Academic Editor: Rafael Franco
Background: Although the occurrence of optokinetic reflex (OKR) adaptation after OKR training is well established, the dynamic properties of OKR adaptation has not been fully studied. This study aimed to examine the difference in the amount of OKR adaptation according to OKR training protocols which have different frequency or amplitude of drum oscillation. Methods: Using C57BL/6N male mice, we induced OKR adaptation by 3 different categories of learning paradigm as follows: (1) Optokinetic drum oscillation for 60 min with same amplitude and different frequency. (2) Optokinetic drum oscillation for 60 min with same frequency and different amplitude. (3) Training with serial combination of different frequency or amplitude. Results: The results show that the amount of OKR adaptation was greater after OKR training with lower frequency or amplitude than that with higher frequency or amplitude. Conclusions: This finding may suggest that the retinal slip signal with lower-velocity OKR stimulation serves as more precise instructive signal for learning, leading to induction of more efficient training effect. Another interesting finding was that the OKR gain increase tended to be greater after training composed of sequential combination of decreasing frequency or amplitude than that composed of sequential combination of increasing frequency or amplitude. Furthermore, the OKR training with high frequency or amplitude eliminated a part of learning effects which have already formed by previous training. We postulate that the stimulation during training with high frequency or amplitude may implement a disturbing instruction for OKR learning when it is conducted in mice with increased OKR gain after previous OKR training.
Optokinetic reflex (OKR) and vestibulo-ocular reflex (VOR) work together to maintain retinal images. The OKR functions well at low frequencies, and VOR functions well at high frequencies [1, 2]. The VOR uses vestibular input to accommodate for retinal slip in advance, while the OKR is controlled by the retinal slide itself. The OKR permits the eyeball to follow a moving image in the same way while the head remains constant, whereas the VOR causes eyeball movements in the reverse direction. The performance of OKR and VOR together is exceptionally well, resulting in minimum visual motion over a diverse variety of movement frequencies. The gain of OKR can be increased by prolonged oscillation of optokinetic drum in the absence of head movements, and this OKR gain adaption has been employed as a good experimental paradigm for researching cerebellum-dependent motor learning [1, 3, 4, 5, 6, 7, 8, 9]. The long-term depression at parallel fibers-Purkinje cell synapses and changes in intrinsic excitability of Purkinje cells in the flocculus have been suggested as mechanism for OKR motor learning [10, 11].
Although the occurrence of OKR adaptation after OKR training is well
established, the dynamic properties of OKR adaptation following training
protocols with different frequency and amplitude of optokinetic drum have not
been studies. For example, when we measure OKR gain with 0.5 Hz/5° drum
rotation, how much learning effect can be obtained if the training for OKR gain
learning is performed with different frequency (0.25 Hz or 1 Hz) or amplitude
(2.5° or 10°)? Furthermore, when we measure OKR gain with 0.5
Hz/5° drum rotation, how much learning effect can be obtained if the
training for OKR gain learning is performed with sequential increase of frequency
(0.25 Hz
The black eye C57BL/6N male mice (body weight 20–25 g, 8 weeks old, OrientBio, Seoul, Korea) were employed in the experiment. Mice were equipped for behavioral tests as mentioned previously [8, 10, 12, 13]. Briefly, under general anesthesia with isoflurane, a minimal incision in the scalp was made after application of lidocaine cream to reduce pain or discomfort. The head fixation pedestal was made with tow nuts and four screws. Nuts were put on bregma and lambda of the cranium, and screws were embedded between the nuts. Then, mice were permitted to recuperate for at least 3 days after surgery. A drop of physostigmine salicylic solution (Eserine, Sigma-Aldrich Korea, Seoul, Korea) was given to the eyes in preparation for eye movement recording to keep the pupil size stable during the recording. Mice were held in a factory animal holder in the middle of a turntable, and they were adapted to constraint in an animal carrier for 15 min in the dark and then 15 min in the light without any excitation. After two days of acclimation, calibration was performed, which is the process of converting 2-demensional linear visual stimuli on the screen into angular eye movement. The radius of pupil, which is necessary for computing eye movement gain, could be measured through the calibration process. The calibration equations and processes were based on those used in Stahl et al. [14], and mice and the container were put at the same location so that calibration could be done at recordings after calibration.
