Sepsis-associated encephalopathy is a common brain diseases, presenting severe
diffuse brain dysfunction. The umbilical cord mesenchymal stem cells have been
reported to have protective role for treating diseases, while its role in
sepsis-associated encephalopathy remained elusive. This brief report investigated
the therapeutic effect of umbilical cord mesenchymal stem cells on
sepsis-associated encephalopathy in mice model and uncovering the underlying
mechanism. The sepsis-associated encephalopathy mice were injected with 3 mg/kg
lipopolysaccharide. An enzyme-linked immunosorbent assay was carried out to
determine the production of inflammatory cytokines. Morris water maze test was
used to evaluate mice’s neurological dysfunction. Cell apoptosis and tissue
injury of the cerebral cortex were assessed using terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay and HE staining. Evans
Blue leakage detection was used to examine the blood-brain barrier integrity. The
protein levels were determined using Western blot. Results showed that
the productions of inflammatory cytokines including interleukin 6 (IL-6),
interleukin-1
Sepsis-associated encephalopathy (SAE) is a severe sepsis-related diffuse brain dysfunction without suffering from direct infection in central nervous system [1]. It is one of the most common brain diseases in intensive care unit (ICU), which seriously threatens patients’ health [2]. The pathogenesis of the disease is based on the invasion of bacteria, viruses, or other pathogens, causing acute infection outside the central nervous system and systemic response syndrome [3]. The clinical manifestations of SAE mainly include somnolence, coma, and cognitive impairment [3]. Previous studies have revealed that SAE was an independent predictor of death [3, 4]. At present, the primary therapeutic strategy for SAE is still limited to managing potential infections [5]. Therefore, it is imperative to explore the effective therapeutic strategy for SAE patients.
In recent years, stem cell therapy has been one of the most promising
therapeutic strategy for treating neurologic diseases [6]. Various stem cells
derived from neural stem cells, mesenchymal stem cells (MSCs), and umbilical cord
blood were considered as therapeutic options for disease treatment [6]. Among
these stem cells, MSCs possessed multilineage differentiation, self-renewal,
proliferation potential, and the small dosage, making it a valuable therapeutic
tool in clinic [7]. MSCs can be isolated from dental pulp, peripheral blood,
umbilical cord and bone marrow [8]. However, Shetty et al. [7] report
that the MSCs from the umbilical cord are dependable sources of an unlimited
number of MSCs for regenerative medicine. At present, umbilical cord mesenchymal
stem cells (UC-MSCs) have shown therapeutic roles for treating many diseases in
animal models. For example, Xiang et al. [9] proved that UC-MSCs can
inhibit inflammation and renal fibrosis, resulting in suppressing the development
of early diabetic nephropathy. Liu et al. [10] found that UC-MSCs
improved the joint damage and osteogenesis in collagen-induced arthritic mice by
suppressing TNF-
Besides, UC-MSCs have exhibited protective effects on sepsis-related diseases. Zhang et al. [12] reported that human UC-MSCs exosomes could attenuate sepsis-associated acute kidney injury through modulating miR-146b expression. A phase 1 clinical trial of UC-MSCs for treatment of severe sepsis showed that UC-MSCs was safe and well-tolerated and had an excellent therapeutic effect on patients without adverse reactions in 15 patients [13]. However, the effect of UC-MSCs on SAE remained elusive. Hence, we investigate the role of UC-MSCs in the SAE mice and explore the underlying mechanism.
Human UC-MSCs were purchased from ATCC (American Type Culture Collection, Manassas, VA, USA). UC-MSCs were cultured with Mesenchymal Stem Cell Basal Media containing growth factors (Mesenchymal Stem Cell Growth Kit, American Type Culture Collection, Manassas, VA, USA).
