1. Introduction
-Synuclein plays an important role in the pathogenesis of several
neurodegenerative disorders, including Parkinson’s disease (PD), multiple system
atrophy, and dementia with Lewy bodies. These disorders are also known as
“synucleinopathies”, which are characterized by abnormally misfolded and
aggregated -synuclein in the nervous system [1]. Currently, only
palliative treatments addressing dopaminergic deficits have been approved, and no
disease-modifying options are available for PD and related
synucleinopathies [2]. However, strategies targeting misfolded
-synuclein aggregates are being considered as new therapeutic
approaches [3].
-Synuclein is a neuronal presynaptic protein regulating
neurotransmitter release. Structurally, -synuclein is a protein formed
by 140 amino acids encoded by -synuclein (SNCA). The A53T point mutation in
SNCA was the first reported pathogenic modification associated with PD
that underlies disease initiation [4]. Post-translational modifications of
-synuclein, such as phosphorylation, favor aggregation. In the healthy
brain, only few -synucleins are phosphorylated, whereas in pathological
inclusions most -synucleins are phosphorylated at serine 129 (Ser129),
as in the Lewy bodies of the PD brain [5]. The polo-like kinase 2 (PLK2), which
is a member of polo-like kinase (PLK) family, has been reported to phosphorylate
-synuclein at Ser129 and modulate its aggregation [6, 7]. The
ubiquitin-proteasome system (UPS) can eliminate unfolded or misfolded proteins
including misfolded -synuclein [8] and parkin plays a key role in the
degradation of -synuclein through the UPS degradation process [9].
Moreover, the expression level of parkin has been reported to decrease due to the
overexpression of -synuclein [10, 11]. Thus, PLK2 and parkin might
partly be involved in the modification and/or degradation of -synuclein
in synucleinopathies, which we investigated in this study.
Herba Epimedii is the dried leaf of the medicinal plant
Epimedii, named Yinyanghuo in Chinese. Flavonoids extracted from
Epimedium constitute the main active ingredient, showing neuroprotective
and anti-inflammatory effects [12, 13]. Among these flavonoids, icariin (ICA) is
the most prominently active flavonoid. In recent years, ICA has been reported to
show beneficial effects in several diseases of the central nervous system,
including Alzheimer’s disease (AD), PD, and multiple sclerosis [14, 15, 16]. Our
previous study indicated that ICA decreases -synuclein expression in
the hippocampus of APPV717I transgenic mice, indicating a potential effect on PD
and other synucleinopathies [17].
Mutant A53T -synuclein transgenic (A53T Tg) mice, which express the
mutant A53T -synuclein, were used to investigate mechanisms and
pharmacology [18]. A53T Tg mice show obvious motor impairments at 8 months of age
due to progressive pathological changes in -synuclein [19, 20]. As we
have previously identified the potential effects of ICA on APPV717I transgenic
mice, we used A53T -synuclein transgenic mice of varied ages to observe
the behavioral and pathological changes after ICA treatment to investigate the
potential effects and mechanisms of ICA on the pathology of -synuclein.
Moreover, we used wild-type -synuclein-transfected SH-SY5Y cells to
investigate the pharmacological effect and potential mechanism of ICA on
synucleinopathies.
2. Materials and Methods
2.1 Drugs
For animal studies, ICA (purity 98%) was purchased from Scidoor Hi-tech
Biology (Xi’an, Shaanxi, China). ICA was diluted with normal saline and
intragastrically administered to mice.
For the cell culture, ICA (purity 99%) was purchased from the National
Institutes for Food and Drug Control (Beijing, China). ICA was added to the cell
culture medium after dilution with phosphate-buffered saline (PBS).
2.2 Animals
A53T Tg mice (B6. Tg (PDGF-h--synuclein A53T)-GC/ILAS, CSTR:
16397.09.0H01000945) were purchased from the Center for Experimental Animal
Research, Chinese Academy of Medical Sciences (Beijing, China) [18]. Age-matched
C57BL/6 (wild type, WT) mice were purchased from Beijing HFK Encapee (Beijing,
China).
All mice were housed under a 12 h light/dark cycle with relative humidity of
55–60% and a temperature of 22 2 °C and free access to water
and food. Animal studies were approved by the Bioethics Committee of Xuanwu
Hospital of the Capital Medical University (approval number:
20120912) and were performed in accordance with the National Institutes of Health
Guide for Care and Use of Laboratory Animals.