CCD camera (IPX – VGA210, IMPERX, Boca Raton, FL, USA) with infrared (IR) filter (LP830) was used to capture the image of the eye, which was then transferred to a desktop PC using a camera link grabber board (PCI – 1426, National Instruments, TX, USA). A single IR-LED was put around the camera to establish a reference corneal reflex for calibration as well as IR illumination was produced using IR-LED. A motor-mounted drum with alternating black and white vertical stripes was used to provide optokinetic stimulation (AKM22E – VBBNR – 00, Kollomorgen, Radford, VA, USA). For the output and input between the PC and the monitor, a data collection (DAQ) PCI board (PCI – 6230, National Instruments, Austin, TX, USA) was utilized. Several virtual instruments built in LabView (National Instruments, Austin, TX, USA) were used to process the obtained images.
To assess the baseline performance of the mice utilized in the research, three
baseline ocular-motor responses were investigated such as OKR, VOR in the light
as well as VOR in the darkness. The horizontal OKR gain was determined by
applying with 0.5 Hz frequency and 50 (peak-to-peak) amplitude of rotation in the
horizontal position in the light to the drum. The hOKR gain was calculated as the
ratio of peak-to-peak eye velocity to peak-to-peak drum oscillation velocity.
Twelve cycles of elicited eye movements were chosen for average from sixty
cycles, free of motion parallax and eye blinking, and the given stimulation and
response were suited to sine curves. As previously described, we used a
custum-made program in LabView to analyze the research data [8, 10, 12, 13]. The
standard deviation (SD) of the group averages is represented in the text. The
changes in OKR gain following hOKR training were compared between groups with
different training methodologies using Mann-Whitney U test or Kruskal–Wallis
test (SPSS v. 17.0, IBM Corp., Armonk, NY, USA), and significant value is
considered with p value
Before induction of OKR adaptation, we examined the baseline performance of OKR in mice. The OKR gain was measured in the light condition. The OKR drum stimulation was given by 5° amplitude-sinusoidal rotation with different frequencies including 0.1, 0.25, 0.5 and 1.0 Hz. The OKR gain was calculated as the ratio of maximal eye velocity to maximal drum velocity. These baseline performance characteristics were consistent with those of our previous observations [8, 12, 13]. Then, we induced OKR adaptation by continuous oscillation of optokinetic drum while the mice are remained stationary with eyes opened. Considering that OKR gain was measured with drum oscillation of 0.5 Hz frequency and 5° amplitude, we used 3 different categories of learning paradigm as follows: (1) Optokinetic drum oscillation for 60 min with same amplitude and different frequency (0.25 Hz and 5°, 0.5 Hz and 5°, and 1 Hz and 5°; see Fig. 1). (2) Optokinetic drum oscillation for 60 min with same frequency and different amplitude (0.5 Hz and 2.5°, 0.5 Hz and 5°, and 0.5 Hz and 10°; see Fig. 2). (3) Training with serial combination of different frequency or amplitude (see Figs. 3,4).
 Fig. 1.
