- Academic Editors
Disruption of lipoprotein metabolism plays an important role in the development of several cardiovascular, inflammatory, and metabolic diseases. This review examines the importance of different types of lipoproteins and the role they play in the development of dyslipidemia in obesity. The causes and consequences associated with the disruption of lipid metabolism and its significance in the pathogenesis of obesity are considered. The relationship between such pathological processes, which occur alongside obesity as dyslipidemia and inflammation, is determined. In view of the current efficacy and toxicity limitations of currently approved drugs, natural compounds as potential therapeutic agents in the treatment of obesity are considered in the review. The complex mechanisms of lipid metabolism normalization in obesity found for these compounds can serve as one of the confirmations of their potential efficacy in treating obesity. Nanoparticles can serve as carriers for the considered drugs, which can improve their pharmacokinetic properties.
Obesity is among the most prevalent diseases considered preventable. Moreover, obesity is a significant issue for public health. Obesity has a multifactorial etiology that includes genetic, environmental, socioeconomic, and behavioral or psychological influences. Obesity results from a chronic positive energy balance, which is regulated by a complex interaction between endocrine tissues and the central nervous system [1].
An estimated 500 million adults worldwide are obese, with 1.5 billion classed as overweight or obese. In particular, the prevalence of obesity, or a combination of overweight and obesity, has increased in Brazil, Canada, Mexico, and the United States. Most of the information on obesity in adults relies on using body mass index (BMI) to define obesity [2].
Being obese raises the risk of several illnesses and ailments that are linked to higher death rates. Among these are non-alcoholic fatty liver disease (NAFLD), depression, osteoarthritis, obstructive sleep apnea, coronary artery disease (CAD), a condition called metabolic syndrome (MS), chronic renal failure, hyperlipidemia, and hypertension. Treating these illnesses can put additional strain on the healthcare system: For example, obese patients are thought to have 30% higher medical costs than people with a normal BMI. Since the corresponding total healthcare costs are doubling every decade, treating the consequences of obesity is a costly problem for patients [3].
Obesity has inflammatory components that are directly or indirectly associated
with major chronic diseases, such as diabetes, atherosclerosis, hypertension, and
some types of cancer. In overweight and obese individuals, circulating levels of
inflammatory cytokines, such as IL-6, TNF-
Obese people frequently have issues related to their lipid metabolism. Between 60 and 70 percent of obese individuals have dyslipidemia. Increased concentrations of oxidized low-density lipoproteins (ox-LDL) result from lipid disorders in obese individuals, which also include raised levels of triglycerides, low high-density lipoproteins (HDL), apolipoprotein B, and very-low-density lipoproteins (VLDL) in the blood [5]. Indeed, ox-LDLs are important pathological mediators in obesity, increasing inflammation and disrupting normal vascular tone [6]. Increased ox-LDL levels result from cellular oxidative stress, which is associated with the development of many chronic diseases [7, 8]. Oxidative stress is a key factor that links the pathogenesis of obesity and metabolic syndrome [6, 9]. Thus, methods for lowering modified lipids and restoring normal lipid metabolism may be appealing targets for managing obesity-related inflammatory processes.
There is ongoing debate over the fundamental causes of obesity. The basic physiological principle that fat storage is caused by a difference between calories ingested and expended is the basis of current medical guidelines for preventing obesity. The rise in energy brought on by the increased availability of foods rich in calories and high in nutrition has played a major role in the obesity epidemic. Diet and various social, economic, and environmental factors associated with nutrition significantly impact the patient’s ability to achieve balance [10]. In a 13-year follow-up study of 3000 young people, it was found that those who consumed much more fast food weighed an average of about 6 kg more and had a larger waist circumference than those who received less fast food. They were also found to be more likely to experience health problems associated with negative weight, such as elevated triglycerides, and were twice as likely to develop metabolic syndromes. These problems are exacerbated in some individuals who have a genetic predisposition for fat storage, which may be due to the significant interplay between homeostatic circuits and brain reward. Accumulation of lipid metabolites, inflammatory signaling, or other mechanisms of hypothalamic neuronal impairment may also lead to obesity, which may explain the biological defense of increased body fat mass [11].