2.3 Animal Treatment and Grouping
A53T Tg and WT mice of two different ages (5 and 10 months old) were allocated
to two experimental groups. (1) 5-month-old A53T mice received a daily dose of
ICA (either 50 or 100 µmol/kg) or saline (as the model group) over a period
of 3 months; coetaneous WT mice were treated with normal saline or 100
µmol/kg ICA; n = 8–10 per group. (2) 10-month-old A53T Tg mice received a
daily dose of ICA (either 50 or 100 µmol/kg) or saline (as the model group)
over a period of 3 months; WT mice of the same age were treated with normal
saline or 100 µmol/kg ICA; n = 10–12 per group. According to the molecular
weight of ICA (676.65), the dose of 100 µmol/kg was converted to 67.7 mg/kg
and 50 µmol/kg was converted to 33.8 mg/kg at the time of administration to
the mice. All treatments were intragastrically administered.
2.4 Rotarod Test
The rotarod test (YLS-4C, Yanyi Life Science, Jinan, Shandong, China) was
applied to evaluate the motor coordination and balance of the mice [21]. Mice
were trained three times in 5-min trials before the test at a speed of 10
rotations per minute (rpm). The mice were then individually placed on the rotarod
with a fixed speed (30 rpm) and cut-off time (180 s). The test was performed five
times at intervals of at least 30 min, and the mean of the results was then
calculated.
2.5 Pole Test
The pole test (XPS-2, Chinese Academy of Medical Sciences and Peking Union
Medical, Beijing, China) was conducted to evaluate the coordination function of
the mice [22]. The mice were placed head-up on the top of a pole with high
rough-surface (height, 50 cm; diameter, 2 cm). The time taken for the 8-month-old
mice to climb down the pole was recorded. The test was then performed in three
trials at intervals of at least 30 min. Trials were excluded if the mouse jumped
off or slid down the pole. The behaviors of the 13-month-old mice were observed
as they descended from the top to the bottom of the pole and evaluated using a
scoring method. Each mouse was allowed to descend the pole three times and the
average score was then calculated. The scoring criteria for the pole test were
set as follows: 5 points, use of all limbs to climb down the pole smoothly; 4
points, step-by-step downward spiral crawling, dragging the hind limbs; 3 points,
pausing several times during the climb down but holding tightly to the pole; 2
points, sliding on the pole and falling off; and 1 point, inability to grab the
pole, directly dropping.
2.6 Tissue Collection and Western Blotting
Four mice from each group were anesthetized using 2.5% Avertin (Sigma-Aldrich,
St. Louis, MO, USA) and euthanized after behavioral testing. The brain was
rapidly removed, and the striatum isolated and homogenized in lysis buffer
containing 20 mM Tris-HCl (pH 7.5), 10% glycerol, 150 mM NaCl, 0.5 mM ethylene
glycol tetraacetic acid, 1 mM ethylenediaminetetraacetic acid, and a protease
inhibitor cocktail (Cat. No. 04693116001; Sigma-Aldrich, St. Louis, MO, USA) [17, 23]. Protein concentration was detected using an RC-DC Protein Assay Kit (Bio-Rad
Laboratories, Hercules, CA, USA), and the protein samples were boiled for 5 min
before storage.
Proteins were separated using sodium dodecyl sulfate (SDS)–Tris-glycine polyacrylamide gel and
transferred onto polyvinylidene difluoride (PVDF) membranes. The following
primary antibodies were used: mouse anti--synuclein (Cat. No. ab1903;
Abcam, Cambridge, UK), rabbit anti-p--syn (Ser129) (Cat. No. ab51253,
Ser129-phosphorylated -synuclein, Abcam), rabbit anti-parkin (Cat. No.
P6248; Merck Millipore, Darmstadt, Germany), rabbit anti-PLK2 (snk, Cat. No.
sc25421; Santa Cruz Biotechnoloy, Santa Cruz, CA, USA), and mouse
anti--actin (Cat. No. A5316, Sigma-Aldrich). PVDF membranes were then
incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG
antibody (1:2000). The immune complex was then visualized using enhanced
chemiluminescence detection reagents (Cat. No. WBLUF0500, Merck Millipore).
Images were captured using the FluorChem E System (Bio-Techne, Minneapolis, MN,
USA) and analyzed using AlphaView software (Bio-Techne).
