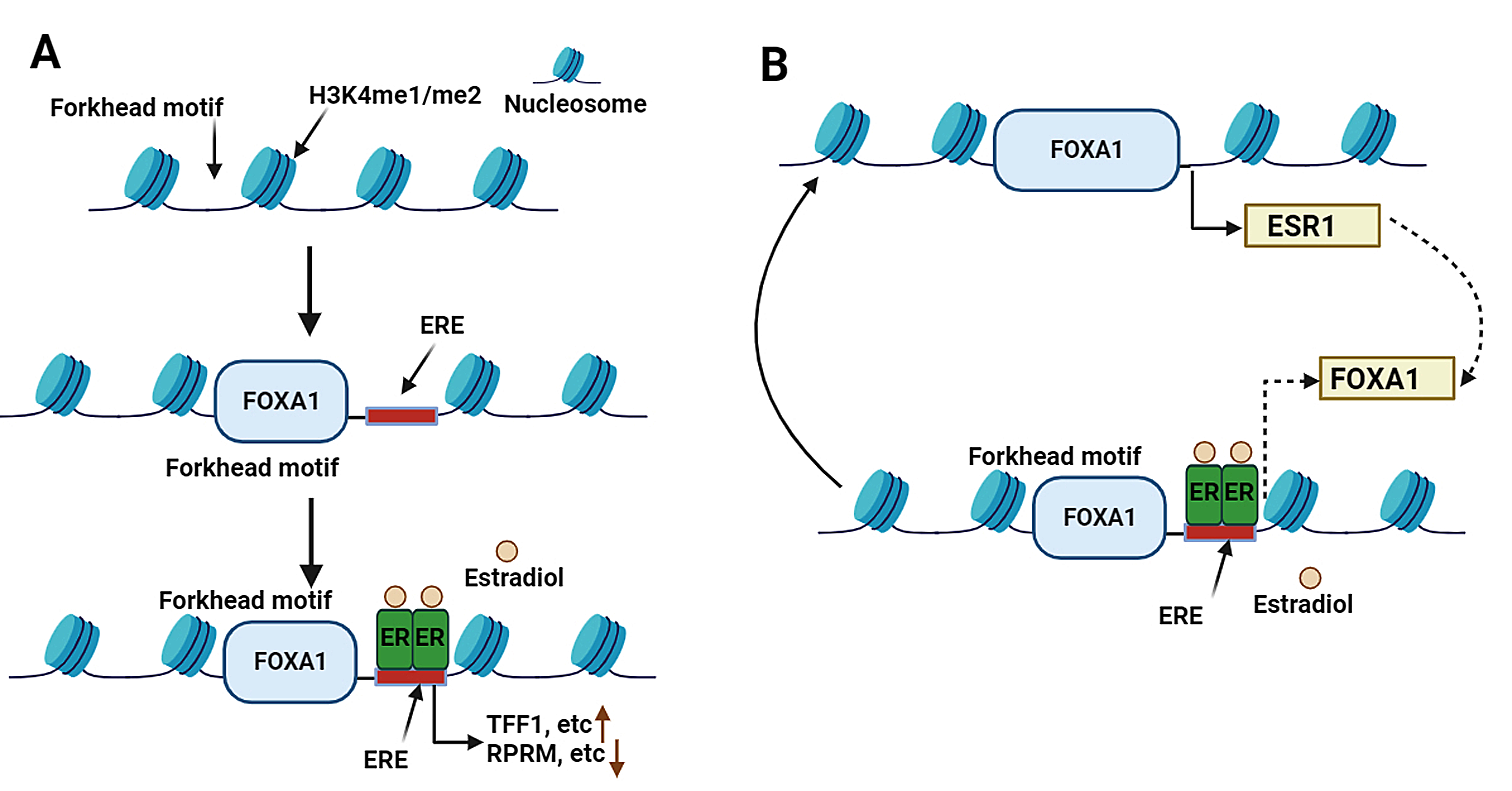
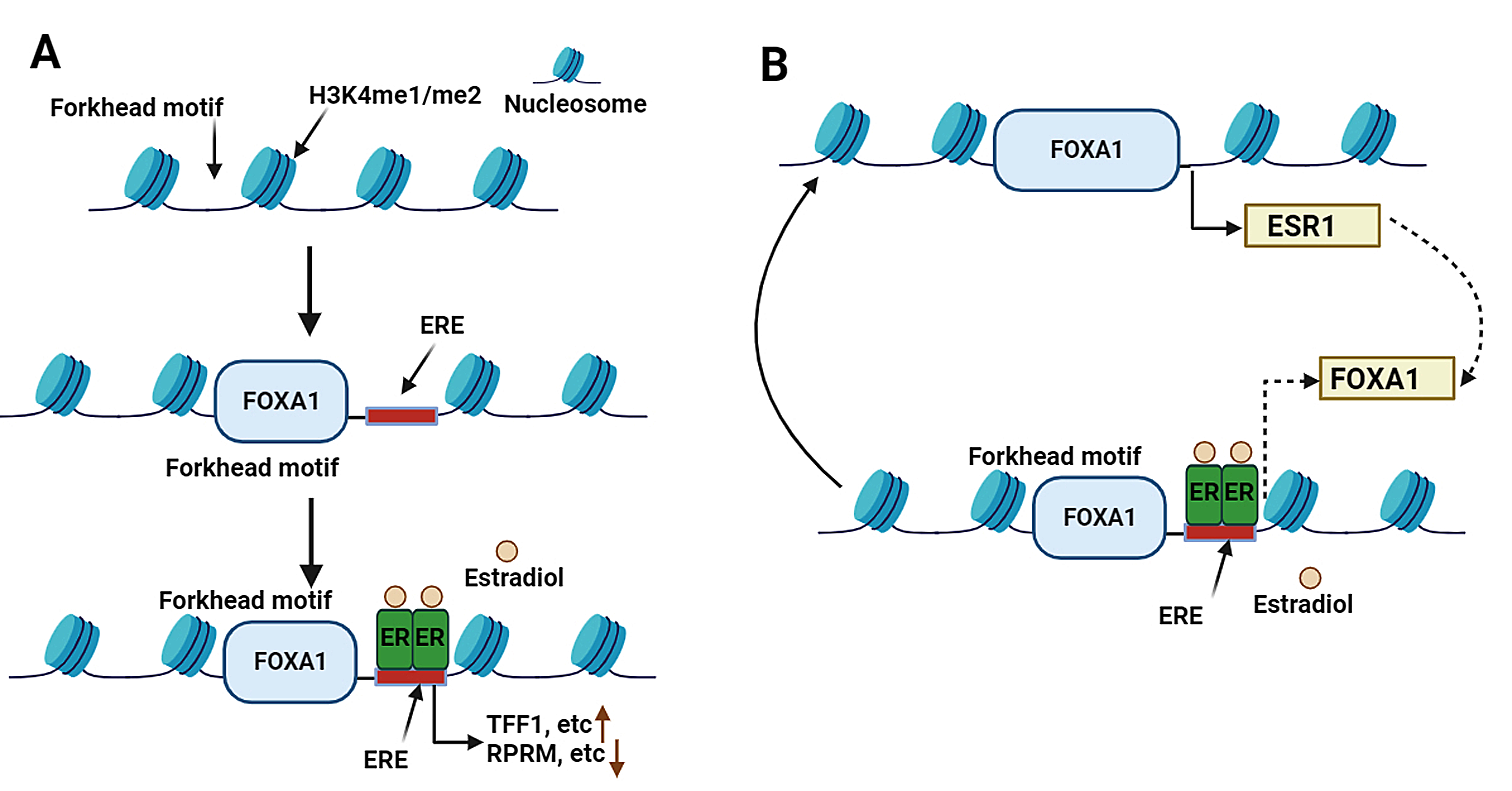 Fig. 1.
Fig. 1.The mechanism of actions of FOXA1 in regulating breast cancer. (A) FOXA1 modulates both estrogen-induced gene transactivation and repression. (B) FOXA1 is important for ER expression, and ER is suggested to regulate FOXA1 expression in an estrogen-dependent manner. ER, estrogen receptor; ERE, estrogen response element; ESR1, estrogen receptor 1; FOXA1, forkhead box A1; RPRM, Reprimo, TP53 dependent G2 arrest mediator homolog.
