1 Department of Interventional Radiology, The University of Texas MD Anderson Cancer Center, Houston, TX 77030, USA
2 College of Medicine, University of the Philippines Manila, 1000 Manila, Philippines
3 Baylor College of Medicine, Houston, TX 77030, USA
4 The University of Texas MD Anderson Cancer Center UTHealth Graduate School of Biomedical Sciences, Houston, TX 77030, USA
Abstract
Mesenchymal stem/stromal cells (MSCs) have emerged as a promising therapeutic
approach for a variety of diseases due to their immunomodulatory and tissue
regeneration capabilities. Despite their potential, the clinical application of
MSC therapies is hindered by limited cell retention and engraftment at the target
sites. Electrospun scaffolds, with their high surface area-to-volume ratio and
tunable physicochemical properties, can be used as platforms for MSC delivery.
However, synthetic polymers often lack the bioactive cues necessary for optimal
cell-scaffold interactions. Integrating electrospun scaffolds and biological
polymers, such as polysaccharides, proteins, and composites, combines the
mechanical integrity of synthetic materials with the bioactivity of natural
polymers and represents a strategic approach to enhance cell-scaffold
interactions. The molecular interactions between MSCs and blended or
functionalized scaffolds have been examined in recent studies, and it has been
shown that integration can enhance MSC adhesion, proliferation, and paracrine
secretion through the activation of multiple signaling pathways, such as FAK/Src,
MAPK, PI3K/Akt, Wnt/
Keywords
- electrospinning
- mesenchymal stem/stromal cell
- polymer
- polysaccharide
- protein
- secretome
Mesenchymal stem/stromal cells (MSCs) have attracted significant interest as a therapeutic option for various clinical conditions due to their immunomodulatory and tissue-regenerating capabilities [1]. However, MSC therapies are challenged by the limited retention of administered cells at the targeted delivery site [2]. Systemic delivery is a reasonable approach, especially for diseases with a more extensive pathology. However, it requires MSCs to go through a series of steps to exit the circulation and migrate to the injury site. Intravenous injection typically causes an initial buildup of MSCs in the lungs followed by redistribution to other organs, such as the liver, spleen, and kidneys [3]. To address this issue, recent studies have focused on local delivery methods, which enable more predictable delivery and higher retention of MSCs. Indeed, the majority of MSC-containing products that have been approved for use across the globe are delivered through local routes (e.g., intraarticular, intracoronary, intralesional, intrathecal, and intramuscular) [4]. Nonetheless, several factors that limit the engraftment, retention, and efficacy of locally delivered cells remain. These include inadequate cell-to-matrix interaction, disruptive mechanical forces, and the pathologic microenvironment at the site of delivery [5, 6]. One strategy that can potentially mitigate these factors is scaffold-based delivery. Specifically, electrospun scaffolds, which can provide a three-dimensional (3D) fibrous framework that resembles a native extracellular matrix (ECM), have emerged as an attractive delivery platform. These scaffolds have a high surface area-to-volume ratio and tunable physicochemical properties that can be optimized to facilitate cell attachment and protect cells from both mechanical and biochemical injury [7]. Because synthetic polymers are comparatively inert, the combination of electrospun scaffolds and natural polymers presents an opportunity to generate a more conducive microenvironment for cell attachment and proliferation. Natural polymers exhibit natural domains for cell recognition, but they often exhibit lower mechanical strength and faster degradation rates compared to synthetic polymers. Hence, recent investigations have explored the integration of natural polymers into synthetic polymers in the context of MSC delivery. In this review, we have summarized the recent progress in the integration of synthetic electrospun scaffolds and biological polymers for enhancing the delivery and efficacy of MSC therapies. Moreover, we have reviewed the molecular and cellular mechanisms that underlie the interactions between MSCs and electrospun scaffolds, as well as the impact of biological polymers in enhancing these mechanisms and interactions.
MSCs are multipotent cells that can be readily isolated from certain
compartments in the body, such as adipose tissue, bone marrow, and umbilical
cord. Nonetheless, all other tissues in the body have been shown to contain MSCs
as part of the microvasculature [8]. The minimum criteria for isolated MSCs
include (a) adherence to plastic when maintained in standard culture conditions,
(b) expression of CD105, CD73, and CD90, and lack of expression of CD45, CD34,
CD14/CD11b, CD79
The MSC niche refers to all the components surrounding the stem cells, such as
ECM, soluble factors, and other niche cells, maintaining the MSCs in their
undifferentiated state [12]. Cell adhesion molecules (CAMs), which are
transmembrane proteins that help anchor cells to surfaces, play an important role
in cell-to-cell and cell-to-ECM interactions in stem cell niches. The two major
families of CAMs involved in stem cell niche interactions are cadherins and
integrins. Cadherins are Ca
Electrospinning is a highly tunable method of fabricating nanofibrous scaffolds
with a porous structure and high surface area mimicking the three-dimensional
(3D) architecture of endogenous ECM [17, 18]. Several products incorporating
electrospun fibers have advanced into human use or testing [19]. Compared to most
bottom-up fabrication techniques, the electrospinning process is economically
advantageous because it is inexpensive, simple, and typically does not require
additional purification techniques. Using high-voltage power, polymer solutions
are charged and ejected through a spinneret following the direction of the
electric field to form nanofibers on the collector plate. The interior bulk
composition and physicochemical properties of nanofibers can be customized for
specific applications by modifying the electrospinning parameters and
implementing integration techniques. The electrospinning variables that influence
the structure and function of nanofibers include solution parameters (e.g.,
molecular weight, concentration, solvent type, surface tension, conductivity, and
viscosity), processing parameters (e.g., applied voltage, flow rate, and spinning
distance), and environmental parameters (e.g., temperature and relative humidity)
[20]. Optimization of the physicochemical characteristics of the scaffolds is
crucial since these have a substantial influence on cell behavior and function.
For example, highly aligned fibers are needed to support the directional growth
of seeded cells through contact guidance, and the stiffness of the scaffolds can
promote the differentiation of MSCs toward specific cell types [21]. MSCs could
be differentiated into neuronal cells on soft substrates, while musculoskeletal
cells could be generated on more rigid scaffolds [21]. Examples of synthetic
polymers that have been used for the production of electrospun scaffolds for MSC
delivery include poly(aniline) (PANI), poly(
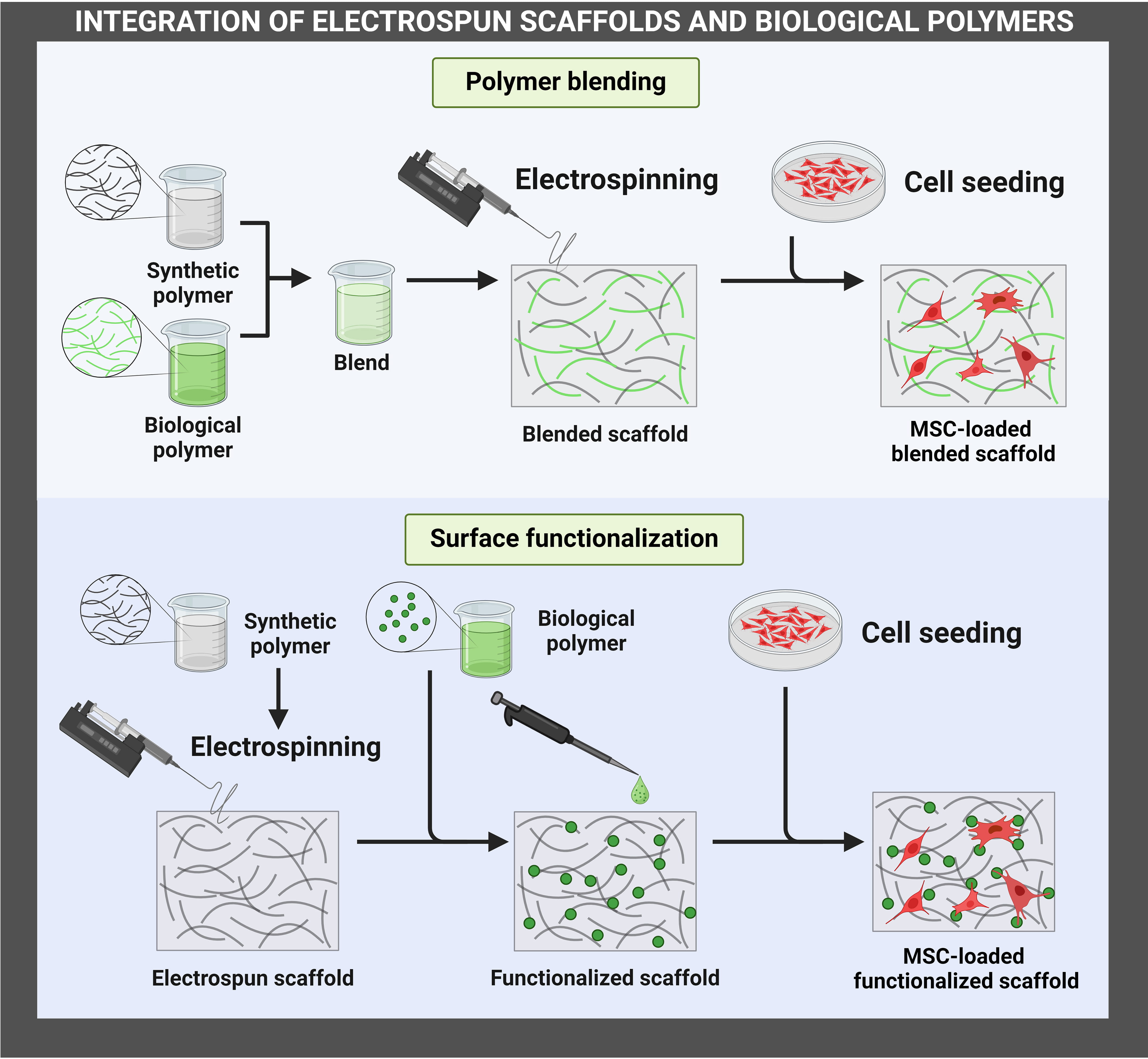
The integration of electrospun scaffolds could be achieved through polymer blending or surface functionalization (Fig. 1). Polymer blending involves mixing two or more polymers in a common solvent to form a homogeneous solution, which is then electrospun into fibrous scaffolds. Solvents that are commonly used to blend synthetic polymers with biological polymers include acetic acid and hexafluoroisopropanol. On the other hand, surface functionalization involves the incorporation of biological polymers on the surface of electrospun synthetic scaffolds. Surface functionalization may be accomplished through immersion of the scaffolds in a solution, plasma treatment, or direct printing onto the scaffolds. Polymer blending and surface functionalization may be employed simultaneously to enhance the integration of biological polymers with synthetic scaffolds.