2.7 Cell Culture
The human dopaminergic cell line SH-SY5Y was purchased from the Cell Resource
Center of Peking Union Medical College (Beijing, China). The cell line was
previously authenticated by STR and tested for Mycoplasma infection by the Cell
Resource Center of Peking Union Medical College. The results indicated that the
cell line was derived from human and showed no mycoplasma contamination. The
cells transfected with Green fluorescent protein (GFP)-tagged WT
-synuclein or vector (gifted by Prof. Hong Ma, Beijing Institute of
Technology, Beijing, China) were maintained in Dulbecco’s modified Eagle’s medium
(DMEM) (Cat. No. A1896701, Thermo Fisher Scientific, Fair Lawn, NJ, USA)
supplemented with 10% fetal bovine serum (FBS) and 0.3 g/L G418 (Cat. No. A1720,
diluted in 0.1 M HEPES, Thermo Fisher Scientific, Fair Lawn, NJ, USA) at 37
°C in an incubator humidified with 5% CO. All experiments were
conducted in triplicate.
2.8 Cell Viability Assay
Cell viability was detected using the 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay. Cells (1 10 cells/mL)
were seeded and incubated with different doses of ICA (1.56, 3.125, 6.25, 12.5,
25, 50, 100, and 200 µM) for 24 or 48 h. After incubation, MTT was mixed
with the medium at a final concentration of 0.5 mg/mL. The MTT solution was
removed after 4 h incubation at 37 °C, and 200 µL dimethyl sulfoxide (DMSO) was added
to completely dissolve the formazan crystals. The absorbance of each well was
measured at 570 nm using a microplate reader (Multiskan Spectrum, Thermo Fisher
Scientific, Fair Lawn, NJ, USA). Cell viability was calculated as follows:
absorbance (optical density, OD) of the drug-treated groups/absorbance of the
vehicle-treated group.
For the western blot assay, 2 10 cells were seeded in a flask
and treated with ICA for 48 h. Cultured cells were harvested and lysed to obtain
the protein.
2.9 Immunocytochemistry
Cells were seeded in 24-well plate and treated with 40 µM ICA for 48 h.
After removing the culture medium, cultured cells were fixed in by 4%
paraformaldehyde and 0.1% Triton X-100 for 30 min and then washed three times in
0.01 M PBS. After blocking with serum, the cultured cells were incubated with
mouse anti--synuclein (1:200, Cat. No. ab1903, Abcam, Cambridge, UK) at 4 °C overnight. After washing
in PBS, the fixed cells were incubated with goat anti-mouse IgG (Alexa Fluor 594,
Cat. No. A-11005, 1:200, Thermo Fisher Scientific) and Hoechst33342 (Cat. No.
R37165, Thermo Fisher Scientific). The cells were covered with mounting medium
before visualization using a Nikon 80i microscope (Nikon, Tokyo, Japan).
2.10 Statistical Analyses
All data were analyzed using SPSS software (version 20.0, IBM Corp, Armonk, NY,
USA). Data were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s post-hoc
comparisons to identify significant differences among groups. Numerical data are
provided as the mean standard error of the mean (SEM). Statistical
significance was set at p 0.05.
3. Results
3.1 ICA Attenuated Behavioral Dysfunction in A53T
-Synuclein Transgenic Mice at 8 and 13 Months of Age
The rotarod test was applied to detect motor balance and coordination in A53T Tg
mice after daily intragastric administration of ICA for 3 months. Both the 8- and
13-month-old A53T Tg mice fell off the rotarod at 30 rpm quicker than the WT
control mice, with the 13-month-old A53T Tg group showing a statistically
significant difference (p 0.05, Fig. 1A,B). ICA (50 and 100
µmol/kg) treatment increased the time to fall off the rotarod in both age
groups of A53T Tg mice, with the ICA (100 µmol/kg) treatment in the
8-month-old A53T Tg group showing a statistically significant difference
(p 0.05, Fig. 1A,B).