In recent years, there has been a significant increase in our understanding of gut microbiota and its intricate link to illness. For instance, compared to thinner hosts, obesity is linked to alterations in the gut milieu that facilitate a wider variety of viral species. The emergence of pathogenic mutations that might lead to more severe illnesses is more likely in this setting [12]. An increasing amount of data indicates that changes in the gut microbiota impact the weight and metabolism of the host. For instance, sterile male mice (without intestinal microbiota) had 42% less total body fat than mice with normal gut bacteria despite consuming 29% more food daily. Conversely, total body fat increased by 57%, lean body mass decreased by 7%, and daily food consumption reduced by 27% in these mice following the colonization of the caecum by microorganisms [13]. A follow-up investigation revealed that these alterations resulted from a decrease in metabolic rate and an increase in adipose tissue deposition, as the microflora colonization caused a 25% increase in capillary density in the small intestine’s distal villi. Comparable outcomes were seen in female mice [14].
One method to categorize the genetic causes of obesity is monogenic, which is caused by mutations in a single gene, usually found in the leptin–melanocortin pathway. Numerous genes, including melano-cortin-4 receptor (MC4R), orexogenic (PYY), and agouti-related peptide (AgRP), have been linked to monogenic obesity. These genes disrupt the system that regulates appetite and weight, as receptors in the arcuate nucleus of the hypothalamus perceive hormonal signals such as insulin, leptin, and ghrelin [15]. Severe obesity brought on by aberrant nervous system development and associated organ/system abnormalities is known as syndromic obesity. The source may be a broader chromosomal area covering numerous genes or alterations to a single gene [16].
Although several genes linked to monogenic types of obesity have been found by this research, the human genome is changing over too long a period for the genome to be significantly involved in the present obesity epidemic. Nonetheless, without requiring a significant alteration to the DNA, epigenetics can provide a reasonable explanation for the rise in the incidence of obesity during the previous few decades [17]. Each cell type may display the genetic code differently in multicellular animals, yet the genetic code is uniform across the body. Nucleotide sequence alterations are unnecessary for inherited regulatory modifications in genomic expression, as demonstrated by epigenetic research [18]. One way to conceptualize epigenetic alterations is as differential DNA packaging that either promotes or inhibits the expression of particular genes in various tissues. The environment and intestinal microbes can influence the epigenetic programming of parental gametes and programming in later life stages [19].
Histone modifications, microRNA-mediated regulation, and DNA methylation are examples of known epigenetic processes, which can be handed down from generation to generation through meiosis or mitosis. Data indicate that the period of perinatal and embryonic–fetal development significantly influences the programming of human organs and tissues [18]. The most significant epigenetic method for controlling gene expression seems to be DNA methylation. Variations in DNA methylation have been linked to several illnesses, including cancer [20]. Leptin, also known as LEP, is crucial for controlling adipose tissue. The DNA methylation in the LEP profile at birth may be influenced by the metabolic remodeling associated with obesity due to the maternal metabolic state [21].
Lipoproteins are complex particles that comprise free cholesterol, phospholipids, and apolipoproteins, which help lipoproteins form and function [22]. The central core of lipoproteins contains cholesteryl esters and triglycerides [23]. Based on size and lipid structure, serum lipoproteins can be categorized into seven classes: apo-LP, VLDL, LDL, HDL, chylomicrons, and chylomicron remnants [24].
The exogenous lipoprotein pathway begins when dietary lipids are incorporated into intestinal chylomicrons. Triglycerides carried by chylomicrons are transformed into free fatty acids, then chylomicrons are transported into the adipose and muscular tissue to produce chylomicron remains. Then, the liver absorbs the chylomicrons remnants [24]. The endogenous lipoprotein pathway starts when VLDL is formed in the liver. After transforming to free fatty acids in muscle and fat tissue, the triglycerides in VLDL are converted to LDL, which is subsequently absorbed by the LDL receptor in various tissues, including the liver [24]. An imbalance in the plasma levels of lipids and lipoproteins is called dyslipidemia and is directly related to the pathogenesis of obesity.