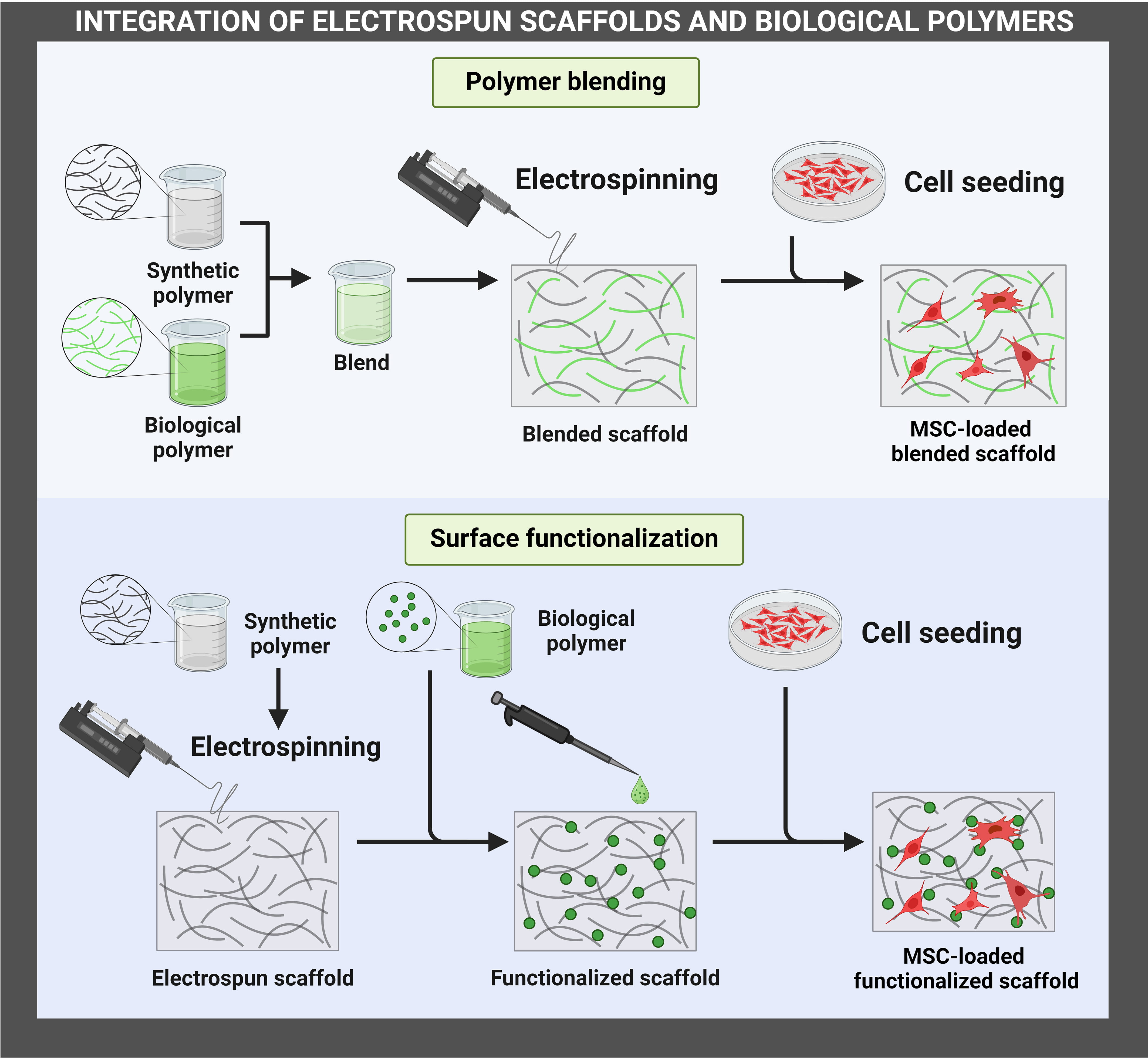 Fig. 1.
Fig. 1.Integration of electrospun scaffolds and biological polymers for enhancing MSC therapies. The figure has been created using https://www.biorender.com/. MSC, Mesenchymal stem/stromal cell.
A wide range of biocompatible synthetic polymers can be used to make electrospun scaffolds. The key advantages of synthetic polymers are spinnability, excellent mechanical integrity, and cost-effectiveness. However, modifications are typically needed for them to become bioactive. On the other hand, natural polymers, such as proteins and polysaccharides, are inherently bioactive and can often readily support cell adhesion and proliferation. Taking advantage of the benefits of both synthetic and natural polymers, recent investigations have focused on the integration of synthetic electrospun scaffolds and biological polymers to generate scaffolds with optimized physicochemical properties for MSC delivery. By improving the interaction between cells and scaffolds, biological polymers can support cell adhesion, stimulate cell proliferation, and promote the secretion of immunomodulatory substances.
3D culture conditions and scaffolds have been shown to enhance the survival of MSCS and amplify their immunomodulatory properties. Specifically, the use of polymer matrices to deliver MSCs has been shown to prevent the rapid loss of MSCs at the transplantation site by promoting cell-to-matrix adhesion and by protecting MSCs from mechanical disruption, biochemical stress, and immune clearance [8]. MSCs can be seeded onto the electrospun scaffolds through a variety of methods. These include passive seeding, electrospraying, centrifugation, and the use of a perfusion system.
Passive seeding, which involves pipetting a cell suspension directly onto the scaffold, is the simplest and most widely used method for attaching cells onto electrospun scaffolds. Electrospraying involves ejecting cell suspensions onto the scaffolds under an electric field, and it may be performed simultaneously with or after the synthesis of the scaffolds. Centrifugation or rotational seeding involves the deposition of the cells on the scaffolds under centrifugal force. Lastly, perfusion systems apply fluid flow to facilitate the delivery of cells into the scaffold’s pores, resulting in more uniform cell distribution throughout the scaffold structure. These methods may produce variable effects on MSCs and must be optimized to maximize cell viability and function [22].
The survival and function of MSCs are significantly influenced by their surrounding microenvironment, which offers physical and biochemical cues crucial for cellular homeostasis. Electrospun scaffolds, both synthetic and natural, transmit mechanical signals to modulate cellular behavior [23]. Consequently, these scaffolds are instrumental in controlling various intracellular signaling pathways, thereby affecting cell adhesion, proliferation, and function. Numerous studies have delved into the effects of the external environment on MSCs, laying the groundwork for our understanding of the interactions between MSCs and electrospun scaffolds [24].
To engage with their surroundings, cells must establish physical connections either by attaching to the ECM or another cell. Adhesion is fundamental for cell communication and regulation, making it a pivotal aspect of biomaterial scaffold design. Effective adhesion facilitates crucial cellular behaviors such as spreading, proliferation, and performance of function [25]. Factors that affect adhesion include hydrophilicity, surface charge, and the presence of natural ligands.