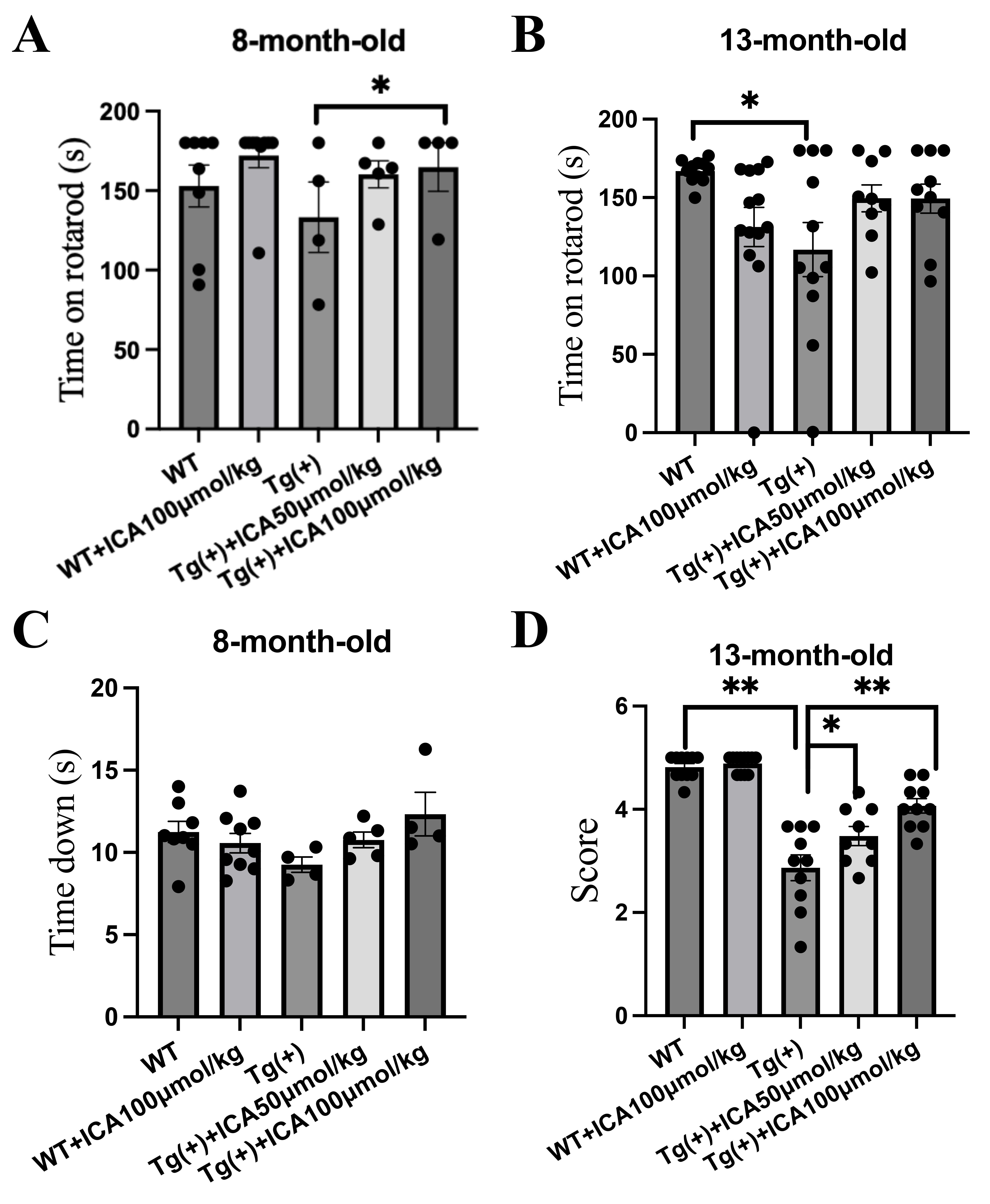 Fig. 1.
Fig. 1.
ICA attenuates behavior dysfunction in young and aged A53T
-synuclein transgenic mice. The rotarod and pole tests were
applied to assess the motor balance and coordination of the A53T Tg mice after
intragastric administration of ICA for 3 months. (A) Time on the rotarod (latency
to fall off the rotarod) of the 8-month-old mice in the rotarod test. (B) Time on
the rotarod of the 13-month-old mice in the rotarod test. (C) Pole test; time
spent climbing down the pole by the 8-month-old mice. (D) Pole test; performance
score for climbing down the pole of mice at 13 months of age. Data are provided
as the mean SEM, n = 4–9 per group (8-month-old mice), n = 9–12 per
group (13-month-old mice). *p 0.05, **p 0.01,
vs. model group. ICA, icariin; SEM, standard error of the mean; Tg (+),
A53T -synuclein transgenic mice; WT, wild type.
