- Academic Editor
Background: Training with inescapable shock (IS; uncontrollable
stressor) is followed by significant decreases in rapid eye movement sleep (REM).
However, controllability is important in the effects of stress. We examined the
effects of escapable shock (ES; controllable stressor) on sleep and whether the
central nucleus of the amygdala (CNA) plays a role in regulating these effects.
Methods: Six Wistar rats implanted with a cannula located in CNA
underwent two days of ES training (20 shock presentations; 0.5 mA; 5.0 s maximum
duration; 1.0 min interstimulus interval). Five days later, they were re-exposed
to the shock context. Results: Following shock training, REM was
significantly increased in both light and dark periods. Non-REM (NREM) and total
sleep (TS) duration were decreased during the light period. Similar effects on
REM and NREM were observed following re-exposure to the training context alone.
Microinjections of saline into CNA immediately following ES also produced similar
increases in REM, whereas microinjections of muscimol (MUS; GABA
Stress-related learning, as modeled by classical fear conditioning, is thought to play an important role in the development of anxiety [1, 2, 3] and trauma and stress-related [4, 5] disorders, and it provides an important avenue through which traumatic memories can produce persisting changes in behavior. However, stressors are commonly encountered without resulting in permanent or pathological changes. The difference between adaptive and nonadaptive coping and whether stress has temporary or lasting effects can vary with the properties of the stressor, including whether or not it is controllable [6].
Stress can affect sleep, and traumatic life events virtually always produce at least transitory problems with sleep that may include insomnia or subjective sleep complaints [7]. Rapid eye movement sleep (REM) may be particularly vulnerable to the effects of stress, and a decrease in REM is initially observed in response to most stressors experienced during the normal sleep period [8, 9]. REM has been hypothesized to be important for processing emotion [10, 11, 12, 13], and we [14, 15] and others [14, 15, 16, 17] have suggested that it is important for adaptive responses to stress [8, 9]. Reduced and disrupted REM is also implicated in the genesis and symptomology of posttraumatic stress disorder (PTSD) [18, 19, 20], whereas greater baseline REM is associated with weaker fear-related activity in the amygdala, hippocampus, and ventromedial prefrontal cortex as well as reduced hippocampal modulation of the amygdala [21]. Thus, sleep, and REM in particular, appears to be important either as an active mediator of the stress response or as a biomarker for adaptive and maladaptive stress responses.
Animal studies have demonstrated that the amygdala can strongly influence REM.
In particular, the central nucleus of the amygdala (CNA) can directly regulate
REM and can mediate the effects of stress and fear memory on REM. For instance,
inactivating CNA with microinjections of the
Several studies have demonstrated that training rats with IS, and re-exposure to the fearful context associated with IS [24, 25], can be followed by significant reductions in REM that can occur without recovery sleep (note that there can be individual differences in responses [15, 27]). In mice, controllable stress modeled by escapable shock (ES) and ES-related fear memories can produce significant increases in REM, whereas IS and IS-related fear memories can produce significant decreases in REM [14, 28, 29, 30]. By comparison, the effect of controllable stress on sleep in rats has rarely been explored though a few studies have examined sleep after training in an avoidance paradigm in which a signal allows the animals to prevent the presentation of a footshock [31, 32]. In the present study, we examined the effects of a controllable stressor on subsequent sleep using an ES paradigm in which an animal always receives a footshock but can terminate it by executing a simple behavioral escape response. As reminders of ES in mice produce changes in sleep similar to those observed when the footshock stressor is presented, we examined sleep after re-exposure to the ES context without footshock presentation to determine whether contextual reminders of ES would also alter sleep in rats. Finally, we assessed whether the CNA, a region implicated in the regulation of spontaneous REM [22, 33, 34, 35, 36, 37, 38] and stress-induced reductions in REM [23], regulates the effects of ES on sleep. Our goal was to establish the effects of ES learning on sleep in rats and to determine whether CNA regulates ES induced increases in REM as well as IS induced decreases as previously reported.
The subjects were 6 nine-week-old male Wistar rats obtained from Harlan Laboratories (Indianapolis, IN, USA). The animals were housed and maintained as previously described [22, 38]. One week after arrival, the animals were surgically prepared for recording sleep and had cannulas implanted for delivering the drug to CNA following established procedures [22, 38]. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School’s Animal Care and Use Committee (Approval number: 16-002).