The hydrophilicity of polymers plays a critical role in cell adhesion. The most commonly used synthetic polymers, such as PCL and PLA, are hydrophobic, and consequently, face challenges in initiating interactions with the hydrophilic surfaces of cell membranes. To overcome this problem, these synthetic polymers can be blended or functionalized with natural polymers that possess hydrophilic domains. These hydrophilic domains often take the form of polar functional groups (e.g., -NH2/-NH3+, -OH, and -COOH) that can facilitate bond formation between the scaffold and cell surface receptors, thus promoting stable cell attachment and proliferation. For example, it has been shown that the addition of zein to electrospun PCL can increase the scaffold’s hydrophilicity and enhance the attachment and growth of adipose tissue-derived MSCs (AMSCs) on the scaffold [26]. Similarly, fibrin has been shown to increase the hydrophilicity of PCL and improve the attachment and proliferation of BMSCs on the scaffold [27]. In another study, gelatin-blended PLGA scaffolds have also been shown to have higher hydrophilicity compared to PLGA alone, and this property translated to enhanced umbilical cord-derived MSCs (UMSC) attachment [28]. The surface charge of polymers can also affect cell adhesion. Synthetic polymers with cationic surfaces can associate with the negatively charged moieties found on the surface of cell membranes. For example, the integration of zein fibers rich in protonated amino groups into PCL has been shown to improve the scaffold’s ability to interact with AMSCs’ anionic cell membranes [26]. Conversely, polymers that are primarily anionic may have difficulties interacting with the cell membrane.
In their native microenvironment, cells constantly interact with adjacent cells and the ECM through protein ligands. CAMs, such as integrins and pattern recognition receptors, act as mechanobiological sensors that identify and bind these ligands. When integrins bind to the ECM, they drive the assembly of focal adhesion complexes as well as the activation of integrin-associated protein kinases to initiate downstream signaling pathways for enhancing cell adhesion and survival [16, 29, 30]. Integrins bind ligands with lower affinity but at higher concentrations on cell surfaces, facilitating the formation of dense plaques upon activation, where multiple integrin molecules are anchored to cytoskeletal filaments to stabilize cell adhesion (Velcro principle) [31]. The most well-understood binding site for integrins is the arginine-glycine-aspartic acid (RGD) sequence, present in fibronectin and other ECM proteins. A study on the combination of gelatin and PLGA as scaffold material led to improved UMSC attachment due to the presence of RGD motifs, which play an essential role in the growth and migration of cells [28, 32].
Following attachment, cells can activate pathways that promote proliferation.
The incorporation of biological polymers that can enhance cell adhesion onto
electrospun scaffolds has been shown to also enhance the proliferation of MSCs
[27, 33, 34]. The binding of integrin to ECM components activates the assembly of
focal adhesion molecules, which not only facilitate stable adhesion but also
trigger an intracellular cascade of signaling pathways that control cell behavior
and survival [15]. In this “outside-in” signaling mechanism, integrins work
with conventional signaling receptors to activate interrelated pathways that
regulate cell proliferation, such as focal adhesion kinase/proto-oncogene c-Src
(FAK/Src), mitogen-activated protein kinases (MAPK), phosphoinositide
3-kinase/protein kinase B (PI3K/Akt), Wingless-related integration
site/
FAK is one of the most well-researched pathways of integrin signaling. Integrins clustering at cell-matrix contacts recruit FAK via intracellular adaptor proteins, and the clustered FAK molecules phosphorylate each other, forming a docking site for cytoplasmic tyrosine kinases from the Src family. Src and FAK reciprocally phosphorylate each other and other proteins within the junction, signaling the cell that it has adhered to a substrate suitable for growth and proliferation [31]. It has been shown that AMSCs seeded on decellularized ECM (dECM) had higher levels of phosphorylated FAK compared to those seeded on standard plates [35]. In another study, the addition of heparin and collagen led to increased phosphorylation of FAK in BMSCs [36].
The MAPK signaling pathway stimulates cells to proliferate or differentiate in response to tyrosine phosphorylation and the activation of Ras. The final kinase in the series is known as extracellular signal-regulated kinase (ERK) and is also a downstream target of FAK. The studies on MSCs seeded on ECM components that demonstrated increased phosphorylation of FAK also showed increased phosphorylation of ERK [35, 36]. In another study, it has been shown that collagen can enhance the adhesion, proliferation, and osteogenic differentiation of BMSCs through Ras homolog proteins [37].
The PI3K/Akt pathway can also be activated by FAK/Src proteins in response to integrin clustering [38, 39]. In this pathway, PI3K generates phosphatidylinositol-3,4,5-trisphosphate, which in turn recruits Akt to the plasma membrane. Once activated, Akt inhibits apoptosis and promotes cell growth and proliferation through downstream signaling events [31]. Integrins play an important role in promoting the adhesion and survival of BMSCs onto ECM components through the activation of Akt [16, 40]. It has also been shown that Akt plays an important role in mediating the proliferation of BMSCs after binding to glycoproteins through the CD44 receptor [41]. Cell growth regulation in the PI3K/Akt pathway relies in part on a protein kinase called mammalian target of rapamycin (mTOR), which promotes ribosome production and protein synthesis. The MAPK and PI3K/Akt pathways converge on mTOR because ERK can also activate this protein kinase.
The Wnt/
The YAP/TAZ pathway is also activated by integrin clustering and focal adhesion maturation. The cytoskeleton integrates mechanical strain arising from cell–ECM and cell–cell interactions. YAP/TAZ is activated through the release from inhibitors and modulation of nuclear–cytoplasmic shuttling. These transcriptional regulators also mediate the effects of Wnt signaling to promote cell proliferation and differentiation [42]. It has been shown that the attachment on dECM via integrins can enhance BMSCs’ expression of YAP1 and proliferation [43].
Under optimal conditions, MSCs secrete a diverse array of soluble (e.g., immunomodulatory cytokines, chemokines, and growth factors) and vesicular factors (e.g., extracellular vesicles), collectively referred to as the MSC secretome. The secretome’s volume and composition are largely influenced by the MSCs’ surrounding environment, prompting research into the effects of blended or functionalized electrospun scaffolds on MSC secretory functions.
The physical characteristics of electrospun scaffolds can enhance the ability of
MSCs to secrete paracrine products. It has been shown that AMSCs cultured on
electrospun PCL had significantly higher secretion of paracrine products, such as
basic fibroblast growth factor (bFGF), hepatocyte growth factor, inducible nitric
oxide synthase (iNOS), prostaglandin E
Recent studies have shown that the addition of biological polymers can improve
the ability to secrete regenerative factors. For instance, PCL nanofibers
combined with platelet-rich plasma have been shown to boost MSC production of
collagen, aiding bone tissue regeneration [46]. In the realm of skin tissue
engineering, scaffolds composed of PCL and collagen have been effective in
stimulating MSCs to release factors that promote wound healing, such as bFGF and
angiopoietin 1 [47]. Additionally, incorporating RGD and TGF-
Polysaccharides, which are polymers composed of monosaccharide units that are connected via glycosidic linkages, are the most abundant biological polymer in nature, and they may function as energy reservoirs, structural components, or mediators of intercellular signaling. Polysaccharides are an important component of the mammalian ECM’s ground substance, which occupies the spaces between the cells and fibers. The ground substance is composed of GAGs, proteoglycans, and glycoproteins. GAGs are the most abundant heteropolysaccharide constituents of the ground substance, and these polymers are composed of repeating disaccharide units composed of a modified sugar (i.e., N-acetylgalactosamine or N-acetylglucosamine) and a uronic acid (i.e., glucuronate or iduronate). GAGs are commonly linked to core proteins to form proteoglycans. Polysaccharides may also act as a component of glycoproteins [13]. Examples of GAGs that have been added to electrospun scaffolds for MSC delivery include chondroitin sulfate (CS) [49] and hyaluronic acid (HA) [50]. Apart from animal-derived polysaccharides, recent studies have also explored the use of other nature-derived polymers, such as alginate [51, 52], cellulose [53], and chitosan [54].
Alginate is a heteropolysaccharide extracted from several genera of brown algae,
such as Sargassum, Laminaria, and Macrocystis [55]. It
is comprised of two building blocks, namely guluronic acid (G-block) and
mannuronic acid (M-block). It is generally regarded as non-toxic and
non-immunogenic, making it an attractive material for tissue engineering,
in vitro modeling, and drug delivery applications. In the presence of
divalent cations, such as calcium (Ca
Cellulose, the most abundant naturally occurring polymer of glucose, is a
homopolysaccharide composed of
Chitosan is a polycationic heteropolysaccharide composed of N-acetyl-D-glucosamine and D-glucosamine units. It is produced from the N-deacetylation of chitin, the second most abundant natural polymer after cellulose and a major component of the cell wall of many fungi and the exoskeleton of insects and crustaceans [60]. The amino and hydroxyl groups on its surface aid in the binding of various substances. Specifically, the addition of carboxyl and methyl groups to chitosan can enhance its similarity to the GAGs found in the ECM. Carboxymethyl chitosan has been blended with PVA to produce an electrospun scaffold that can support the attachment and proliferation of placenta-derived MSCs (PMSCs) and facilitate complete closure of full-thickness wounds in rats after 14 days [54]. Chitosan has also been blended with PVA to produce an electrospun scaffold for the delivery of AMSCs in rat calvarial defects. The scaffold was also incorporated with platelet-rich plasma (PRP), and the addition led to significantly higher bone regeneration [61]. Chitosan nanoparticles have also been used to encapsulate brain-derived neurotrophic factor (BDNF) and to functionalize the surface of an electrospun PLGA scaffold for the delivery of AMSCs in a rat model of sciatic nerve injury. However, the MSC-loaded scaffolds had a significantly lower sciatic functional index (SFI) and gastrocnemius mass ratio than the autograft at 12 weeks [62].