Fig. 1.The OKR adaptation after training protocols with different
frequency of drum rotation. (A) After 60 min OKR training with 0.25 Hz and
5° drum oscillation, OKR gain was enhanced from 0.190
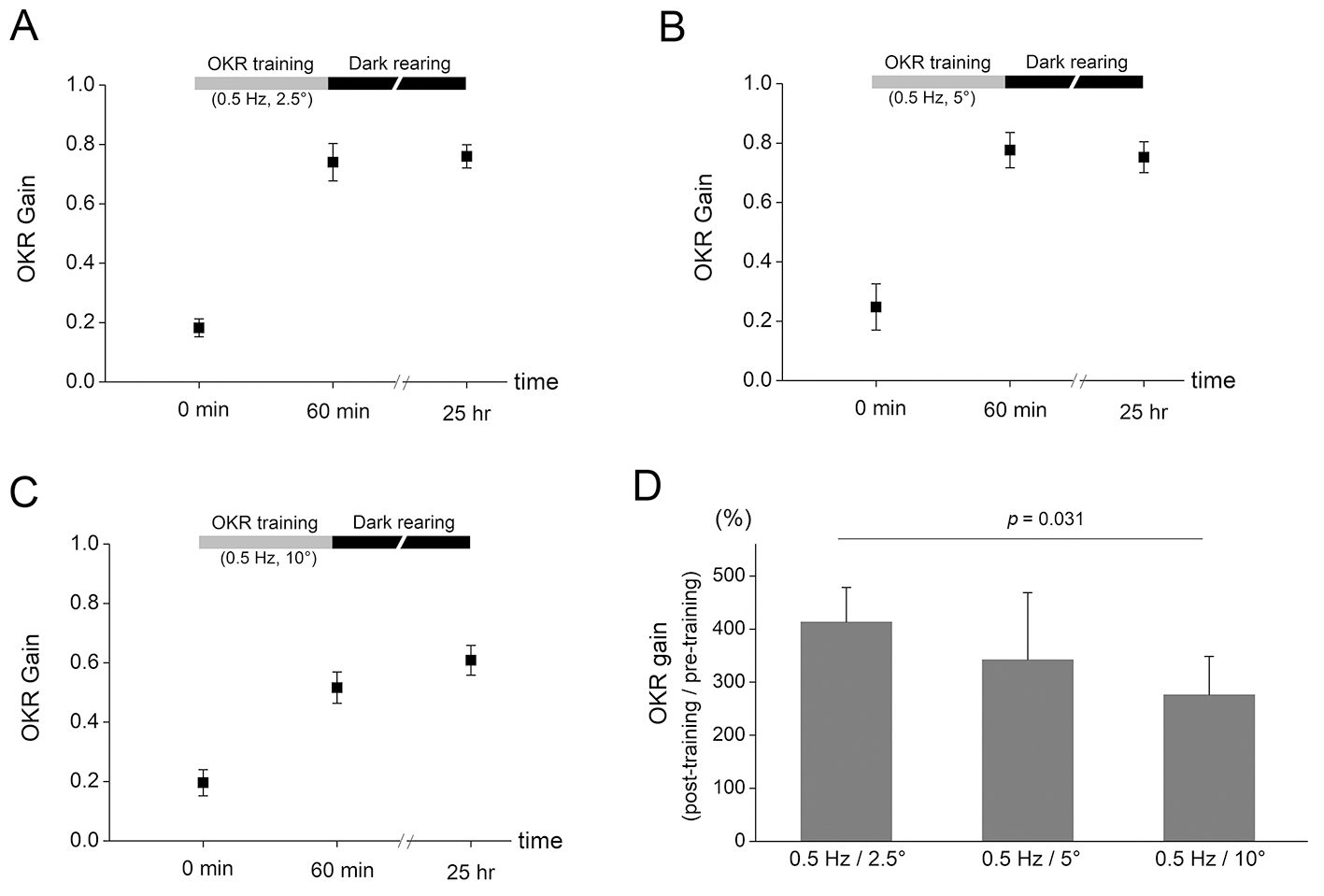 Fig. 2.
Fig. 2. The OKR adaptation after training protocols with different
amplitude of drum rotation. (A) After 60 min OKR training with 0.5 Hz and
2.5° drum oscillation, OKR gain increased from 0.182
 Fig. 3.