After the rats had recovered from surgery for 14 days, they were habituated to the recording setup over the course of two days. Subsequently, sleep following a mild, 5-min restraint stressor was obtained for use as the control sleep condition. This control condition was used instead of an undisturbed baseline because stress produces an immediate period of arousal [39] that is typically followed by a period of increased REM and/or non-REM (NREM) after the stressor is removed [40, 41, 42, 43]. Brief restraint handling such as that we used produces initial arousal followed by a small but significant increase in subsequent REM [44].
On experimental days 1 and 2 (ES1 & ES2), the rats were placed in the shuttlebox shock chambers (Coulbourn Instruments shuttlebox (Model # E10-15SC, Coulbourn Instruments LLC, Holliston , MA, USA) equipped with a grid floor) for 5 min of habituation. They then received footshock once every min for 20 min (0.5 mA, up to 5 s duration). Once the shock began, the animal could terminate it by moving into the opposite chamber of the shuttlebox.
On experimental day 7, the rats were placed back in the shock chambers (context re-exposure, CR) and allowed to explore freely for 30 min with no shock presented before being returned to their home cage for sleep recording.
On experimental days 11 & 17, the rats underwent two additional sessions of the escapable shock paradigm described above. These sessions were immediately followed by an injection into CNA of either saline (ES-SAL) or muscimol (ES-MUS: 0.1 µM, muscimol hydrobromide, 5-aminomethyl-3-hydroxyisoxazole obtained from Sigma–Aldrich (St. Louis, MO, USA)). Microinjections had a volume of 0.2 µL and were infused over a 3 min period.
After each day of training or testing, the rats were immediately transferred to their home cage, and sleep was recorded for 20 h.
Wakefulness, NREM, and REM were scored based on established procedures [22, 38]
and NREM amounts (min), REM amounts (min); total sleep amounts (REM + NREM, TS),
latency to NREM or REM, and REM percentage (REM%: REM/TS
Escape latencies were calculated by subtracting the time of shock onset from the time of shock termination. These values were averaged across the entire training period on days 1 and 2.
Comparisons involving the entire light or dark periods across ES days were
conducted using one-way within subjects analysis of variance (ANOVA) tests.
Analyses of the entire light or dark periods across treatments were conducted
using paired t-tests. Comparisons of 4 h blocks of time (light period:
B1 and B2, dark period: B3, B4, and B5) across experimental days were conducted
using two-way within subject (condition