CS is a GAG that is commonly found in cartilage, bone, and heart valves. It is composed of repeating disaccharide units of N-acetylgalactosamine sulfate and D-glucuronic acid. It is a fundamental component of aggrecan, the proteoglycan that confers shock-absorbing capacity to articular cartilage [13]. CS has been blended with PCL-PTHF to produce an electrospun scaffold for the delivery of BMSCs into full-thickness patellar cartilage defects in rats. After 8 weeks, the MSC-loaded CS-containing scaffold demonstrated superior repair of the cartilage defects compared to no treatment (International Cartilage Repair Society [ICRS] score of 10.67 versus 2.33) [49].
HA is a GAG that is commonly found in synovial fluid, vitreous humor, and ECM of most connective tissues in the body. It is composed of repeating disaccharide units of N-acetylglucosamine sulfate and D-glucuronic acid. Unlike the other GAGs, HA is composed of a very long chain of sugars. Hence, HA can hold large amounts of water and can act as either a lubricant or shock absorber. It is also a major component of aggrecan [13]. Another important function of HA is to immobilize growth factors in the ECM. Indeed, HA was recently used to improve the integration of BDNF with PLA to generate an electrospun scaffold for the delivery of BMSCs into the hemisected spinal cords of rats. The addition of HA did not change the scaffold’s fiber diameter and stiffness. The surface of the scaffold was also functionalized with collagen, and the scaffold demonstrated a better improvement in motor function compared to the cell-free scaffold at 8 weeks [50].
Proteins are the most functionally diverse polymers found in living organisms, and they play various important roles, such as structural components, contractile elements, transporters, receptors, enzymes, hormones, and antibodies. More importantly, proteins are an essential component of the ECM’s structural fibers and ground substance (i.e., proteoglycans and glycoproteins). Proteoglycans are formed through the covalent attachment of GAGs to a core protein. The number of GAGs that can attach to the protein core can vary from 1–200, which gives rise to proteoglycans’ remarkable diversity. On the other hand, glycoproteins represent a smaller group of proteins in the ECM. Nonetheless, multidomain glycoproteins play an important role in stabilizing the ECM by providing a structural framework as well as binding sites for cells and other substances in the ECM [13]. Examples of glycoproteins that have been integrated with electrospun scaffolds for MSC delivery include collagen [49, 50, 51, 63, 64, 65, 66], gelatin [51, 67, 68, 69], laminin [62], and fibrinogen [70]. Apart from glycoproteins, growth factors, peptides, and plant-derived proteins have also been blended with synthetic polymers to optimize MSC delivery (Table 1, Ref. [49, 63, 64, 65, 66, 67, 68, 69, 71, 72, 73]).
| Protein | Synthetic polymer | Integration technique | MSC type | Seeding technique | In vitro results | Target tissue | Animal model | In vivo results | Ref |
| Collagen | PCL | Polymer blending, surface functionalization | AMSC | Direct seeding | After 21 days, the MSC-seeded blended scaffold showed around 1.2 times higher GAG content than the surface-functionalized scaffold. | Trachea | Rabbit | After 4 weeks, the blended scaffold showed a higher cell density in the regenerated tracheal tissue compared to the surface-functionalized scaffold. | [66] |
| Collagen | PCL-PTHF | Polymer blending | BMSC | Direct seeding | The blended scaffold showed around 1.4 times higher cell viability compared to the tissue culture plate after 21 days. | Cartilage | Rat | After 8 weeks, the MSC-loaded blended scaffold demonstrated better repair of full-thickness patellar cartilage defect compared to no treatment (ICRS 15.68 versus 2.33). | [49] |
| Collagen | PCT | Polymer blending | BMSC | Perfusion system | The MSCs maintained their stem cell properties following seeding on the blended scaffold. | Heart | Rat | Around 15% of the cells on the cardiac patch survived 5 weeks after transplantation. After 5 weeks, the blended scaffold significantly improved cardiac function compared to control (LVEF: 40.8% versus 28.9%). | [64] |
| Collagen | PLA | Polymer blending | BMSC | Electrospraying | The blended scaffold supported the proliferation of MSCs. | Bone | Rat | After 4 weeks, total cell count, total callus formation, and osteocalcin staining in the skull defect were around 1.6, 6, and 1.3 times higher in the cell-seeded scaffolds compared to cell-free scaffolds, respectively. | [63] |
| Collagen | PLCL, PANI | Polymer blending | AMSC | Direct seeding | The addition of collagen significantly increased the metabolic activity and neuronal differentiation of the seeded cells. | Nerve | Rat | After 6 months, there was no significant difference in the SFI and muscle mass ratio between the MSC-loaded and cell-free scaffolds. However, the MSC-loaded scaffold showed around 1.1 times higher nerve density compared to the cell-free scaffold. | [65] |
| CTGF | PCL | Surface functionalization | Not specified | Direct seeding | None reported. | Muscle | Rat | After 24 weeks, the cell-free scaffold had a significantly higher collagen deposition and abundant abscess formation. On the other hand, no complications were observed for the MSC scaffolds. | [72] |
| Gelatin | PCL | Surface functionalization | PLMSC | Direct seeding | The scaffolds supported the proliferation of MSCs. | Bone | Rat | After 6 weeks, the MSC scaffolds had greater collagen deposition and periostin expression in the periodontal defects compared to no treatment. | [69] |