The dyslipidemia that is observed in obese people is caused by a variety of anomalies [5]. These anomalies result from a confluence of factors, including insulin resistance, increased liver absorption of free fatty acids due to visceral and total adiposity, and an inflammatory state brought on by macrophages entering adipose tissue [25]. The primary abnormality is the overproduction of VLDL particles by the liver, which plays a significant role in the increase in blood triglyceride levels [26]. The number of fatty acids available for triglyceride synthesis in the liver determines the availability of triglycerides, which in turn affects the secretion rate of the VLDL particles. Triglycerides are abundant because they inhibit the liver’s ability to break down apoB100, which increases its synthesis and release of VLDL.
Fatty acids in the hepatocytes come from three primary sources, all of which can change in obese individuals [5]. First, the number of fatty acids sent to the liver increases with a rise in the bulk of adipose tissue, particularly visceral tissue. Furthermore, insulin increases the number of triglycerides that are delivered to the liver by inhibiting the lipolysis of triglycerides into free fatty acids in adipose tissue [26]. Additionally, de novo fatty acid synthesis is the second source of fatty acids for the liver. The hyperinsulinemia observed in individuals with insulin resistance may act as a mediating factor for this rise [27]. Specifically, insulin enhances the expression of enzymes needed for fatty acid synthesis by activating the SREBP-1c transcription factor. The liver is susceptible to the effects of insulin, which promotes fat synthesis, even if it is resistant to the effects of insulin on the metabolism of carbohydrates [28]. The absorption of high triglyceride lipoproteins by the liver is the third way of obtaining fatty acids. Research has indicated that increased fatty acid production in the colon, along with elevated chylomicron secretion in obese individuals, results in enhanced fatty acid transport to the liver. Increased triglyceride synthesis and the preservation of apoB100 from degradation result from a boost in fatty acids in the liver via these three routes, which increases VLDL production and secretion [29]. Moreover, consuming more calories may enhance the circulation of triglycerides via two different mechanisms: either dietary fat raises chylomicron triglyceride levels as well as facilitates the supply of fatty acids to the liver, or dietary carbohydrates stimulate de novo hepatic lipogenesis [30].
Apart from the liver and intestines producing excessive amounts of lipoproteins that are high in triglycerides, there are also disruptions in the following metabolism of these lipoproteins [5]. The concentration of apoC-III is elevated in obese individuals [27]. Insulin inhibits the expression of apoC-III; hence, the rise in apoC-III levels in obese individuals may be explained by insulin resistance [31]. Since apoC-III inhibits lipoprotein lipase activity, it may decrease the clearance and cellular absorption of lipoproteins high in triglycerides, which raises the levels of triglycerides in the blood of obese individuals [27].
Other lipoproteins are impacted by a rise in triglyceride-rich lipoprotein levels. Specifically, the equimolar exchange of chylomicrons and triglycerides from triglyceride-rich VLDL for cholesterol from LDL and HDL is mediated by cholesterol ester transfer protein (CETP) [5]. Triglycerides rise, CETP-mediated metabolism increases, and LDL and HDL cholesterol drop when triglyceride-rich lipoproteins increase in and of themselves. Furthermore, obesity raises the bulk and activity of CETP. The widely noted reciprocal link between low HDL cholesterol and high triglycerides and raised HDL cholesterol and lowered triglycerides is caused by this CETP-mediated exchange [32].
Subsequently, hepatic lipase and lipoprotein lipase hydrolyze the triglyceride of LDL and HDL, forming tiny, dense particles for the two lipoproteins [5]. Individuals with adiposity and increased visceral obesity have significantly higher levels of hepatic lipase activity, which helps to separate triglycerides from LDL and HDL and forms lipoprotein particles [33]. Reduced sensitivity of apo A-I for small HDL particles causes apo A-I to dissociate, which the kidneys remove and destroy [26]. In obese persons, these modifications lead to decreased levels of HDL cholesterol and apo A-I.