Fig. 3.The OKR adaptation after training protocols with combination of
different frequency of drum rotations. (A) The OKR training protocol was
composed of serial combination of 20 min OKR training with 0.25 Hz and
5° drum oscillation, 20 min OKR training with 0.5 Hz and 5°
drum oscillation, and 20 min OKR training with 1 Hz and 5° drum
oscillation. The OKR gain was enhanced from 0.176
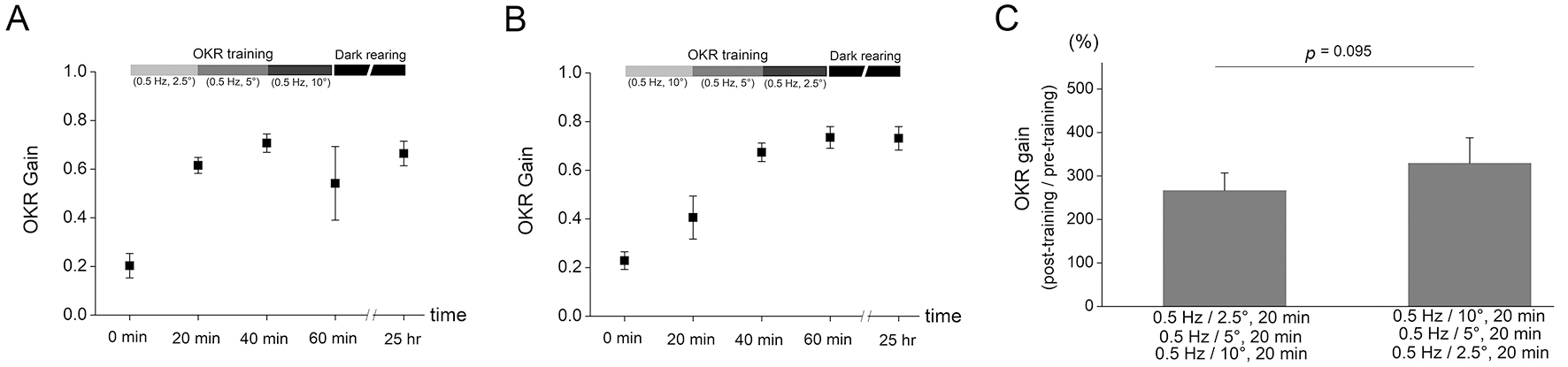 Fig. 4.
Fig. 4. The OKR adaptation after training protocols with combination of
different amplitude of drum rotations. (A) The OKR training protocol was
composed of serial combination of 20 min OKR training with 0.5 Hz and
2.5° drum oscillation, 20 min OKR training with 0.5 Hz and 5°
drum oscillation, and 20 min OKR training with 0.5 Hz and 10° drum
oscillation. The increase in OKR gain was obtained from 0.203
The OKR adaptation was investigated by measuring OKR gain change after 60 min
training of which the protocol consisted of optokinetic drum oscillation with
same amplitude and different frequency. After 60 min OKR training with 0.25 Hz
and 5° drum oscillation, OKR gain increased from 0.190
Then, we investigated the amount of OKR adaptation after training protocols with
different amplitude of drum oscillation. After 60 min OKR training with 0.5 Hz
and 2.5° drum oscillation, OKR gain increased from 0.182
The amount of OKR adaptation after training with serial combination of different
frequency was tested. Training protocol consisted of three consecutive 20 min
drum oscillations with different frequency while keeping the amplitude of drum
rotation constant. First, the OKR training was conducted by serial combination of
20 min OKR training with 0.25 Hz and 5° drum oscillation, 20 min OKR
training with 0.5 Hz and 5° drum oscillation, and 20 min OKR training
with 1 Hz and 5° drum oscillation (Fig. 3A). The increase of OKR gain
was obtained from 0.176
The amount of OKR adaptation after training with serial combination of different
amplitude was tested. Training protocol consisted of three consecutive 20 min
drum oscillations with different amplitude while keeping the frequency of drum
rotation constant. First, the OKR training was conducted by serial combination of
20 min OKR training with 0.5 Hz and 2.5° drum oscillation, 20 min OKR
training with 0.5 Hz and 5° drum oscillation, and 20 min OKR training
with 0.5 Hz and 10° drum oscillation (Fig. 4A). The increase in OKR gain
was obtained from 0.203
Sustained oscillation of optokinetic drum evokes remarkable retinal slip, and
consequently induces the increase in OKR gain to reduce the retinal slip. The OKR
is activated by genuine retinal slip, and due to the relatively considerable
latency in visual processing, this OKR reaction responds late [15]. The adaptive
change of OKR occurs during continuous optokinetic stimulation, which is under
regulation of the cerebellar flocculus. The present study investigated if OKR
adaptation is training frequency- and amplitude-specific. A frequency-specific
change in gain is one of the most interesting characteristics of VOR adaptation
in rabbits and monkeys [16, 17], although the neural mechanisms underlying this
frequency specificity is not clear. Hübner et al. [18] reported that
when VOR gain was measured at the same peak velocity and acceleration utilized
during training in mice, the efficacy of VOR adaption training was maximized.