Brain sections through CNA were cut at 40-µM, stained with a 0.5% cresyl violet solution, and injection site locations were verified using a stereotaxic atlas [45]. One rat was not used in the microinjection study due to degraded electroencephalogram (EEG) signals and was not processed for histology.
The rats could terminate the shock by moving into the opposite chamber of the
shuttle box. The average escape latency during ES1 was 1.03
Significant changes in sleep were found following ES in both the light and dark
periods, with the most profound effects observed in REM. There was a significant
treatment main effect for the 8 h light period (F(2,10) = 9.33, p =
0.006) and 12 h dark period (F(2,10) = 24.95, p
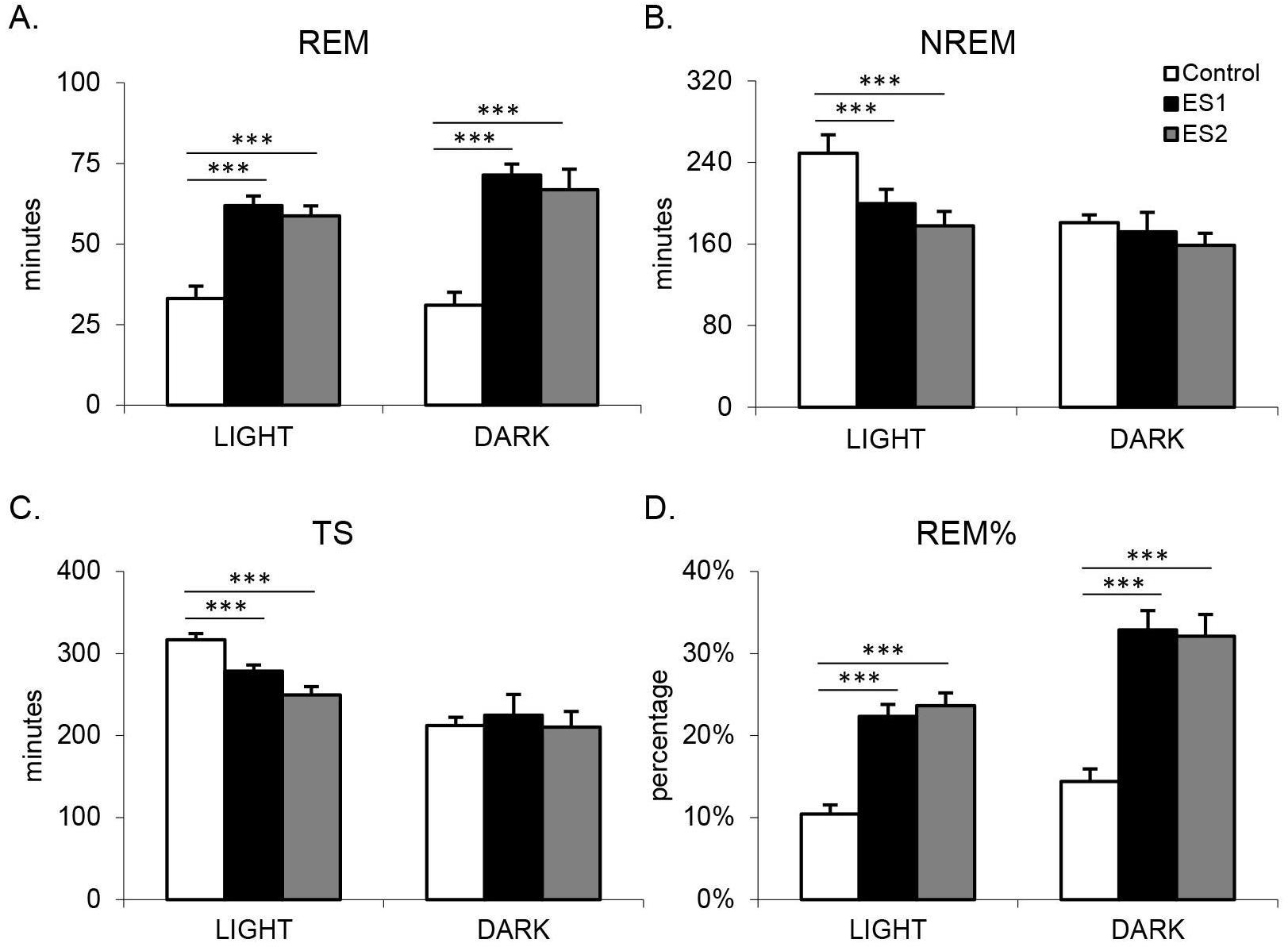 Fig. 1.
Fig. 1.Amount of sleep on control day or following shock training days
during the light period (8 h) and dark period (12 h). (A) REM duration; (B) NREM duration; (C) total sleep (TS) duration; (D) REM%. Values are mean
| REM Duration | |||||||
| B1 | B2 | ANOVA | B3 | B4 | B5 | ANOVA | |
| Control | 19.61 |
21.73 |
F(2,10) = 14.221 (i) | 11.39 |
10.64 |
8.92 |
F(2,10) = 24.948 |
| ES1 | 19.11 |
36.31 |
25.53 |
24.72 |
21.08 | ||
| ES2 | 22.84 |
32.34 |
26.33 |
16.92 |
23.59 | ||
| Context | 20.95 |
29.56 |
F(1,5) = 2.217 | 22.00 |
18.36 |
16.69 |
F(1,5) = 20.350 |
| ES-SAL | 23.78 |
32.30 |
F(2,8) = 21.529 | 23.12 |
17.75 |
14.42 |
F(4,16) = 3.398 (i) |
| ES-MUS | 15.87 |
20.37 |
14.27 |
11.87 |
12.5 | ||
| NREM Duration | |||||||
| B1 | B2 | ANOVA | B3 | B4 | B5 | ANOVA | |
| Control | 130.6 |
144.6 |
F(2,10) = 7.444 | 52.64 |
60.92 |
67.58 |
F(2,10) = 4.387 |
| ES1 | 118.22 |
132.3 |
67.19 |
75.44 |
79.59 | ||
| ES2 | 125.97 |
121.94 |
56.03 |
59.78 |
82.03 | ||
| Context | 127.44 |
143.22 |
F(1,5) = 5.533 | 60.58 |
62.47 |
79.95 |
F(1,5) = 1.763 |
| ES-SAL | 132.59 |
148.11 |
F(2,8) = 4.575 | 74.39 |
67.25 |
77.81 |
F(2,8) = 4.849 |
| ES-MUS | 127.20 |
140.37 |
62.16 |
66.37 |
79.63 | ||
| Total sleep duration | |||||||
| B1 | B2 | ANOVA | B3 | B4 | B5 | ANOVA | |
| Control | 150.22 |
166.37 |
F(2,10) = 0.724 | 64.03 |
71.55 |
76.50 |
F(2,10) = 10.581 |
| ES1 | 137.34 |
168.61 |
92.77 |
100.17 |
100.67 | ||
| ES2 | 148.81 |
154.28 |
82.36 |
76.69 |
105.61 | ||
| Context | 148.39 |
172.78 |
F(1,5) = 0.0847 | 82.58 |
80.83 |
96.64 |
F(1,5) = 6.334 |
| ES-SAL | 156.37 |
180.42 |
F(2,8) = 2.311 | 97.50 |
85.00 |
92.22 |
F(2,8) = 10.281 |
| ES-MUS | 143.07 |
160.73 |
76.43 |
78.24 |
92.13 | ||
| REM% | |||||||
| B1 | B2 | ANOVA | B3 | B4 | B5 | ANOVA | |
| Control | 13% |
13% |
F(2,10) = 19.106 (i) | 18% |
14% |
11% |
F(2,10) = 26.429 |
| ES1 | 14% |
22% |
29% |
25% |
21% | ||
| ES2 | 16% |
21% |
32% |
22% |
22% | ||
| Context | 14% |
18% |
F(1,5) = 2.217 | 27% |