| Gelatin | PLA | Surface functionalization | BMSC | Direct seeding | After 7 days, the functionalized scaffold showed a 1.2 times higher proliferation than the non-functionalized scaffold. | Bone | Rat | After 8 weeks, the MSC-loaded functionalized scaffold demonstrated around 1.7 and 1.4 times higher bone regeneration as seen on digital mammography and histological analysis, respectively, compared to the non-functionalized PLA scaffolds. | [67] |
| Gelatin | PLCL | Polymer blending | EMSC | Direct seeding | The addition of gelatin enhanced the hydrophilicity of the scaffold and the proliferation of MSCs on the scaffold. | Muscle | Mouse | After 6 weeks, the blended scaffold had around 1.6 times higher cell infiltration compared to the non-blended scaffold. The MSC-loaded blended scaffold also showed a high M2 macrophage and minimal M1 response. | [68] |
| Laminin | PLA | Surface functionalization | DPMSC | Direct seeding | The scaffolds supported the proliferation of MSCs. | Skin | Mouse | After 9 days, the MSC-loaded scaffolds stimulated the production of a complete epidermal layer in some of the animals. Functionalization with laminin did not appear to provide an advantage. | [71] |
| Soya protein isolate | PEO | Polymer blending | BMSC | Direct seeding | The blended scaffold supported the adhesion, proliferation, and preconditioning of MSCs. | Bone | Rat | After 2 weeks, the MSC-loaded scaffold showed enhanced new bone formation in the skull defect compared to the cell-free scaffold and no treatment. | [73] |
Abbreviations: AMSC, adipose tissue-derived mesenchymal stem/stromal cell; BMSC,
bone marrow-derived mesenchymal stem/stromal cell; CTGF, connective tissue growth factor; DPMSC, dental pulp-derived
mesenchymal stem/stromal cell; EMSC, endometrial tissue-derived mesenchymal
stem/stromal cell; ICRS, International Cartilage Repair Society; PANI,
poly(aniline); PCL, poly(
Collagen, the most abundant protein in the body and the most abundant structural
component of the ECM is composed of three
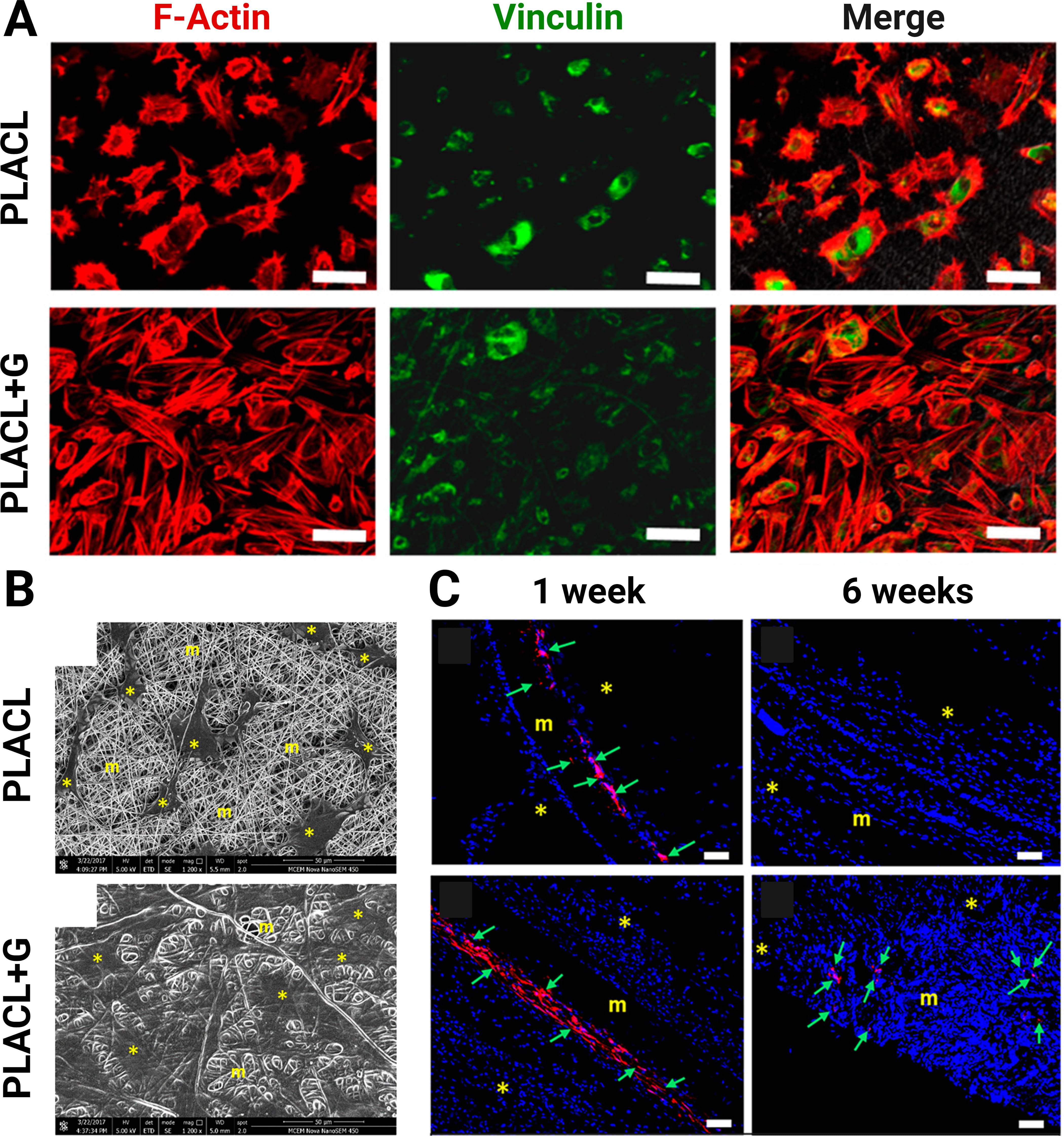
Gelatin is derived from the denaturation of collagen. Although gelatin does not possess the triple helical structure of collagen, certain functional attributes, such as biocompatibility and hydrogel-forming capability, are retained in gelatin. Recent investigations have demonstrated the applications of MSC-seeded gelatin-containing scaffolds for tissue regeneration. Common tissue types that have been regenerated utilizing MSCs in gelatin-containing scaffolds include musculoskeletal and integumentary tissues [51, 67, 69]. It has been shown that the addition of gelatin increases the scaffold’s hydrophilicity [28, 68]. It may also increase the scaffold’s fiber diameter and pore size [68]. In the context of bone regeneration, a recent study reported MSC-seeded gelatin-functionalized scaffolds that facilitated higher levels of BMSC proliferation in vitro and superior bone regeneration in vivo when used to repair rat calvarial defects compared to MSC-seeded non-functionalized scaffolds [67]. Similarly, the repair of periodontal fenestration defects was also demonstrated in a rat model using an MSC-seeded gelatin-functionalized scaffold. The scaffolds generated greater collagen deposition and periostin expression in the periodontal defects compared to no treatment at 6 weeks [69]. Another study examined the potential of gelatin for pelvic muscle repair and found that the addition of gelatin can enhance the hydrophilicity of the scaffold and the proliferation of EMSCs on the scaffold. After 6 weeks, the MSC-loaded gelatin-blended scaffold had a significantly higher cell infiltration compared to the MSC-loaded but non-blended scaffold (Fig. 2, Ref. [68]). The gelatin-blended scaffold also showed high M2 macrophage and minimal M1 macrophage responses [68].
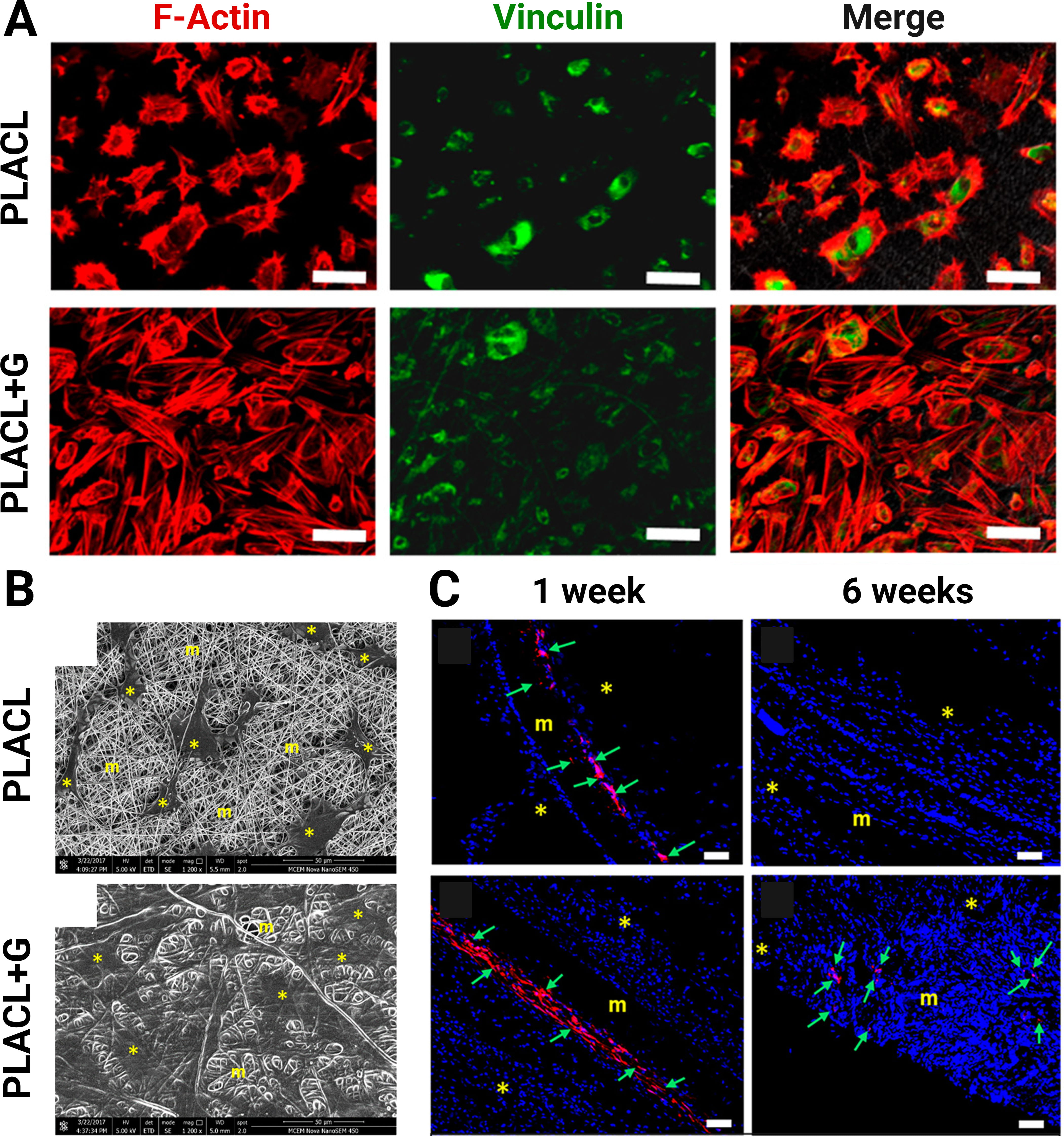 Fig. 2.
Fig. 2.Integration of electrospun mesh with gelatin. (A) The endometrial mesenchymal stem cells (EMSCs) on the gelatin-blended scaffold (PLACL+G) showed continuous spreading of filamentous F-actin and vinculin over the mesh surface (bar = 50 µm). (B) PLACL+G allowed more growth and penetration into the scaffold as shown on scanning electron microscopy images compared to PLACL. (*, EMSC; m, mesh). (bar = 50 µm). (C) PLACL+G also had higher in vivo retention of EMSCs than PLACL (*, host tissue; m, mesh; green arrow, EMSCs; bar = 100 µm). Reproduced with permission from Mukherjee S et al. [68], Biomacromolecules; published by American Chemical Society, 2019.
Laminin is a glycoprotein found in the basal lamina, and it possesses binding sites for various ECM fibers and ground substance components. It plays an important role in supporting the adhesion, migration, growth, and differentiation of various cell types. Laminin has been blended with PLGA to produce an electrospun scaffold for the delivery of AMSCs in a rat model of sciatic nerve injury. Although the scaffolds had a significantly lower SFI and gastrocnemius mass ratio than the autograft at 12 weeks, they produced a comparable mean fiber diameter and myelin sheath thickness to those of the autograft [62]. Laminin has also been used to functionalize PLA scaffolds for the delivery of DPMSCs to full-thickness skin defects in mice. The delivery of MSCs stimulated the production of a complete epidermal layer in some of the animals. However, functionalization with laminin did not appear to enhance the efficacy of delivered MSCs [71].