Owing to the infiltration of macrophages into adipose tissue, obesity is considered an inflammatory condition. Lipid metabolism is also influenced by adipokines generated by fat cells and cytokines made by macrophages [34].
Lipid metabolism is regulated by adipokines, such as resistin and adiponectin. Obese people have lower amounts of adiponectin in their blood. Reduced HDL-cholesterol (HDL-C) levels and elevated blood triglycerides are linked to decreased adiponectin levels [34]. Mice studies have demonstrated that overexpressing adiponectin reduces triglycerides and raises HDL-C, while adiponectin-knockout animals, on the other hand, increase triglycerides and decrease HDL-C. These data suggest that the link between the two variables is causative. Increased degradation of triglyceride-rich lipoproteins due to elevated lipoprotein lipase activity and decreased levels of apoC-III, a molecule that inhibits lipoprotein lipase, mediates the adiponectin-induced reduction in triglyceride levels. Increases in hepatic apo A-I and ABCA1 facilitate the rise in HDL-C level caused by adiponectin, increasing HDL particle synthesis [35].
Human monocytes and macrophages produce the adipokine resistin. Obese people have higher resistin levels, and there is a clear correlation between resistin and plasma triglyceride levels. Furthermore, resistin has been demonstrated to increase the production of apoB, triglycerides, and cholesterol in the liver, stimulating the creation and secretion of VLDL [36]. Resistin is also linked to decreased levels of apo A-I and HDL cholesterol. It is also commonly recognized that resistin plays a part in inflammation. By promoting resistin gene expression, inflammatory cytokines cause macrophages to secrete more resistin, which encourages the synthesis of proinflammatory cytokines [37].
Tumor necrosis factor
TNF-
The effects of cytokines and adipokines on lipid metabolism and other manifestations of obesity pathogenesis are shown in Table 1 (Ref. [34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45]). The effects of pro-inflammatory cytokines are depicted on the Fig. 1.
| Cytokine (adipokine) | Level changes in obesity | Direct impact | Implications for the pathogenesis of obesity |
| Adiponectin | Decreased | (1) Increased apoC-III level and decreased lipase activity [35] | (1) Increased triglyceride level [34] |
| (2) Decreased levels of apo A-I and ABCA [35] | (2) Decreased HDL-C level [34] | ||
| Resistin | Increased | (1) Increased synthesis of apoB, triglycerides, and cholesterol [36] | (1) Increased VLDL level [36] |
| (2) Increased expression of proinflammatory cytokines [37] | (2) Increased inflammation [37] | ||
| TNF- |
Increased | (1) Decreased expression of GLUT4 [38] | (1) Insulin resistance [38] |
| (2) Increased oxidative stress [40] | (2) Enhanced lipid peroxidation [40] | ||
| (3) NF- |
(3) Increased inflammation [39] | ||
| TNF- |
Increased | (1) Increased synthesis of fatty acids and triglycerides [41] | (1) Increased secretion of VLDL [41] |
| (2) Decreased expression of lipase and ANGPLT4 [42, 43] | (2) Decreased clearance of triglyceride-rich lipoproteins [44] | ||
| Other proinflammatory cytokines | Increased | (1) Decreased production of apo A-I [45] | (1) Decreased HDL level [45] |
| (2) Decreased expression of ABCA1 and ABCG1 [45] | (2)–(5) Decreased reverse cholesterol transport [45] | ||
| (3) Decreased production of LCAT [45] | |||
| (4) Decreased CEPT level [45] | |||
| (5) Decreased expression of SR-B1 [45] |
*The numbers in the column: ⟪Direct impact⟫ correspond to the numbers in the column: ⟪Implications for the pathogenesis of obesity⟫. TNF-
 Fig. 1.
Fig. 1.The influence of proinflammatory cytokines on lipoproteins in obesity. TG, Triglyceride.