22% |
18% |
F(1,5) = 12.767 |
| ES-SAL | 15% |
18% |
F(2,8) = 18.035 | 23% |
21% |
15% |
F(2,8) = 5.832 |
| ES-MUS | 11% |
13% |
19% |
15% |
14% | ||
All ANOVAs indicate comparisons are relative to the control. F-values indicate
significant main effects or interactions (i). Light period: B1-B2; dark period:
B3-B5. Differences are relative to control determined by post hoc Tukey tests
(*p
REM, rapid eye movement sleep; ANOVA, analysis of variance; ES, escapable shock; ES-SAL, escapable shock saline; ES-MUS, escapable shock muscimol.
There were also significant treatment effects for the analyses of the 8 h light
period for NREM (F(2,10) = 36.08, p
To examine the REM increase further in relation to the decrease in TS, we
analyzed REM% (Fig. 1D) and found a significant main effect of treatment on
REM% during the 8 h light period (F(2,10) = 78.92, p
Context reexposure (CR) produced changes in sleep during both the light and dark periods that were similar to those observed after ES. There was a significant increase in REM (Fig. 2A) during the 8 h light period sleep (t = 5.16, p = 0.004) and 12 h dark period (t = 4.51, p = 0.006) following CR compared to control. The increase in REM during the light period after CR appeared to be primarily due to increased REM amounts in B2 of the light period, but the difference did not reach significance (p = 0.051). During the dark period, REM amounts in all 4 h blocks (B3–B5) after CR was significantly increased compared to control sleep (see Table 1).
 Fig. 2.
Fig. 2.Amount of sleep on control day or following context reexposure
during the light period (8 h) and dark period (12 h). (A) REM duration; (B) NREM
duration; (C) total sleep (TS) duration; (D) REM%. Values are mean
NREM was also altered following CR. During the 8 h light period, there was a significant decrease in NREM duration (t = 2.57, p = 0.05; Fig. 2B) and a trend towards a decrease in TS duration (t = 2.14, p = 0.086; Fig. 2C). NREM and TS during the dark period were not significantly altered.
Again, analyses of REM% (Fig. 2D) revealed a significant increase during the 8 h light period (t = 4.64, p = 0.006) and 12 h dark period (t = 5.89, p = 0.002) following CR compared to the control. This was observed as a trend towards an increase in REM% during the second 4 h of the light period and a significant increase in REM% during all 4 h blocks of the dark period compared to control sleep (see Table 1).
Similar changes in sleep in both the light and dark periods were observed
following ES-SAL. Comparing control sleep to ES-SAL and ES-MUS, there was a
significant main effect of condition on REM duration (Fig. 3A) during the 8 h
light period (F(2,10) = 35.791, p
 Fig. 3.