Studies have also proposed several mechanisms linking diabetes with breast
cancer, including activation of the insulin pathway, insulin-like growth factor
(IGF) pathway, and modulation of endogenous sex hormones [91]. Chronic
hyperglycemia, known as the Warburg effect, may contribute to increased breast
cancer risk [92]. Elevated levels of insulin and IGF-1, coupled with inflammatory
cytokines, directly impact cancer cell behavior, influencing proliferation,
apoptosis, and metastasis [93]. Specifically, IGF-1 binds to IGF-binding protein
3, affecting breast cancer risk, particularly in premenopausal women. Insulin,
another mitogen, stimulates the insulin receptor and promotes malignant
transformation of breast epithelial cells [94]. Moreover, insulin resistance can
lead to hyperinsulinemia, altering androgen synthesis and estrogen production,
thereby affecting breast cancer risk, especially in postmenopausal women [95]. On
the other hand, Vatamaniuk et al. [96] demonstrated that Foxa1-deficient
mice exhibited impaired insulin secretion due to uncoupled oxidative
phosphorylation. Zhu et al. [97] showed that FOXA1 suppressed the
transcription of special AT-rich sequence binding protein 1 and inactivated the
Wnt/
FOXA1 plays an essential in prostate development. FOXA1 regulates prostate morphogenesis and cell differentiation by interacting with androgen signaling [44]. An experiment conducted in the prostate of mice revealed that the expression of mouse FOXA1 occurs throughout the development and adulthood of the urogenital sinus epithelium, whereas Shh and FOXA2 expression is restricted to the prostate budding basal cells. In Foxa1-deficient mice, the prostate exhibits a profound altered ductal pattern resembling primitive epithelial cords surrounded by a thick stroma layer. The characteristics of these mutated cells suggest the presence of a group of basal-like cells comparable to embryonic urogenital sinus epithelium, with a lack of mature or differentiated ductal epithelial cells in Foxa1-deficient epithelium. In epithelial cells lacking Foxa1, the prostate-specific homeobox protein Nkx3.1 is downregulated, and several androgen-regulated markers and novel Foxa1 target genes specific to prostate cancer are absent. These results imply that FOXA1 exerts key functions in regulating cell differentiation and prostate morphogenesis, exhibiting a tumor-suppressive role in the prostate epithelium [98].
Regarding how FOXA1 regulates the occurrence, development, and/or metastasis of tumors in the prostate, there is currently much research on this topic. Studies have speculated that FOXA1 interacts with ARs in prostate-derived cells to enhance AR target genes expression. The interaction between these TFs may initiate prostate development [98]. Most prostate cancers are driven by abnormal AR signaling, and there is currently a limited understanding of underlying mechanism. Research has shown the differential expression of FOXA2 and FOXA1 proteins in epithelial cells during mouse prostate development. A study using immunoblot analysis in LPB-Tag LADY mouse prostate cancer model, human prostate cancer specimens, and various prostate cancer cell lines examined the expression of FOXA proteins. Results showed high FOXA1 expression in the regions of prostatic intraepithelial neoplasia in both androgen-dependent 12T-7f and metastatic androgen-independent 12T-10 LADY models. Significant expression of FOXA2 and FOXA1 was observed in invasive undifferentiated neuroendocrine carcinoma, hormone-independent and metastatic tumors originating from 12T-10, NE-10 xenograft tumors, and all metastatic lesions isolated from 12T-10 mice. FOXA1 protein can be observed in human prostate, and its expression level is indistinguishable from benign tissue. FOXA2 can only be detected in neuroendocrine small cell carcinomas and certain high Gleason grade adenocarcinomas, while it is undetectable in low-grade prostate adenocarcinomas. In vitro functional assays suggest that FOXA2 can activate ARs and androgen-dependent prostate-specific genes in a ligand-independent manner. These results indicate importance of FOXA proteins in prostate cancer, and the possible involvement of FOXA2 in prostate cancer progression toward androgen independence [99].