The current approach to obesity therapy includes drugs such as orlistat, rimonabant, sibutramine, dexfenfluramine, fenfluramine, or phenylpropylamine [46]. However, these drugs have low efficacy and cause systemic drug toxicity and multiple side effects associated with mental disorders and cardiovascular diseases, which limits their use [47]. To overcome these limitations, innovative drug delivery vehicles, such as nanoparticles based on gold, phosphatidylcholine, and cholesterol; PLGA-b-PEG, PLGA, dextran, and dextran-PEG; liposomes based on peptides; nanoemulsions and lipase-sensitive nanocarriers have been developed to improve the therapeutic efficacy of targeted synthetic drugs [30].
A recent study described nanotechnology-based therapy as an alternative strategy using three different strategies: Inhibiting angiogenesis in white adipose tissues (WAT), converting WAT to brown adipose tissue (BAT), and photothermal lipolysis WAT. Generic nanocarriers (liposomes, polymeric, and gold nanoparticles) demonstrated high tolerability, reduced side effects, and increased efficacy in a reproducible manner, thus, supporting the concept of the hypothesis that targeted nanotherapy may act as a feasible tactic to combat obesity and prevent its comorbidities [48].
Many medicinal plants have been studied to treat obesity, dyslipidemia, and diabetes. Herbal medicines are considered an effective treatment strategy due to their established mechanisms of action, which are directed to lipid metabolism normalization [49]. A previous study showed [50] that synthetic drugs and natural compounds could modulate GLP1 expression. Such compounds, including curcumin, berberine, resveratrol, and cinnamon, can be effectively used as GLP1 agonists to reduce hyperglycemia. It was demonstrated in a clinical trial [51] that combination therapy with resveratrol and myoinositol, compared with metformin and pioglitazone, resulted in significantly better results in weight loss and increased adiponectin concentrations in obese women with polycystic ovary syndrome. Curcumin positively reduced the inflammatory cytokine monocyte chemoattractant protein 1 (MCP-1) in obese patients, although there was no change in resistin and visfatin levels [52]. In a clinical study [53], patients in the experimental group received capsules containing Momordica charantia extract. They had significant reductions in triglycerides and very low-density lipoprotein (VLDL) levels compared to the placebo group, although the decrease in body weight was not statistically significant. Therefore, these compounds can be potentially used in treating diseases whose pathogenesis is connected with impaired lipid metabolism, including obesity. Discovered mechanisms of action of herbal medicinal compounds are shown in more detail in Fig. 2.