Fig. 3.Amount of sleep on the control day or following microinjection
of saline (ES-SAL) or muscimol (ES-MUS) prior to shock during the light period (8
h) and dark period (12 h). (A) REM duration; (B) REM%. Values are mean
Analyses of REM% (Fig. 3B) revealed a significant main effect of the condition
during the 8 h light period (F(2,10) = 14.44, p = 0.001) and condition
Fig. 4 presents line drawings illustrating cannulae placement in the 5 rats receiving microinjections. Histological analysis indicated that MUS or saline was microinjected into CNA, though the drug could have diffused into other amygdala nuclei as well.
 Fig. 4.
Fig. 4.Line drawings illustrating microinjection sites in animals (n = 5). The shaded area refers to the central nucleus of the amygdala and the triangles indicate the end of the cannula tract.
ES resulted in significant increases in total REM and REM%. These increases occurred across training days that also had significant decreases in light period total NREM and TS. The changes in total REM and REM% also were not due to a recovery of lost REM but were long-lasting increases over the entire recording period. Re-exposure to the shock chamber alone was followed by changes in sleep that resembled those observed following ES, with significant increases in total REM and REM% in both light and dark periods. Thus, reminders of the controllable stressor produced changes in subsequent sleep directionally similar to those observed following the original stressor. These results are similar to the increases in REM observed after ES and ES-associated memories in mice [14, 28, 29, 30].
To our knowledge, this study provides the first description of sleep in rats after an “escapable” shock paradigm in which animals always experience the stressor, but are given the opportunity to learn and execute a behavioral response that terminates it. The term “escapable” has been used in the literature to describe a variety of paradigms, but most are actually “avoidance” paradigms [32]. In avoidance paradigms, the animal can learn a behavioral response, which, if executed in response to a signal, will prevent them from experiencing the stressor. Avoidance training is also a stress paradigm, but it has historically been utilized to examine the potential role of sleep in learning and memory, and studies typically have been interpreted as providing evidence that the post-training increases in REM (either % or duration) played a role in memory consolidation [46, 47, 48].
Our results with ES demonstrate substantial increases in REM duration and REM% despite the fact that the footshock stressor was always experienced. This contrasts with the commonly observed decrease in REM duration that can occur following training with IS [24, 25, 26]. In addition, in the case of ES, the increases in REM occur throughout the dark period, and in the case of IS the significant decrease in REM can occur without an apparent period of recovery REM [24]. Moreover, re-exposing the animal to the ES training context, a situation that should bring about recall of memories associated with the stressor, also increased REM. Re-exposure to the training context alone also produces alterations in sleep in IS paradigms that are similar to those observed after training with IS [24]. Thus reminders of controllable and uncontrollable stress produce alterations in sleep similar to those produced by the original footshock stressor. Indeed, parallel increases or decreases in REM can occur across multiple days with ES and IS training, respectively. It is certainly possible that memories are recalled and reconsolidated across training days, but this also involves directionally different changes in REM. Another consideration for the ES and IS training paradigms is that the changes in sleep appear disproportionally large for learning relatively simple associations and responses. The increases in REM with ES were larger than increases in REM reported after avoidance training in a shuttlebox in which the animals had to learn to respond to a cue to avoid the presentation of footshock [47, 48].
While the escape latencies as well as altered sleep after re-exposure to the ES context alone demonstrate that learning occurred, the ES paradigm also involves a strong emotional component. Our microinjection study shows that the alterations in REM following ES are regulated by CNA, a region involved in the regulation of emotional behavior [49]. Microinjections of saline into CNA following ES did not alter the increase in REM observed across the light and dark periods. However, the inactivation of CNA with microinjections of MUS following ES prevented the increase of REM. Performing the microinjection after footshock exposure was completed ensured that behavioral responses during ES were not altered and that the effect of inactivation of CNA on post-stress sleep was being tested instead of on behaviors that occur in stressful situation. The involvement of the amygdala in regulating post-stress REM suggests that differences in REM after ES and IS could reflect differences in modulating the long-term effects of stress-related emotion after the experience with controllable and uncontrollable stressors.