Eighteen male C57BL/6 mice aged 4–6 weeks were acquired from Beijing Laboratory
Animal Research Center (Beijing, China). All mice were divided into three groups
(6 mice in each group): sham group, LPS group, and LPS + UC-MSC group. To
determine the effect of UC-MSCs on SAE, the SAE model was first established by
injecting mice with lipopolysaccharide (LPS). Except for the mice in the sham
group, 3 mg/kg LPS were injected into all mice intraperitoneally to induce SAE
[4]. After 1 h post LPS injection, the mice in the LPS + UC-MSC group were
injected with UC-MSCs (1
The secretion of inflammatory cytokines in SAE mice’s brain tissues after
administrating with UC-MSCs was determined by ELISA assay. After euthanasia of
mice, brain tissues of mice were collected to measure the production of
interleukin 6 (IL-6), tumor necrosis factor-
Brain tissue cell lysate was isolated with RIPA lysis reagent (Beyotime
Biotechnology, Shanghai, China). The lysate concentration was quantified by the
BCA kit (Beyotime Biotechnology, Shanghai, China). The lysate was then subjected
to SDS-PAGE and transferred to the PVDF membrane. Subsequently, the PVDF
membranes were blocked with 5% non-fat milk and probed with primary antibodies
including anti-p-NF-
Cell apoptosis of cerebral cortex in SAE mice after administration with UC-MSCs
was detected using TUNEL assay. The Click-iT
Morris water maze test was carried out to evaluate mice’s neurological
dysfunction as previously described [14, 15, 16]. The water maze consisted of a
circular black pool (100 cm diameter, 38 cm deep), and it was filled with opaque
water (25 cm deep). The water was prepared as black through adding non-toxic
pigment, and the temperature of the water was kept at 23
HE staining was conducted to assess cerebral cortex neuron injury in SAE mice
after administration of UC-MSCs. After euthanasia of mice, brain tissues of mice
were collected to detect neuron damage of the cerebral cortex. Tissue samples
were fixed in 10% formalin and embedded into paraffin. Subsequently, 4
To evaluate blood-brain barrier (BBB) integrity, mice in the different groups were collected to conduct Evans Blue staining. After mice were anesthetized, 2% Evans Blue (3 mL/kg) in sterile saline solution was injected into the tail vein of mice. After 1 h circulation, mice were transcardially perfused with cold saline. After that, mice were sacrificed, and the brain tissues were removed and weighed. The tissues were then prepared as homogenate, which was centrifuged for 20 min at 10000 g. After centrifugation, the supernatant was collected and the absorbance was determined at 620 nm.
All data were presented as mean
The production of IL-6, IL-1
 Fig. 1.
Fig. 1.UC-MSCs inhibited the production of inflammatory factors in SAE
mice. (A) The productions of IL-6, IL-1
Cell apoptosis was remarkably induced by LPS in the cerebral cortex of SAE mice,
which was inhibited by UC-MSCs treatment (p
 Fig. 2.
Fig. 2.UC-MSCs inhibited cell apoptosis of the cerebral cortex in SAE
mice. (A) Cell apoptosis of cerebral cortex in UC-MSCs-treated SAE mice was
evaluated using TUNEL assay. (B) The protein levels of cleaved caspase-3, Bax,
and Bcl-2 were detected by Western blot. **: p
The average body weight was significantly decreased in SAE mice compared to sham
mice (p
 Fig. 3.
Fig. 3.UC-MSCs alleviated the cognitive dysfunction of SAE mice.
(A) The average body weight of mice in sham, LPS, and LPS+ UC-MSCs
groups was determined. (B) The latency to find the platform of mice in sham, LPS,
and LPS+ UC-MSCs groups were recorded during the Morris water maze test.
(C) Times of crossing the platform of mice in sham, LPS, and LPS+
UC-MSCs groups were recorded during Morris water maze test. (D) The swimming
speed mice in sham, LPS, and LPS+ UC-MSCs groups were determined during Morris
water maze test. (E) The movement routes of mice in sham, LPS, and LPS+
UC-MSCs groups were recorded during the Morris water maze test. **: p
The cerebral cortex of sham mice presented a normal histological structure with
regular architecture and clear boundary (Fig. 4A). In SAE mice, cerebral cortex
neuron injury was observed, characterized by condensed and hyperchromic nuclei,
smaller cell bodies with perineuronal vacuolations around the degenerative
neurons (Fig. 4A). However, UC-MSCs administration improved the abnormal
morphology of the cortex and decreased the number of degenerated neurons (Fig. 4A).