The level of AR in primary prostate cancer predicts a poorer prognosis, with higher mortality rates. FOXA1 not only activates the AR pathway but depletion of FOXA1 also causes a significant redistribution of AR binding on chromatin in LNCaP-1F5 cells, corresponding to changes in the expression characteristics of androgen-dependent genes. Research has shown the overlap between AR and FOXA-binding sites, which account for approximately 70% of AR-binding sites, whereas AR-binding sites account for about 25% of FOXA1 binding sites. This suggests FOXA1-mediated transcriptional regulation in prostate cancer cells. Establishing a model with silenced Foxa1 revealed an increase in the number of sites occupied by other ARs, doubling the number of binding events. Therefore, these findings suggest that FOXA1 has a dual function in both promoting and inhibiting AR. Based on AR-binding sites, prostate cancer can be classified into three types: independent of FOXA1, dependent on FOXA1 as a pioneer factor, and masked by FOXA1 and activated when FOXA1 is depleted. High expression of FOXA1 is associated with a poor prognosis, while low FOXA1 expression, even with high AR expression levels, indicates a favorable prognosis of patients with prostate cancer. The prognosis of prostate cancer is worse when both FOXA1 and AR proteins are expressed simultaneously compared to when AR is expressed alone [100].
Late-stage prostate cancer can progress to systemic metastatic tumors, which are often insensitive to androgens and ultimately lead to death. Whole-genome sequencing in metastatic prostate tumors has identified amplifications in the gene locus 14q21, which includes the Foxa1 gene [65]. Understanding the basic mechanisms by which FOXA1 regulates AR binding is crucial for