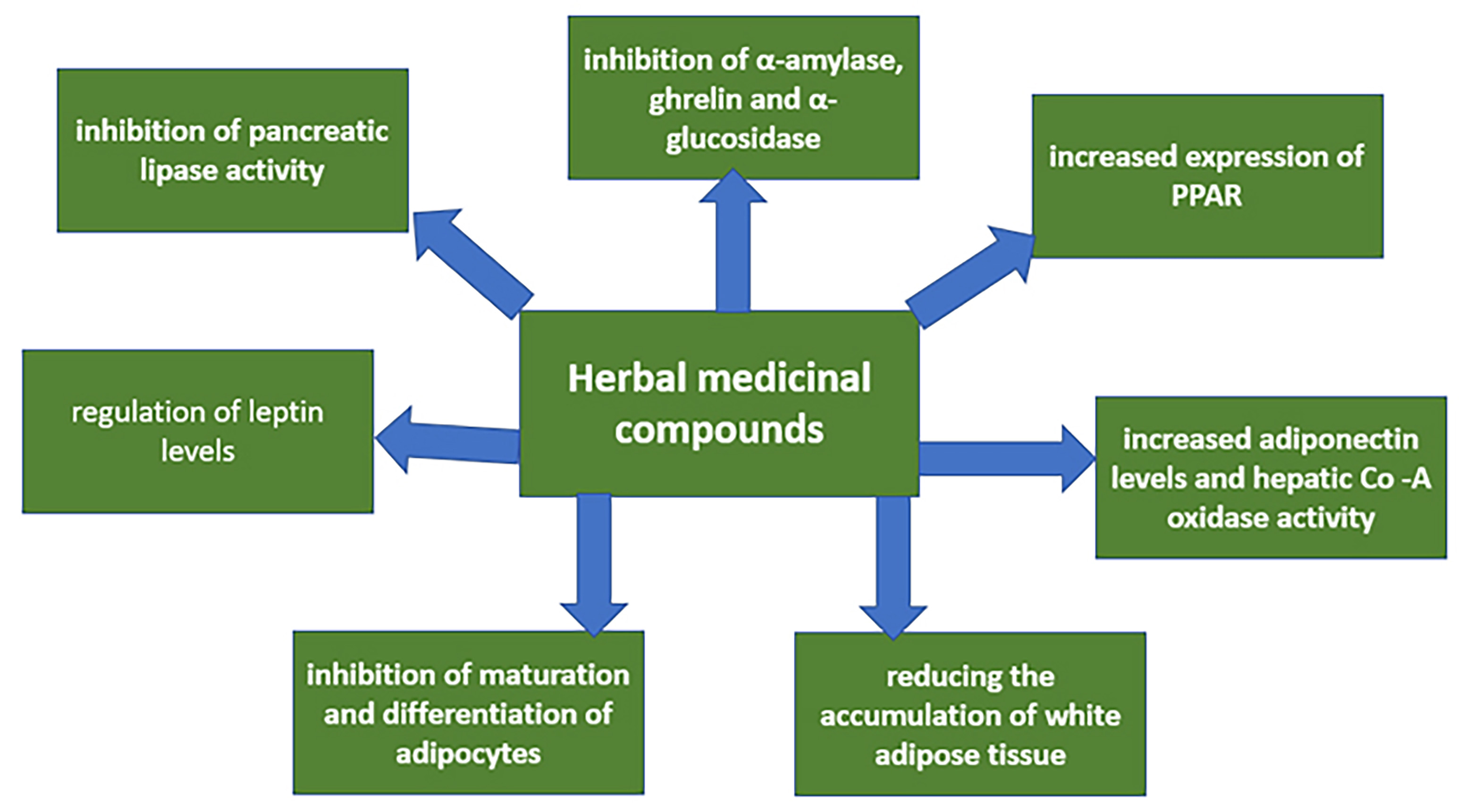 Fig. 2.
Fig. 2.Discovered mechanisms of action of herbal medicinal compounds. Legend: As can be seen from this diagram, specific molecular mechanisms of action of these compounds have been identified for a number of medicinal plant extracts. These mechanisms can be aimed at different processes but have a common result—decreased dyslipidemia. This allows us to consider plant compounds an effective and safe alternative to existing obesity therapy.
Based on the features of the influence of lipoproteins on the pathogenesis of
obesity described in this review, several important issues that were not
addressed in this review require further, more detailed consideration in future
research. First of all, it is of interest to study in detail the changes in the
concentration, distribution, and metabolic transformation of some types of
lipoproteins, such as chylomicrons, which are not fully described in this
article. In terms of the development of new therapeutic agents, considering the
multiple effects of TNF-
Dyslipidemia is a comorbid condition of various metabolic conditions, including
obesity. Initially, it manifests as an increase in the content of VLDL particles,
which is caused, conversely, by the overproduction of triglyceride-rich
lipoproteins and, on the other hand, by an imbalance in the processes of their
subsequent distribution, which is associated with inhibition of lipoprotein
lipase activity. These disorders, in turn, affect the metabolism of other
lipoproteins, including cholesterol ester transport proteins. Concurrently,
dyslipidemia is also strongly influenced by inflammatory factors, including
adipokines, such as resistin and adiponectin, and proinflammatory cytokines,
primarily TNF-
AB, VS and AO designed the review plan. KR, AG, EP and KI performed the data collection and analysis. AG, EP and AB wrote the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to its accuracy or integrity. All authors contributed to editorial changes in the manuscript.
Not applicable.
Not applicable.
This work was supported by the Russian Science Foundation (Grant # 22-65-00005).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