Fibrinogen is the largest plasma protein, and it acts as an important component of the coagulation cascade. The cleavage of fibrinogen gives rise to fibrin monomers that rapidly polymerize to form an impermeable mesh, which binds red blood cells and platelets at the side of vascular injury [13]. It has been shown that the addition of fibrin increases the scaffold’s hydrophilicity [27]. Fibrinogen has been used to functionalize the surface of PCL for the delivery of MSCs into abdominal wall defects in rats. It has been shown that the MSC-loaded functionalized scaffold can provide adequate support without major complications for up to 53 weeks [70]. Fibrin has also been used for the delivery of MSCs into the injured sciatic nerves of rats. However, the fibrin-containing scaffold showed the lowest SFI and gastrocnemius mass ratio of all the experimental groups, which suggests that the fibrin matrix could interact with critical regenerative factors and reduce the overall efficacy of MSC-loaded scaffolds [62]. Indeed, it has been shown that fibrinogen may promote cell adhesion at low densities, but it may also prevent cell adhesion at high loading densities [75], which could limit the ability of MSC to exert regenerative effects at the site of injury.
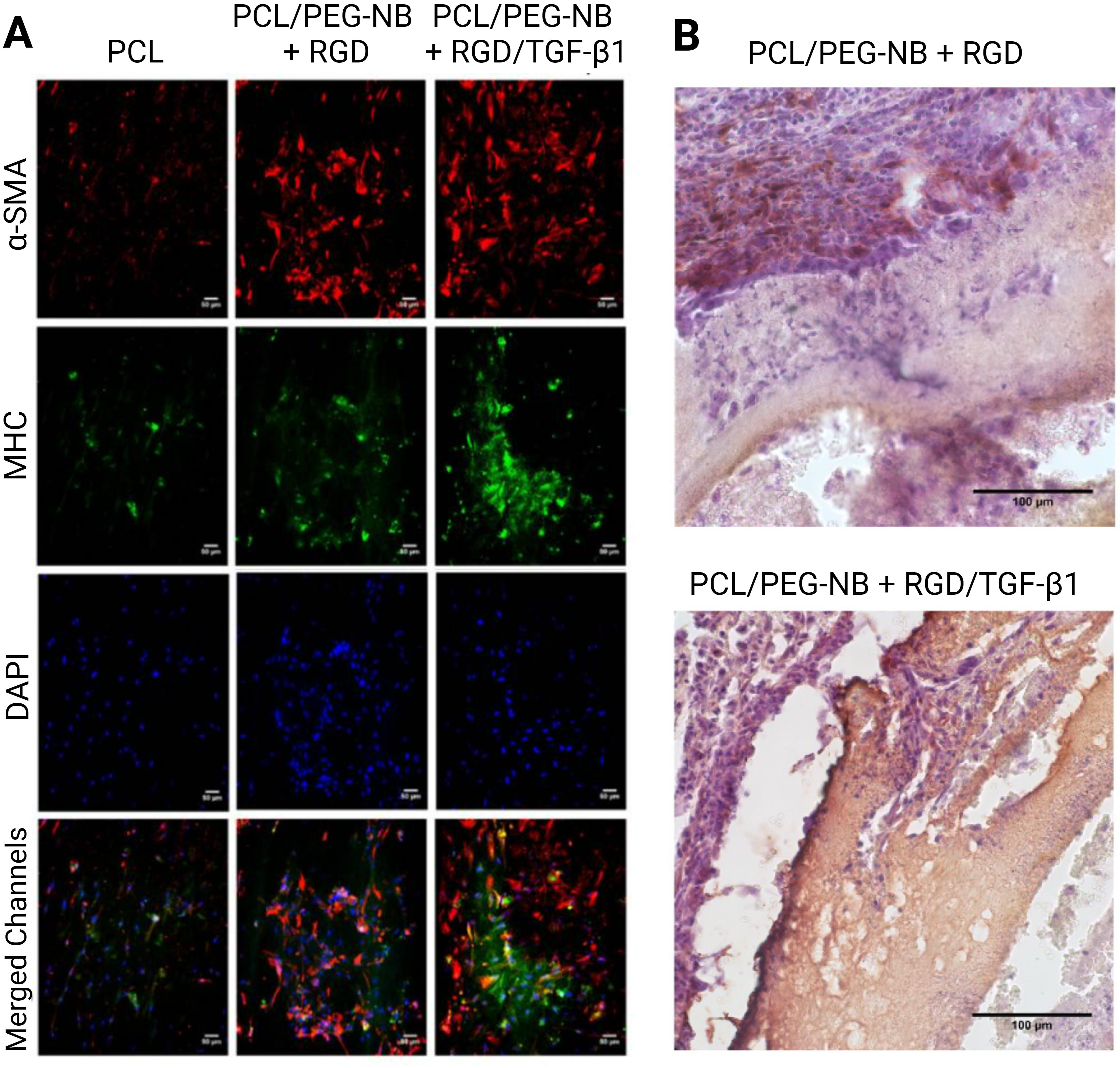
Peptides are short sequences of amino acids that are easily synthesized or produced via recombinant protein expression, making them attractive agents for the fabrication of biomaterials. The RGD peptide was initially discovered as the essential structure recognized by cells in fibronectin, the most abundant glycoprotein in connective tissues [76]. Significant advancements have been achieved in comprehending the molecular interactions responsible for cell adhesion, and it is now established that this peptide serves as a cell recognition site for several proteins in the ECM and the bloodstream, such as collagen, fibrinogen, laminin, osteopontin, thrombospondin, vitronectin, and von Willebrand factor. The RGD sequences in these proteins are recognized by integrins. RGD-functionalized scaffolds were recently utilized to reconstitute the mechanical and cellular profile required for vascular tissue reconstruction. The addition of RGD improved the adhesion and proliferation of seeded MSCs in vitro (Fig. 3, Ref. [48]). The MSC-loaded RGD-functionalized grafts had significantly higher cell infiltration and ECM production compared to the bare grafts 1 week after implantation in rats [48].
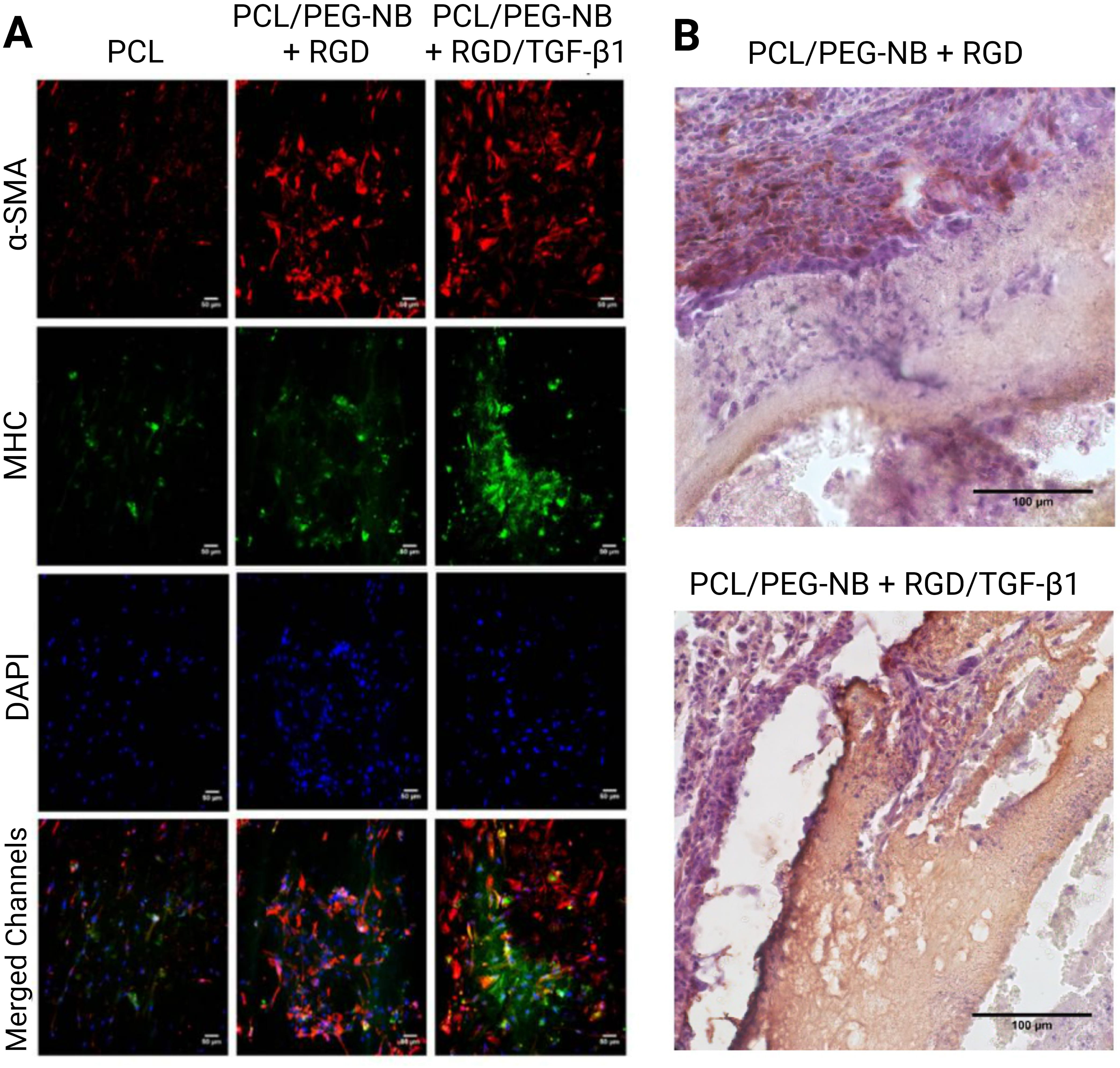 Fig. 3.