determining the FOXA1’s impact on androgen-dependent transcriptional modulation, providing effective diagnosis and treatment for prostate cancer patients. Research has shown that FOXA1 is recruited to chromatin modifications associated with active chromatin, thereby promoting nuclear receptors recruitment and activation of transcription [41].
Most prostate cancers are androgen-dependent, meaning that they respond to androgen deprivation therapy. The selected treatment methods are prostatectomy or radiation therapy. However, despite androgen deprivation, these tumors eventually develop into androgen-independent forms, posing a challenge for treatment when they recur or have already metastasized at the time of diagnosis [101]. Previous studies have indicated FOXA1 expression in epithelial cells of prostate gland, where it regulates prostate-specific genes transcription. Another study reported FOXA1 as a novel inhibitory factor for AR in prostate cells. FOXA1 inhibits the targeted transcriptional activation of androgen response elements by AR in a dose-dependent manner. FOXA1 physically interacts with the AR to inhibit its transcriptional activation. Moreover, overexpression of FOXA1 reduces of prostate-specific antigen expression, induced by androgens, in LNCaP cells. These findings illustrate that FOXA1 is key for androgen-activated expression and serves as a novel inhibitor of AR [102].
Studies have suggested that FOXA1 plays a crucial role in the neuroendocrine
differentiation of prostate cancer. Downregulation of FOXA1 in prostate cancer
cells results in an upregulation of IL-8 expression. IL-8 can activate MAPK/ERK
pathway, leading to phosphorylation of ERK1/2 and subsequently upregulating
17
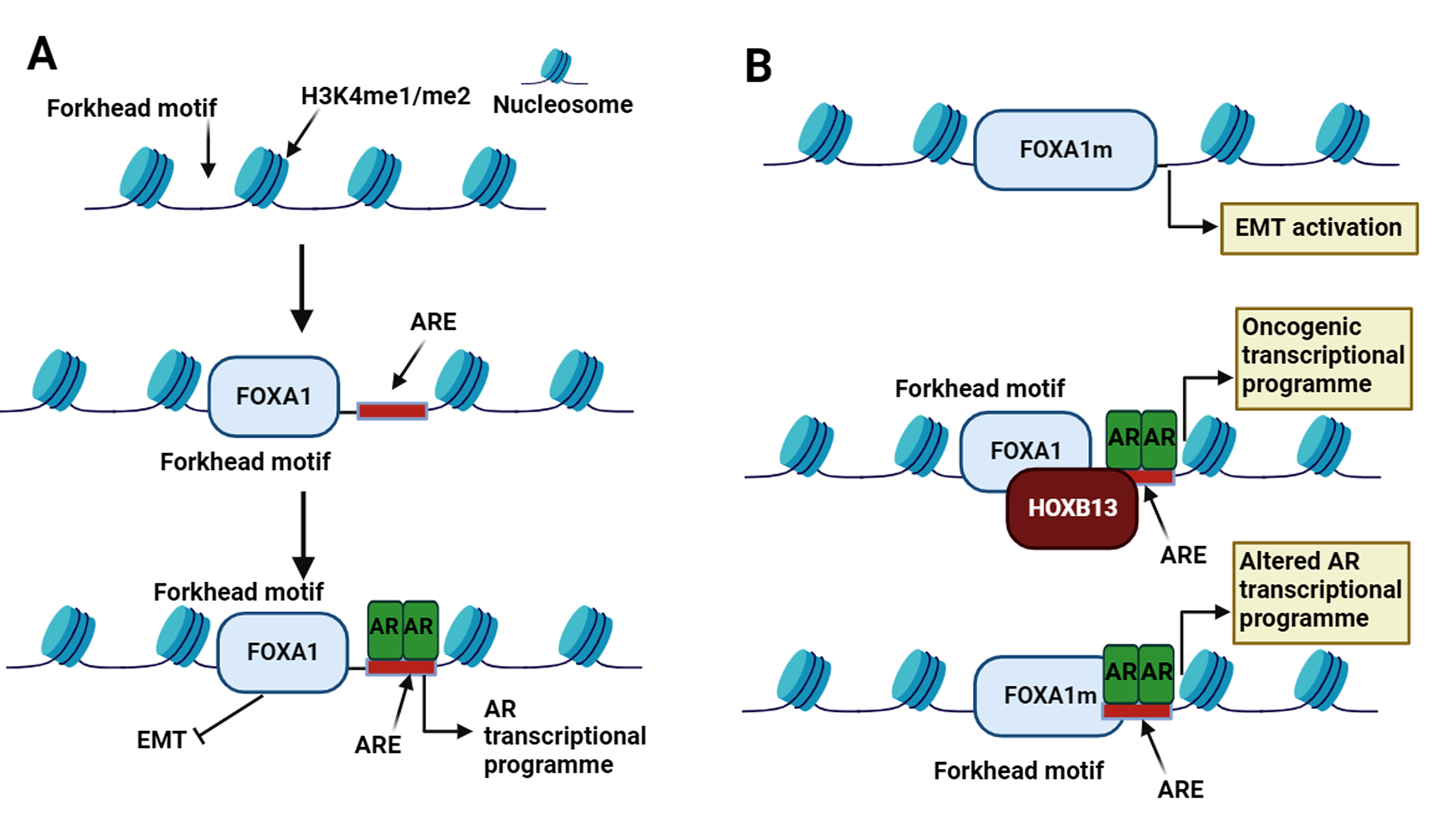