The pole test assesses the coordination ability of mice. The 8-month-old A53T Tg
mice climbed down the pole quicker than the WT control mice, whereas ICA-treated
A53T Tg mice took longer to climb down the pole than the vehicle-treated A53T Tg
mice, albeit without statistically significant differences (Fig. 1C). For
13-month-old mice, we evaluated the pole test performance score. A53T Tg mice had
lower scores than the WT group (p 0.01), and ICA (50 and 100
µmol/kg) treatment significantly increased the scores of A53T Tg mice
(p 0.05, p 0.01, Fig. 1D). These results indicate that
ICA was able to alleviate impaired motor function and coordination in A53T Tg
mice at 8 and 13 months of age.
3.2 ICA Decreased the Expression Level and Aggregation of
-Synuclein in the Striatum of A53T Tg Mice
We used western blotting to detect the expression levels of -synuclein
in the striatum of 8-month-old and 13-month-old mice. The results showed that the
levels of -synuclein monomers and polymers were increased in the
striatum of A53T Tg mice compared with WT mice at 8 months of age (p
0.05, Fig. 2A,B). ICA treatment at doses of 50 and 100 µmol/kg
significantly decreased the level of -synuclein monomers (p 0.01), with 100 µmol/kg ICA treatment significantly decreasing the
level of -synuclein polymers in the striatum of A53T Tg mice at 13
months of age (p 0.05, Fig. 2C,D). These results indicate that ICA
reduced the expression and aggregation of -synuclein in the striatum of
A53T mice.
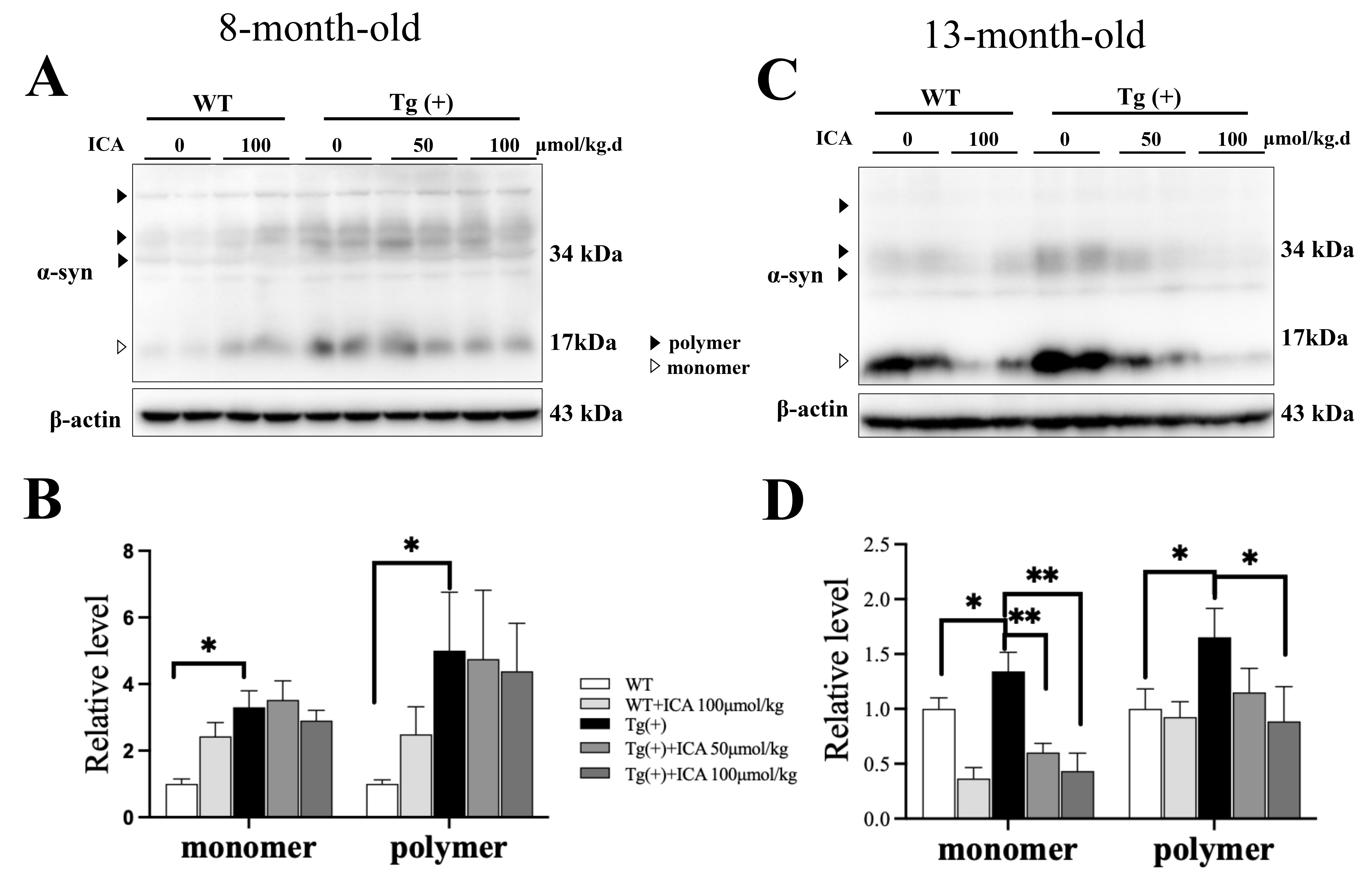 Fig. 2.