Other work also supports a role for CNA in the initiation and maintenance of REM in both undisturbed and post-stress conditions. For example, noradrenergic denervation of CNA significantly reduces REM rebound after sleep deprivation [37]. Our lab has demonstrated, in non-stressful situations, that inhibition of CNA by microinjections of MUS produced significant reductions in the light period [22] but not the dark period [38], REM. By comparison, activation of CNA by microinjections of BIC increased REM [22]. Additionally, optogenetic activation of CNA projections to the nucleus reticularis pontis oralis, but not pedunculopontine tegmentum or nucleus subcoeruleus, during the dark period also promotes REM [38]. As noted above, microinjections of BIC into CNA blocked REM reductions found following IS, whereas microinjections of MUS did not [23]. Taken together, these studies suggest that CNA plays a significant role in regulating REM in both stressful and non-stressful situations and that activation of CNA can promote REM, possibly via specific projections to brainstem REM generator regions.
CNA modulation of the effects of stress on REM could also involve projections to the locus coeruleus (LC) and dorsal raphe nucleus (DRN), which have roles in regulating REM [50] and have been linked to the effects of stress. For example, IS in rats increases norepinephrine turnover in several brain areas whereas norepinephrine utilization is reduced after the coping response is acquired with ES [51]. IS activates 5-HT (5-hydroxytryptamine) DRN neurons to a greater degree than does ES thereby increasing 5-HT in DRN and in target areas [52, 53, 54, 55]. Differential activation of LC and DRN, possibly by CNA, after ES and IS could play a significant role in the differences in amounts of REM as activation of these regions may be inhibitory and inactivation permissive to REM [50].
There are some limitations to this study that need to be considered. First, though we have previously shown that ES and IS and related memories consistently produce differential effects on REM in inbred mice [14, 28, 29, 30], the story may be more complicated in outbred rats where we have found individual differences in the REM response in Wistar strain rats [15, 27]. Vulnerable (Vul) rats show decreases in REM, and resilient (Res) rats increase in REM after the same IS and IS-related fear memories [15, 27]. We did not distinguish Vul and Res rats in the current study suggesting that an increase in REM might have occurred in some animals even without the possibility of escape. However, REM increases in Res rats do not approach the level of increases observed after ES in this study, indicating that the level of increase was impacted by ES regardless of potential differences in phenotype.
Our results also demonstrate that the ES-induced increase in REM was clearly blocked by post-training inactivation of CNA. By comparison, post-training MUS inhibition of the basolateral amygdala (BLA) in Res rats did not alter either IS-induced or fear-conditioned alterations in REM [15], whereas post-training inhibition of the BLA with MUS did not alter IS-induced alterations in REM but did block subsequent fear conditioned reductions in REM in Vul rats [15]. Indeed, several lines of research demonstrate that the different REM responses in Vul and Res rats are regulated by BLA [15, 27, 56], which projects to and through CNA [57]. We previously found that the inactivation of fibers of passage in CNA with tetrodotoxin produced a significant reduction in REM (and an increase in NREM) [38]. Those fibers likely originate in BLA and project, at least partially, to the bed nucleus of the stria terminalis (BNST) [57], which has downstream projections similar to those of CNA [58, 59]. This suggests that the amygdala may increase or reduce REM and/or reduce NREM via BLA control of CNA and BNST and their projections to brainstem REM regulatory and generator regions.
In conclusion, emotional stress can contribute to mental disorders. However, even traumatic stress can be encountered without having a permanent negative impact. Interactions between stressor parameters, sleep and learning are likely important mediators of stress outcomes. This study demonstrates that ES, and ES associated fear memory, can produce significant increases in REM as well as decreases in NREM and TS. The REM increases are regulated by the amygdala as post-training inactivation of CNA can block ES-induced alterations in sleep. These data in rats, along with similar findings in mice, demonstrate that stressor controllability is important in determining the effects of stress and stress-related memories on sleep and that the amygdala is an important mediator of these alterations in sleep.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
LDS, LLW, and XT designed the research study. LLW and LY performed the research. LLW, AMA and LY analyzed the data. LLW, and LDS wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Experimental Animals and were approved by Eastern Virginia Medical School’s Animal Care and Use Committee (Approval number: 16-002).
Not applicable.
This research was supported by NIH research grant MH64827 (LDS).
The authors declare no conflict of interest. Larry D. Sanford and Laurie L. Wellman are serving as the Guest editors of this journal. We declare that Larry D. Sanford and Laurie L. Wellman had no involvement in the peer review of this article and had no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Gernot Riedel.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