Fig. 3.Functionalization of electrospun vascular grafts with
RGD and TGF-
Growth factors are biologically active molecules that can affect the growth and
differentiation of cells. Many growth factors are proteins, and these types of
growth factors typically bind with a high affinity to a specific plasma
membrane-bound receptor to exert their effect [77]. Methods that utilize this
class of molecules to functionalize scaffolds capitalize on the extensively
researched impacts of these molecules on the maintenance of homeostasis in native
tissues. Examples of growth factors that have been used to functionalize
electrospun scaffolds to enhance MSC delivery include BDNF [50, 62], connective
tissue growth factor (CTGF) [70, 72], insulin, and transforming growth
factor-beta 1 (TGF-
Biological polymers can be combined to form artificial composites with improved
physicochemical properties and higher biocompatibility. Artificial composites can
be formed by combining polymers from similar (e.g., protein-protein) or different
classes (e.g., polysaccharide-protein). Examples of protein combinations that
have been used to functionalize electrospun scaffolds for the delivery of MSCs
include the following: CTGF and fibrinogen [70]; RGD and TGF-
| Composite | Synthetic polymer | Functionalization techniques | MSC type | Seeding technique | In vitro results | Target tissue | Animal model | In vivo results | Ref |
| Alginate, aloe vera powder | PCL | Surface functionalization | EMSC | Direct seeding | The functionalized scaffold supported the proliferation of MSCs. | Muscle | Mouse | After 7 days, the MSC-loaded functionalized scaffold had significantly lower M1 macrophage and higher M2 macrophage infiltration than the bare scaffold. | [52] |
| Alginate, collagen, gelatin | PCL | Polymer blending, surface functionalization | AMSC | Direct seeding | The scaffold supported the attachment of MSCs. | Skin | Rat | After 21 days, the scaffold showed significantly higher wound closure (around 95%) compared to the control (around 65%). | [51] |
| BDNF, chitosan, fibrin, laminin, | PLGA | Surface functionalization | AMSC | Direct seeding | None reported. | Nerve | Rat | After 12 weeks, the MSC-loaded scaffolds had a significantly lower SFI and muscle mass ratio than the autograft. The MSC scaffolds with laminin and BDNF had a comparable mean fiber diameter and myelin sheath thickness to those of the autograft. | [62] |
| BDNF, collagen, HA | PLA | Polymer blending, surface functionalization | BMSC | Direct seeding | The addition of collagen increased the scaffold’s hydrophilicity and MSC adhesion. | Spinal cord | Rat | After 8 weeks, the MSC-loaded functionalized scaffold demonstrated a greater improvement in motor function compared to cell-free scaffolds (BBB score: around 14 versus 12). | [50] |
| Cellulose, insulin | PCL | Polymer blending, surface functionalization | BMSC | Rocking | None reported. | Tendon | Rat | After 8 weeks, the MSC-loaded insulin-functionalized scaffold had significantly higher collagen compared to the MSC scaffold without insulin. | [53] |
| Chitosan, PRP | PVA | Polymer blending | AMSC | Direct seeding | Blending improved the proliferation and osteogenic differentiation of MSCs. | Bone | Rat | After 8 weeks, the MSC-loaded PRP-blended scaffold had a higher area of bone regeneration in the calvarial defect as seen on CT imaging (around 80% versus 60%) and histology (around 65% versus 40%) compared to the MSC-loaded non-PRP-blended scaffold. | [61] |
| CTGF, fibrinogen | PCL | Surface functionalization | Not specified | Direct seeding | None reported. | Muscle | Rat | After 53 weeks, the MSC-loaded functionalized scaffold exhibited sufficient support, biocompatibility, and no mesh-related complications in a rat model of abdominal wall defect. | [70] |
| dECM | PCL, PGMA | Surface functionalization | AMSC | Centrifugation | The functionalized scaffold supported the proliferation and chondrogenic differentiation of MSCs. | Bone | Rat | After 12 weeks, there was no significant difference between the MSC-loaded dECM-functionalized scaffold and the MSC-loaded non-functionalized scaffold in terms of osteochondral regeneration. | [80] |
| Macrophage cell membrane | PLGA | Surface functionalization | BMSC | Direct seeding | The functionalized scaffold had a 4 times higher cell viability than the control. | Skin | Mouse | After 15 days, the functionalized scaffold showed significantly higher wound closure (97.06%) than the non-functionalized scaffold (86.91%). | [82] |
| PRP | PCL | Surface functionalization | AFMSC | Direct seeding | The scaffold had a lower cell viability compared to the tissue culture plate. | Bone | Rat | After 8 weeks, all the cranial defects with the MSC-loaded functionalized scaffold had blood vessels and improved collagen deposition compared to the non-functionalized scaffold. | [81] |
| RGD, TGF- |
PCL | Surface functionalization | Not specified | Direct seeding | RGD improved cell adhesion and proliferation, while TGF-B1 promoted hMSC differentiation into vascular smooth muscle cells. | Vascular tissue | Rat | After 1 week, the MSC-loaded peptide-functionalized grafts had significantly higher cell infiltration and ECM production. Compared to MSC-loaded RGD grafts, the MSC-loaded RGD/TGF- |
[48] |
Abbreviations: AFMSC, amniotic fluid-derived mesenchymal stem/stromal cell;
AMSC, adipose tissue-derived mesenchymal stem/stromal cell; BBB, Bresnahan,
Beattie, and Basso; BDNF, brain-derived neurotrophic factor; BMSC, bone
marrow-derived mesenchymal stem/stromal cell; CTGF, connective tissue growth
factor; dECM, decellularized extracellular matrix; EMSC, endometrial
tissue-derived mesenchymal stem/stromal cell; HA, hyaluronic acid; PCL,
poly(
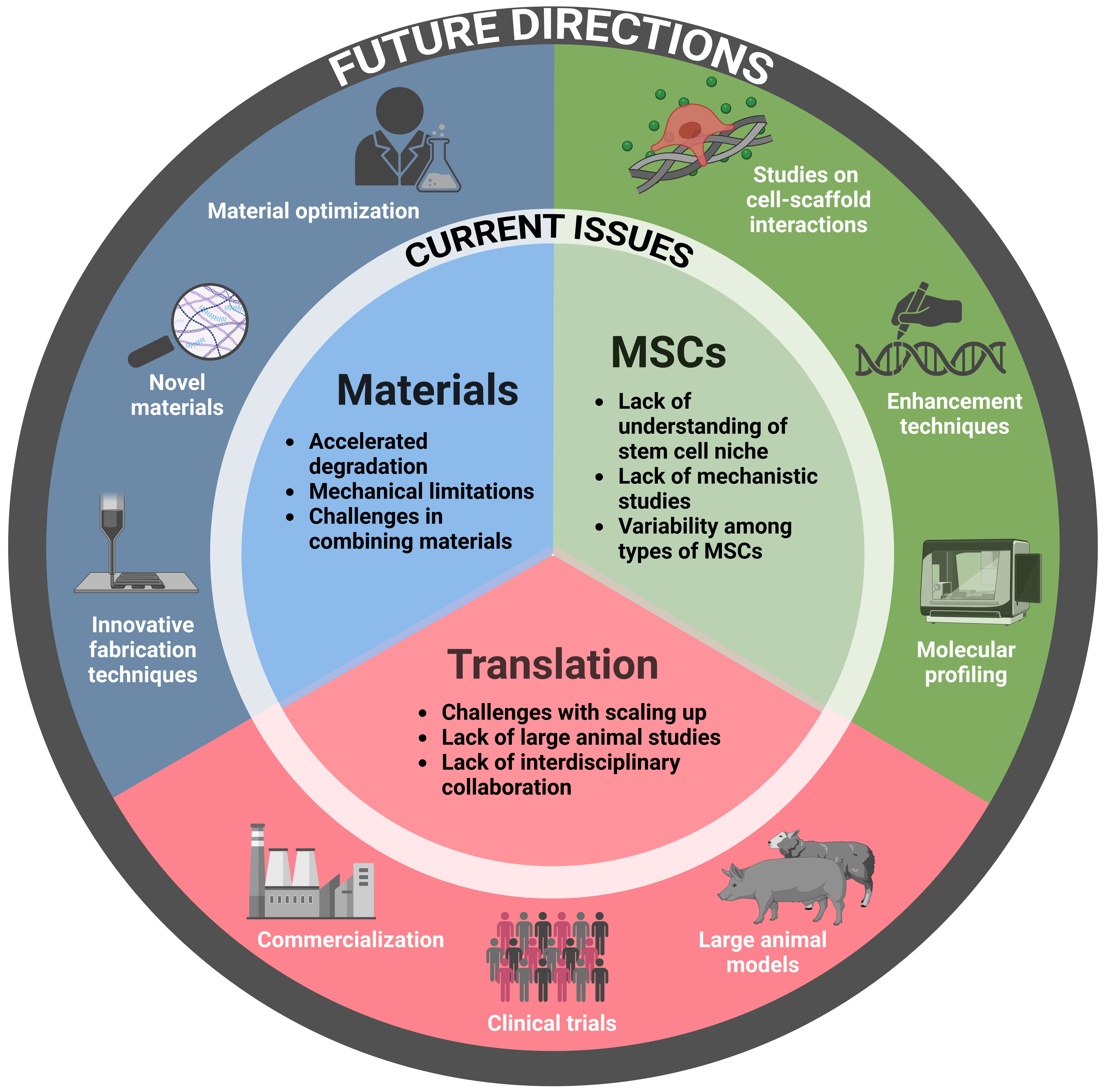
Moving forward, future investigations in the field of scaffold-based MSC delivery should focus on addressing crucial issues regarding the scaffold materials, MSCs, and clinical translation (Fig. 4).