Fig. 2.
Effects of ICA on the expression level and aggregation of
-synuclein (-syn) in the striatum of A53T Tg mice (western
blotting). (A) Representative blot images of -synuclein in 8-month-old
mice. (B) Quantitative analysis of the -synuclein monomer and polymer
forms in 8-month-old mice. (C) Representative blot images of -synuclein
in 13-month-old mice. (D) Quantitative analysis of the -synuclein
monomer and polymer forms in 13-month-old mice. The ratio of -synuclein
to -actin was taken as 1. Data are provided as the mean SEM, n =
4. *p 0.05, **p 0.01, vs. Tg (+) model group.
ICA, icariin; SEM, standard error of the mean; Tg (+), A53T -synuclein
transgenic mice; WT, wild type.
3.3 ICA Decreased the Phosphorylation of -Synuclein at
Serine 129 in the Striatum of A53T Tg Mice
Phosphorylation of -synuclein at Ser129 is an important marker of
pathological forms of PD and related synucleinopathies. A53T Tg mice at 8 and 13
months of age showed elevated phosphorylation levels of -synuclein at
Ser129 in the striatum compared with WT mice (p 0.01, Fig. 3). ICA
treatment (100 µmol/kg) significantly decreased the phosphorylation level
of -synuclein at Ser129 in the striatum of A53T Tg mice at 8 months old
(p 0.01, Fig. 3A,B), but no significant difference was observed in
A53T Tg mice at 13 months old (Fig. 3C,D). These results indicate that ICA
decreased the phosphorylation of -synuclein, which might inhibit the
formation of pathological -synuclein.
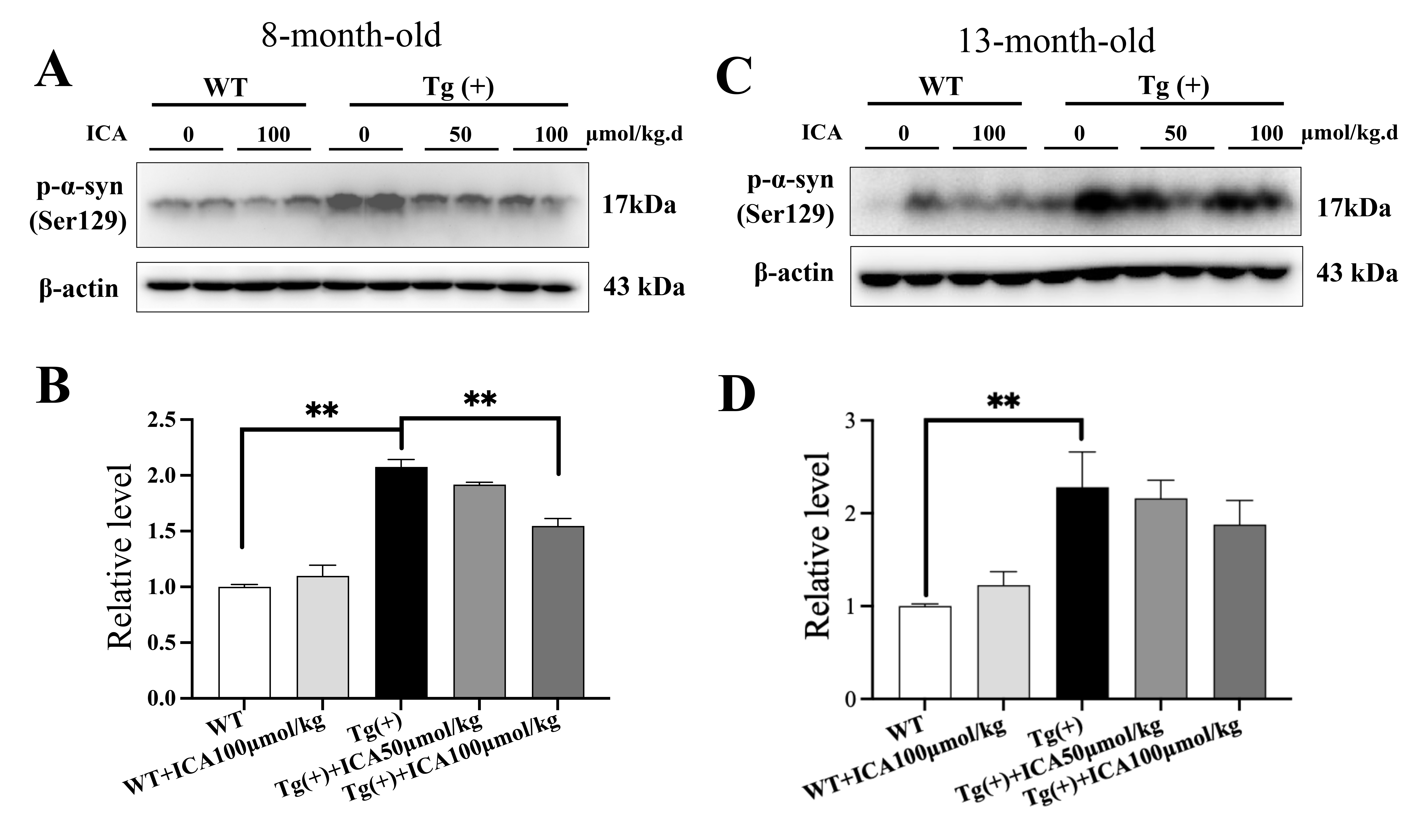 Fig. 3.
Fig. 3.
Effects of ICA on the phosphorylation of -synuclein at
serine 129 in the striatum of the A53T Tg mice (western blotting). (A,B)
Representative blots and quantitative analysis of p--syn (Ser129) in
the striatum of mice at 8 months of age. (C,D) Representative blots and
quantitative analysis of p--syn (Ser129) in the striatum of mice at 13
months of age. The ratio of p--syn (Ser129) to -actin was