Moreover, Evans Blue leakage detection experiment was conducted to evaluate
BBB integrity. It was observed that Evans Blue leakage was increased in SAE mice
(p
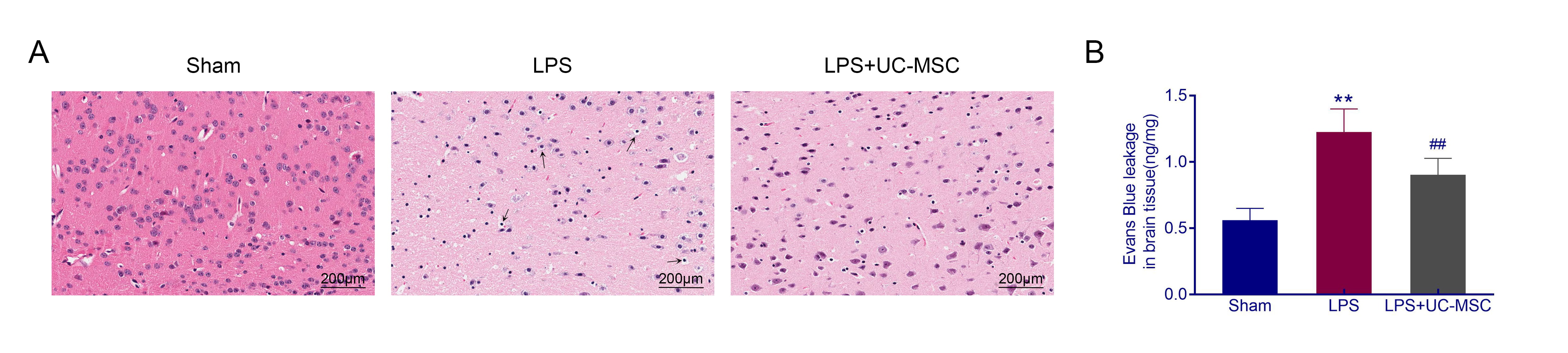 Fig. 4.
Fig. 4.UC-MSCs alleviated the cerebral cortex neuron injury of SAE
mice. (A) HE staining was conducted to assess cerebral cortex neuron injury of
UC-MSCs-treated SAE mice. (B) BBB integrity was assessed by Evans Blue leakage
detection experiment. The black arrows indicated damaged neurons. **: p
The phosphorylation of PI3K and AKT were decreased in SAE mice (p
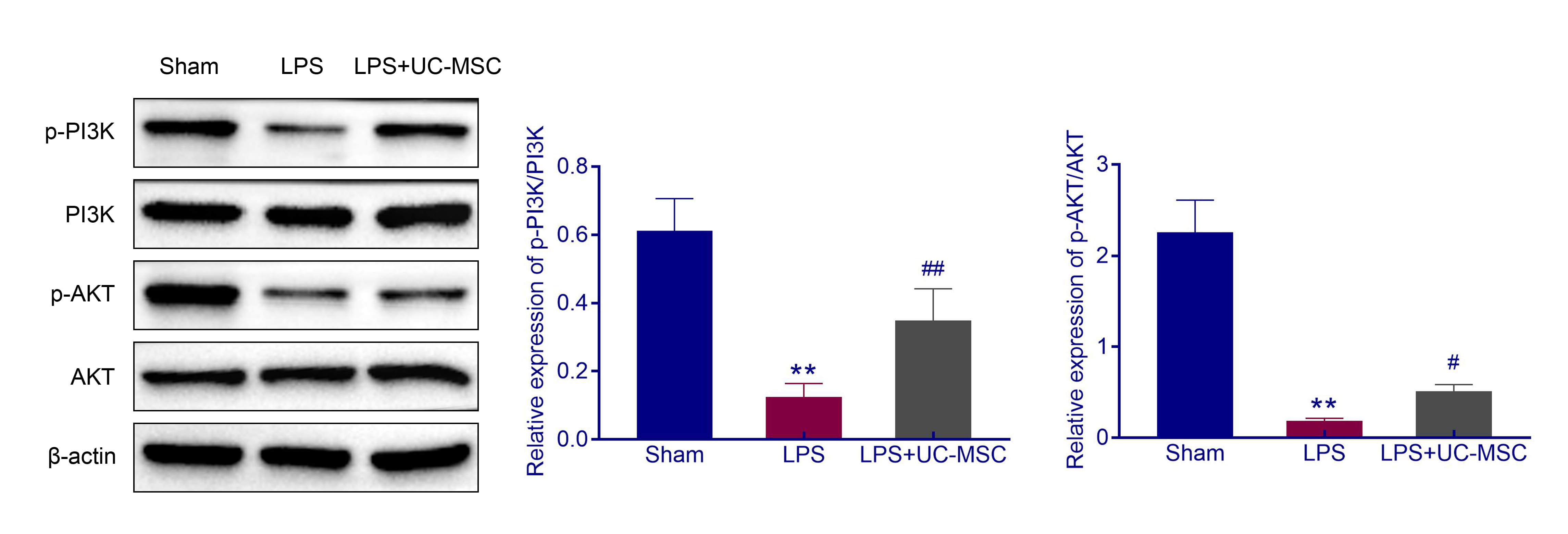 Fig. 5.
Fig. 5.UC-MSCs activated PI3K/AKT pathway. The protein levels of
p-PI3K, p-PI3K, p-AKT, and AKT in brain tissues were detected using Western blot.