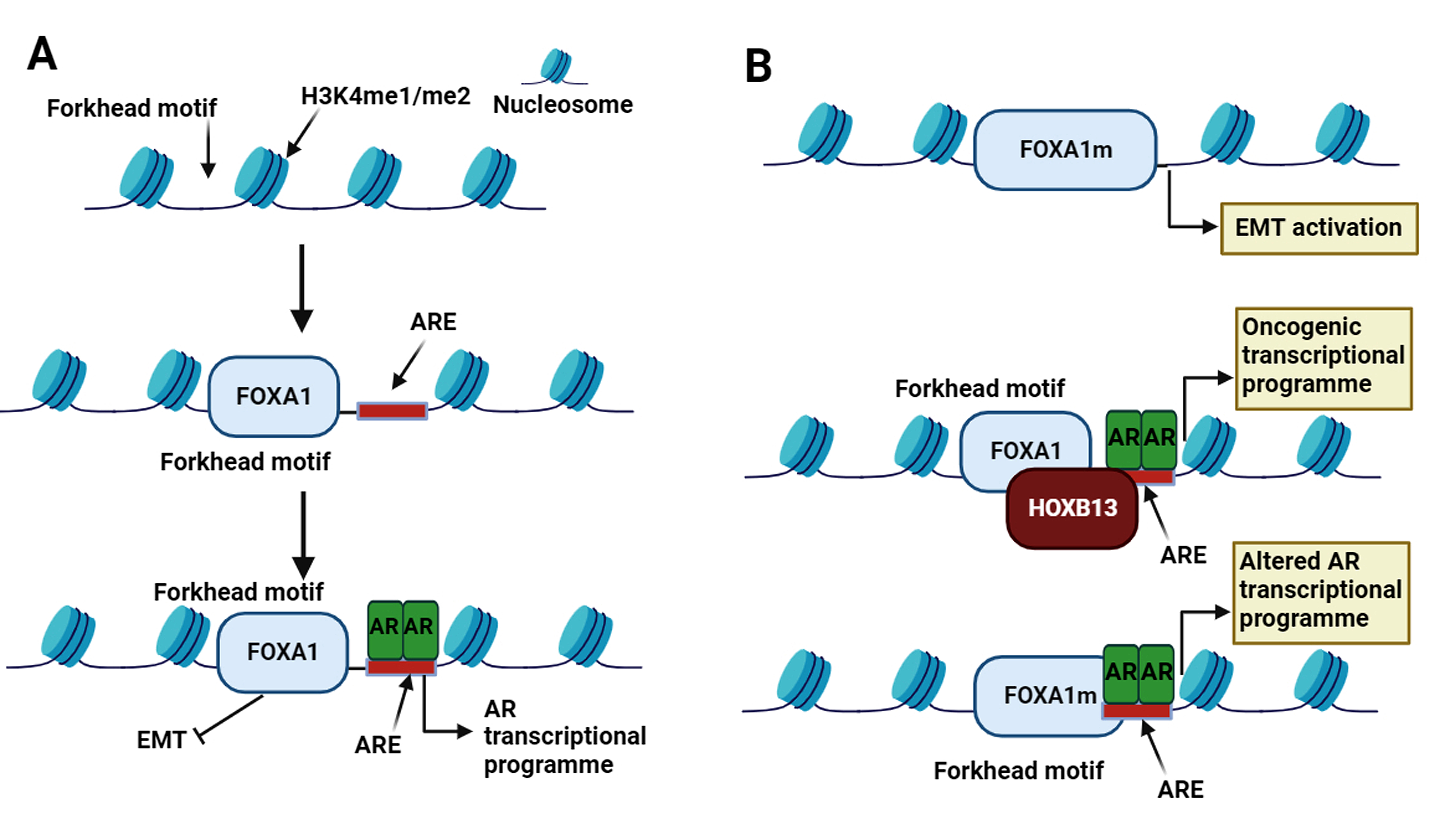 Fig. 2.
Fig. 2.The interactions between FOXA1 and AR in prostate cancer. (A) FOXA1 modulates both androgen-induced gene transactivation and repression. (B) FOXA1 mutants can enhance EMT to promote prostate cancer metastasis. FOXA1 can modulate AR cistrome along with HOXB13. FOXA1 mutations can result in enhanced AR binding leading to altered AR transcriptional programs. AR, androgen receptor; ARE, androgen response element; FOXA1, forkhead box A1; EMT, epithelial-mesenchymal transition; HOXB13, Homeo Box B13.
FOXA1 in the prostate not only participates in the regulation of AR
transcriptional activity but is also involved in other signaling pathways during
prostate development and progression. FOXA1/2 positively regulates the
developmental gene anterior gradient 2 (AGR2). Overexpression of AGR2
promotes the migration and invasive behavior of non-metastatic human prostate
cancer cells (LNCaP), while silencing of AGR2 inhibits tumor cell invasion. ErbB3
binding protein 1 (EBP1) may act as an inhibitor of AGR2 and is underexpressed in
prostate cancer. Studies have shown that the anti-invasive effect of EBP1 is due,
at least in part, to the inhibition of AGR2 expression. Functional experiments
have demonstrated that EBP1 can downregulate the transcription of AGR2 stimulated
by FOXA1 and FOXA2, reducing prostate tumor metastasis behavior. Conversely,
knockout of EBP1 increases activity of the AGR2 promoter stimulated by FOXA1 and
FOXA2, leading to increased prostate tumor metastasis. There is a negative
correlation between EBP1 and AGR2 levels in primary prostate tumors and prostate
cancer cell lines. These results demonstrate that FOXA1 plays a regulatory role
in metastatic prostate cancer through the EBP1-FOXA-AGR2 signaling pathway [105].