taken as 1. Data are provided as the mean SEM, n = 4 per group.
**p 0.01, vs. Tg (+) model group. p--syn
(Ser129), -synuclein phosphorylated at serine 129.
3.4 Effects of ICA on the Expression and Phosphorylation Levels of
-Synuclein in SH-SY5Y Cells Transfected with -Synuclein
Wild-type -synuclein-transfected SH-SY5Y cells were used to
investigate the potential effects of ICA on -synuclein expression and
phosphorylation in vitro. -Synuclein-transfected SH-SY5Y cells
were treated with different dosages of ICA for 48 h. Cell viability after 24 h or
48 h of treatment with ICA was assessed using an MTT assay. The results indicated
that ICA did not exhibit any toxic or inhibitory effects on SH-SY5Y cells at a
dose range of 1.56–200 µM (Fig. 4A,B).
 Fig. 4.
Fig. 4.
Effects of ICA on the expression and phosphorylation of
-synuclein (-syn) in SH-SY5Y cells. (A) Cell viability after
a 24-h treatment with ICA at different doses. (B) Cell viability after a 48-h
treatment with ICA at different doses; cell viability = optical density (OD) in
ICA-treated group/OD in control group. (C,D) Representative western blot images
and quantitative analysis of -synuclein in SH-SY5Y cells. (E,F)
Representative blots and quantitative analysis of Ser129-phosphorylated
-synuclein in SH-SY5Y cells. The ratio of -syn and
p--syn (Ser129) to -actin in the vehicle-treated
-syn-transfected group was taken as 100%. (G) Representative images of
immunocytochemistry staining for -synuclein (-syn, red), GFP
(green), and nucleus (Hoechst33342, blue) as well as the merged images; scale bar
= 50 µm. Data are provided as the mean SEM, n = 3.
p 0.01, vehicle-treated -syn-transfected
group vs. vector control group; *p 0.05, **p
0.01, ICA-treated -syn-transfected group vs. vehicle-treated
-syn-transfected group. GFP, green fluorescent protein;
p--syn (Ser129), -synuclein phosphorylated at serine 129.