Considering frequency-specific VOR adaptation, two possibilities have been
offered. According to the “frequency-channel hypothesis” parallel adaptable
filters with overlapping bandwidths transport data about the frequency components
of head movements and central adaptive mechanisms change the tuning of these
particular “channels” [19]. The “context-specific adaptation” postulates that
VOR adaptation is dependent upon modification of the tonic (velocity sensitive)
and phasic (acceleration sensitive) afferent signals from the vestibular
periphery [20, 21]. In contrast to VOR adaptation, it has been controversial
regarding frequency-specificity of OKR adaptation. Collewijn and Grootendorst [3]
measured OKR gain increase after 4 hr optokinetic training with 1/6 Hz and
20° in rabbits, and showed that the amount of OKR adaptation was greater
at 1/6 Hz- than 1/3 Hz- and 1/12 Hz-OKR gain test. Nagao measured OKR gain
increase after 4 hr optokinetic training with 0.33 Hz and 2.5° in
rabbits, and also showed that the amount of OKR adaptation was greater at the
same frequency of testing than other frequencies [22]. Iwashita et al.
[1] conducted OKR training with 0.4 Hz and 1.8° for 2 hr using mice, and
reported that the OKR gain increase was not restricted to the oscillation
frequency used for the training. The increase in OKR gain obtained from 0.58
Another interesting finding of the present study was that the OKR gain increase tended to be greater after training which is composed of sequential combination of decreasing frequency (Fig. 3) or amplitude (Fig. 4) than that composed of sequential combination of increasing frequency or amplitude, although the difference was not significantly different. Furthermore, increased OKR gain was decreased by subsequent OKR training with high frequency (1 Hz and 5° for 20 min, Fig. 3A) or with high amplitude (0.5 Hz and 10° for 20 min, Fig. 4A) stimulation. Thus, the OKR training with high frequency or amplitude may eliminate part of learning effects which have already been formed by previous training. We postulate that the stimulation during training with high frequency or amplitude may implement a disturbing instruction for OKR learning when it is conducted in mice with increased OKR gain by previous OKR training. In addition, the results demonstrated that the amount of OKR adaptation was, although the maximum velocity is same between drum rotation with 0.5 Hz/10° and that with 1 Hz/5°, greater after training with 0.5 Hz/10° (Fig. 2C) than that with 1 Hz/5° (Fig. 1C). This may suggest that even when training velocity is same, the lower frequency OKR stimulation serves as more precise instructive signal.
The OKR gain increase was greater after OKR training with lower frequency or amplitude than that with higher frequency or amplitude, which may be postulated that the retinal slip signal with lower-velocity OKR stimulation serves as more precise instructive signal for learning than higher-velocity OKR stimulation.
Conceptualization of the study—SJK, C-HK. Drafting the manuscript—NCP, C-HK. Data collection—NCP, YGK. Data interpretation—NCP, YGK, SJK, C-HK. Revision of the manuscript for intellectual content—SJK, C-HK.
All procedures were approved by the Institutional Animal Care and Use Committee of Seoul National University College of Medicine. The approval number is SNU-131028-1.
Not applicable.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (2021R1F1A1062019, 2018R1A5A2025964).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