Another study showed that EBP1 can inhibit AR-mediated gene transcription, thus
participating in the tumorigenesis of prostate cancer [105]. Based on these
results, it can be inferred that EBP1 may inhibit AR transcriptional activation
by suppressing the transcriptional function of AGR2 stimulated by FOXA1. Song
et al. [106] demonstrated that inhibiting FOXA1-mediated repression of
TGF-
FOXA1 plays a crucial regulatory role in the proliferation and differentiation of normal cells, organ and embryo development, and tumors. It is expressed and functional in various organ tissues including the pancreas, breast, prostate, liver, lungs, brain, gastrointestinal tract, and kidneys [20]. Numerous studies have indicated overlapping and compensatory roles of FOXA1 and FOXA2 in the development and differentiation of the pancreas, lungs, liver, and nervous system. The tissue-specific regulation of the Shh pattern by FOXA1 is evident, as the loss of FOXA1 reduces Shh in the lungs and brain but increases Shh in the prostate [53, 61]. Considering the presence of abnormal Shh signaling in various cancer types, further evaluation of the interaction between FOXA1 and Shh is needed. Additionally, Zrt- and Irt-like protein 9 (ZIP9) has been recognized as a newly identified membrane AR as well as a zinc transporter protein [114]. ZIP9 functions via multiple signal transduction pathways, activating a stimulatory G protein in granulosa cells, an inhibitory one in cancer cells, and a Gq11 pathway in spermatogenic cells, without involvement in AR signaling [114]. The G protein-coupled ER (GPER) is characterized by its seven-transmembrane-domain structure, and its nongenomic activities mediated by GPER are independent of the estrogen nuclear receptor [115]. Upon ligand binding, GPER initiates various downstream pathways that elicit diverse biological effects, regulating cell growth, migration, and programmed cell death across different tissues [116]. Estrogen-induced activation of GPER, along with the GPER-selective agonist G-1, can activate the phosphoinositide 3-kinase/Akt pathway, leading to subsequent inactivation of FOXO3a and thereby promoting cell survival [117]. However, the interaction between FOXA1 and ZIP9/GPER signaling remains largely unexplored, necessitating further investigation.
FOXA1 is overexpressed in breast, prostate, lung, thyroid, and esophageal cancers [64, 65, 66, 67, 68]. By contrast, FOXA1 is underexpressed in advanced late-stage bladder cancer, resulting in increased tumor cell proliferation [69]. Therefore, FOXA1 has a complex function, exhibiting both oncogenic and tumor-suppressive roles. In breast cancer, the expression level of FOXA1 is significantly associated with luminal-type breast cancer and serves as an important prognostic factor for the survival of ER-positive breast cancer, suggesting a potential role for FOXA1 in the treatment of luminal-type breast cancer [75]. FOXA1 also plays a crucial role in the occurrence and development of prostate cancer, directly influencing the development of prostate cancer through regulation of the AR, and can exert its effects through other pathways that do not affect AR expression levels. The expression level of FOXA1 is associated with the malignancy and metastasis of prostate cancer. FOXA1 functions not only in hormone-dependent tumors but also in hormone receptor-negative, non-hormone-dependent tumors. Hormone receptor-negative diseases are more invasive and represent a challenging characteristic of cancer to treat, emphasizing the need for further research in this direction. Considerable advancements have been achieved in understanding the biological significance of FOXA1 in the development, differentiation, and progression of breast and prostate cancers. These intricate findings underscore the necessity for further investigation aimed at exploring the potential therapeutic avenues targeting FOXA1 through tailored treatment strategies. While much of our understanding of FOXA1 in cancer pertains to its involvement in hormone receptor-driven breast and prostate cancers, it is imperative to extend future research efforts beyond the realm of hormone signaling. There is an urgent need to delve into the role of FOXA1 in hormone-independent breast and prostate cancers, as well as cancers affecting other tissues where FOXA1 has been implicated in normal developmental processes.
ZY, YM, and JZ conceived/designed the study. YW, FD and YZ contributed to review literature. JZ wrote the manuscript. ZY and YM reviewed and edited the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This work was supported by Hainan Provincial Natural Science Foundation of China (No. 822RC836), Hainan Province Science and Technology Special Fund (No. ZDYF2020121), National Natural Science Foundation of China (81960283), The specific research fund of The Innovation Platform for Academicians of Hainan Province, Hainan Province Clinical Medical Center.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.