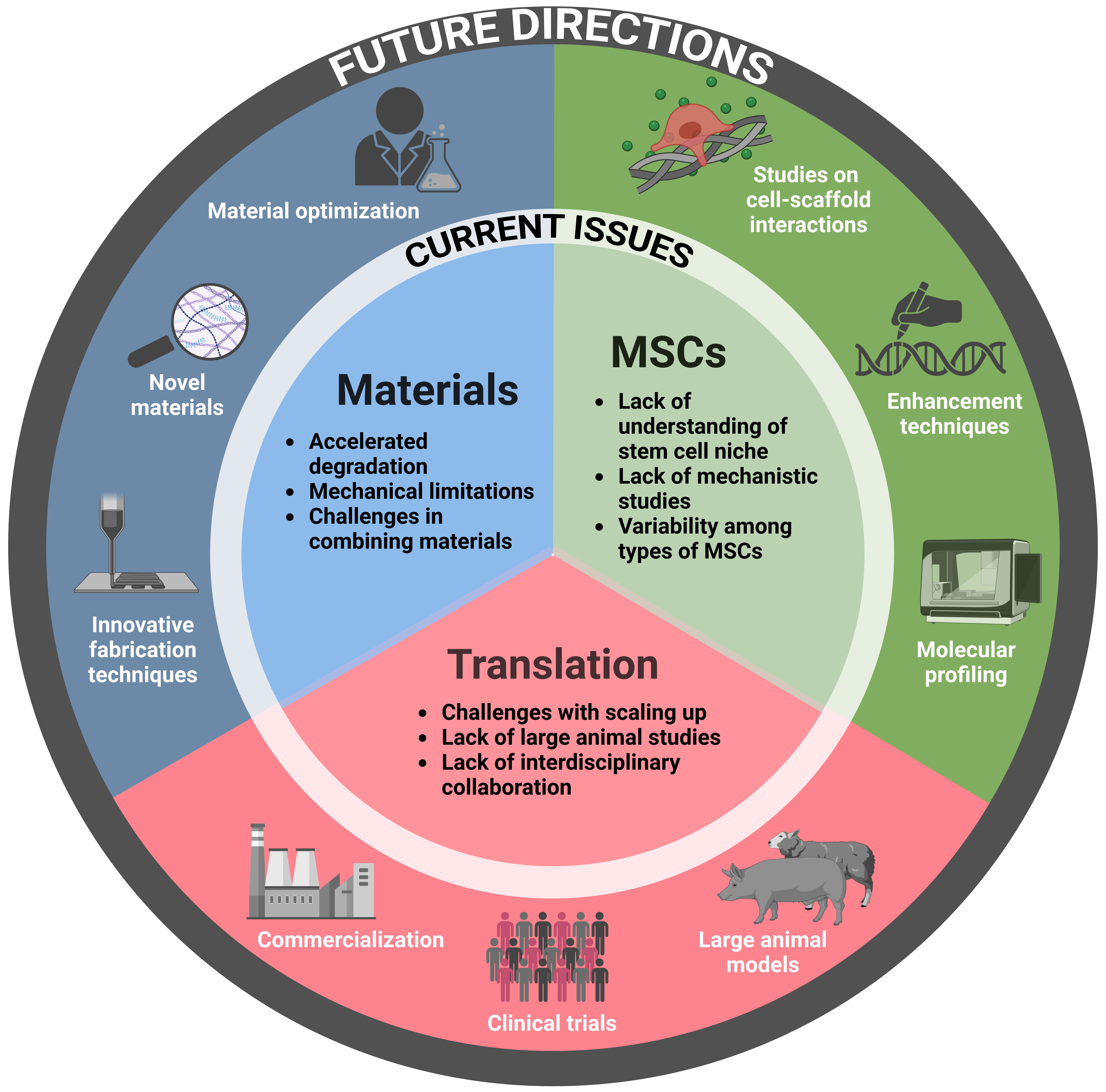 Fig. 4.
Fig. 4.Current issues in the integration of electrospun scaffolds and biological polymers and the future research directions to improve its utility for MSC therapies. The figure has been created using https://www.biorender.com/.
Firstly, the combination of different scaffold materials should be optimized to address the challenges of accelerated degradation, mechanical limitations, and physicochemical incompatibility. Although recent studies have shown that the addition of biological polymers does not affect the initial mechanical properties of electrospun scaffolds, the rapid in vivo degradation of biological polymers can compromise both scaffold stability and the sustained delivery of MSCs and secreted factors. Hence, optimization studies must be performed to balance in vivo degradation and mechanical stability. Furthermore, the hydrophilic nature of most biological polymers makes blending with more mechanically stable hydrophobic polymers challenging. To overcome this, novel materials and innovative fabrication techniques, such as 3D printing, microfluidics, and click chemistry, should be explored to enable the integration of polymers with diverse physicochemical properties and the creation of more complex and functional scaffolds.
Secondly, further research is needed to address our limited understanding of stem cell niches, cell-scaffold interactions, and variability among MSC types. A fundamental step is to investigate the specific matrix composition and architecture that best preserves the beneficial properties of different MSC types. Mechanistic studies should also be conducted to delve more deeply into the complex interactions between various MSC types and polymers. The results of these studies can guide scaffold design for enhancing material biocompatibility and cell-to-matrix interactions, providing a more conducive microenvironment for MSC growth and release of paracrine signals. Additionally, integrating emerging stem cell technologies, such as priming and genetic modification, can help prevent premature cell death and further enhance therapeutic outcomes. Finally, to standardize therapeutic outcomes, comprehensive molecular profiling of various MSC types should be performed to identify key markers for selecting optimal cell types for specific applications.
Thirdly, several critical issues must be tackled to facilitate the clinical translation of MSC-loaded electrospun scaffolds. Although electrospinning in itself has been shown to be scalable, with several products incorporating electrospun fibers advancing into human use or testing, some blending and functionalization methods remain challenging to scale for mass production. Establishing collaborations between academia and industry can help overcome this hurdle, facilitating the translation of promising technologies into commercially viable products. In addition, preclinical studies in large animal models are crucial for assessing safety and efficacy in human trials. Therefore, immediate actions must be taken to initiate large-scale animal investigations. Nonetheless, rigorous clinical trials are needed to evaluate the safety and efficacy of MSC-based therapies, and successful conduct of human studies requires close collaboration among materials scientists, stem cell biologists, clinicians, and regulatory experts.
By addressing these challenges and leveraging interdisciplinary expertise, scaffold-based MSC delivery holds great promise for revolutionizing regenerative medicine and improving patient outcomes.
In conclusion, the integration of electrospun scaffolds and biological polymers represents a promising strategy for enhancing the delivery and efficacy of MSC therapies. By leveraging the advantages of both synthetic and natural polymers, researchers have developed techniques to optimize scaffold properties, thereby creating a conducive microenvironment for MSC adhesion, proliferation, and function. Through polymer blending, surface functionalization, and the development of composite materials, scaffold-based delivery systems offer an ability to control the presentation of bioactive cues to MSCs, enhancing cell-scaffold interactions and therapeutic outcomes. Moreover, investigations on the molecular interactions between MSCs and integrated scaffolds have provided valuable mechanistic insights, guiding the design of next-generation scaffolds. Future endeavors should focus on integrating emerging technologies in biofabrication and stem cell therapy to further elevate the potential of MSC therapies in regenerative medicine.
Conceptualization, AJRB, AM, DKMB, BMM, and MPM; data curation, AJRB, AM, DKMB, and BMM; writing—original draft preparation, AJRB, AM, DKMB, and BMM; writing—review and editing, AJRB, AM, DKMB, BMM, and MPM; supervision, MPM; funding acquisition, MPM; All authors have read and agreed to the published version of the manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity. All authors contributed to editorial changes in the manuscript.
Not applicable.
Not applicable.
This research was funded by the National Institutes of Health-National Heart, Lung, and Blood Institute (1R01HL141831-01 and 1R01HL159960-01A1).
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.