**: p
As one of the most common brain diseases in the ICU, SAE presented as a severe diffuse brain dysfunction accompanied by cognitive dysfunction [1, 2]. At present, controlling inflammation is still the primary treatment for SAE patients, although long-term neurocognitive deficits remain to be resolved [5]. Therefore, it is essential to search for a promising strategy for treating neurocognitive deficits in SAE patients. The protective roles of UC-MSCs in treating diseases including sepsis have been found [12]. However, its role in SAE was unclear. Therefore, this work focused on investigating the effect of UC-MSCs on SAE and uncovering the potential mechanism.
To determine the role of UC-MSCs in SAE, the SAE were constructed in mice. As a
component of gram-negative bacteria cell wall, LPS is a mediator
of sepsis [1, 5]. LPS has been extensively used to induce sepsis and its related
complications in vitro and in vivo [5]. Therefore, in this
work, SAE mice models were established through LPS administration. The
pathogenesis of SAE is based on the invasion of bacteria, viruses, or other
pathogens in very old cases, young cases, pregnant women, or cases with severe
injuries, weakened immune systems, catheters, or a breathing tube. Therefore,
neuroinflammation was found in SAE [17] accompanied by increased production of
pro-inflammatory factors such as early pro-inflammatory factor IL-6,
IL-1
Previous research revealed that the pathology and histopathology of SAE were mainly involved in the cerebral cortex, while rarely affected the deeper structures and the spinal cord [23, 24]. Thus, the cerebral cortex damage in SAE mice was evaluated. Results showed that cerebral cortex cell apoptosis and neuron injury were observed in SAE mice, consistent with [25, 26] results. The previous study revealed that UC-MSCs could inhibit cell apoptosis of injured neurons induced by hypoxic-ischemic injury [27]. UC-MSCs alleviated neurological disorders via suppressing mitogen-activated protein kinase pathway-mediated apoptosis [28]. To further investigate the potential of UC-MSCs in treating SAE, the effect of UC-MSCs in cerebral cortex cell apoptosis and neuron injury was assessed. It was observed that UC-MSCs administration inhibited cell apoptosis and cerebral cortex neuron injury of cerebral cortex in SAE mice.
Cognitive dysfunction was a significant symptom of SAE clinically related to increased mortality [5]. Therefore, the protective effect of UC-MSCs on cognitive dysfunction in SAE mice was explored. Results indicated that the UC-MSCs alleviated the cognitive dysfunction of SAE mice. The beneficial effect of UC-MSCs on improving cognitive dysfunction has been reported previously [29]. Zhou et al. [29] found that UC-MSCs transplantation effectively improved cognitive and neurological function caused by traumatic brain injury. These evidences confirmed the function of UC-MSCs in alleviating cognitive impairment. In addition to controlling inflammation, UC-MSCs also improved cognitive impairment, which may be an advantage for UC-MSCs as a treatment strategy for SAE.
Finally, the potential mechanism of UC-MSCs’ protective effect on SAE was explored. The findings revealed that UC-MSCs increased the phosphorylation of PI3K and AKT. In other words, UC-MSCs activated PI3K/AKT pathway. PI3K/AKT pathway has been reported to participate in the SAE development, and the inhibition of this pathway contributed to improving SAE [15, 30]. Tang et al. [30] revealed that Metformin attenuated sepsis-induced brain injury by suppressing oxidative stress, neuroinflammation and apoptosis via regulating the PI3K/AKT pathway. Therefore, these findings suggested that UC-MSCs might protect mice from SAE via activating the PI3K/AKT pathway.
UC-MSCs alleviated inflammation, cell apoptosis and neuron injury of the cerebral cortex, and cognitive dysfunction of SAE, making UC-MSCs therapy a promising therapeutic strategy for SAE treatment.
In this study, the survival time of mice after LPS injection and long-lasting effect of administration of UC-MSCs in SAE model remained elusive. These problems will be furtherly explored in the future study.
SAE, Sepsis-associated encephalopathy; UC-MSCs, umbilical cord mesenchymal stem cells; LPS, lipopolysaccharide; ELISA, enzyme-linked immunosorbent assay; BBB, blood-brain barrier; MSCs, mesenchymal stem cells.
ZZ and LW designed the study, supervised the data collection, FL analyzed the data, interpreted the data, XQ, ZH, LW, YJ and HH prepare the manuscript for publication and reviewed the draft of the manuscript. All authors have read and approved the manuscript.
Ethical approval was obtained from the Ethics Committee of Zhejiang Chinese Medicine University (Approval No. 201809-0290).
Thanks to all the peer reviewers for their opinions and suggestions.
This work was supported by the Science and Technology Development Program of Hangzhou City, Zhejiang Province (Grant No. 20201203B56).
The authors declare no conflict of interest.