The expression and phosphorylation levels of -synuclein were detected
using western blotting. Compared with vehicle-treated cells transfected with
GFP-tagged -synuclein, ICA decreased the elevated expression level of
GFP-tagged -synuclein (p 0.05, p 0.01, Fig. 4C,D). Moreover, the level of Ser129-phosphorylated -synuclein was
significantly increased in vehicle-treated SH-SY5Y cells transfected with
-synuclein, and ICA treatment dose-dependently decreased the level of
the Ser129-phosphorylated -synuclein (p 0.01, Fig. 4E,F).
In the western blot assay, ICA at a dose of 40 µM decreased the levels of
Ser129-phosphorylated -synuclein without decreasing the elevated
expression level of GFP-tagged -synuclein. Since Ser129-phosphorylated
-synuclein tends to aggregate into its pathological form, we assessed
-synuclein aggregation in SH-SY5Y cells using immunocytochemistry.
Morphologically, GFP staining was similar in the two groups of cells, indicating
comparable expression levels of transfected -synuclein (shown in green,
Fig. 4G). However, detection with the -synuclein antibody revealed that
ICA treatment (40 µM) of SH-SY5Y cells transfected with
-synuclein reduced the deposition of aberrant -synuclein
compared with the vehicle-treated cells (shown in red, Fig. 4G).
These results suggest that ICA may inhibit the overexpression and aggregation of
-synuclein in SH-SY5Y cells.
3.5 ICA Increased the Expression Level of Parkin and Decreased the
Expression Level of PLK2
To explain the possible mechanisms through which ICA affects protein expression
and phosphorylation, we applied western blotting to detect the expression levels
of parkin and PLK2. The expression of parkin was decreased in the brain of A53T
Tg mice compared with WT mice at 13 months of age (p 0.05) and ICA
treatment significantly increased the expression level of parkin (p
0.05, p 0.01, Fig. 5A,B). This result suggests that ICA may activate
parkin-related pathways, including the UPS-related pathways.
 Fig. 5.
Fig. 5.
Effects of ICA on the expression of Parkin and PLK2. (A,B)
Representative blots and quantitative data of parkin in the brain of 13-month-old
A53T Tg mice; n = 4; *p 0.05, **p 0.01, vs. Tg
(+) model group. (C,D) Representative blots and quantitative data of PLK2 in the
-synuclein-transfected SH-SY5Y cells; n = 3; p 0.05, vehicle-treated -syn-transfected group vs. vector
control group; *p 0.05, **p 0.01, ICA-treated
-syn-transfected group vs. vehicle-treated
-syn-transfected group. The ratio of parkin and PLK2 to -actin
was taken as 1. Data are provided as the mean SEM. PLK2, polo-like kinase
2.
In SH-SY5Y cells transfected with -synuclein, the expression level of
PLK2 was significantly increased (p 0.05), while ICA treatment
decreased its expression (p 0.05, p 0.01, Fig. 5C,D).
This result suggests that ICA inhibits the phosphorylation of
-synuclein by regulating PLK2.
4. Discussion
In the present study, A53T mutant -synuclein transgenic mice and
SH-SY5Y cells transfected with wild-type -synuclein were used to
examine the potential effect of ICA on pathological -synuclein in PD
and synucleinopathies. The results indicated that intragastric treatment of ICA
for 3 months improved the impaired motor function and coordination in A53T Tg (+)
mice at 8 and 13 months of age. ICA decreased the expression, Ser129
phosphorylation, and aggregation of -synuclein in the striatum of A53T
mice and -synuclein-transfected cells. Moreover, ICA increased parkin
expression and decreased PLK2 expression.
Usually, the pathology of neurodegenerative diseases, including PD, is
progressive. Initially, abnormal neuronal activity and pathology are not evident
owing to the capacity of the cell or neighboring cells to compensate. Eventually,
clinical and pathological prodromes emerge with the failure of compensatory
effects and cell degeneration [24]. To observe the effects of ICA on different PD
stages, we used two groups of A53T Tg mice at the ages of 8 and 13 months. It has
been previously reported that the motor function of 5-month-old A53T Tg mice is
not impaired [18, 25]. However, A53T Tg mice show obvious motor impairments at 8
months of age due to progressive pathological changes in -synuclein
[19, 20]. In the present study, A53T Tg mice showed impaired motor function and
coordination in the rotarod and pole tests. Treatment with ICA for 3 months
improved the behavioral performance of A53T Tg mice at 8 and 13 months,
indicating that both earlier intervention and therapeutic treatment with ICA had
beneficial effects on -synuclein-induced motor impairment.
High expression of -synuclein has been found in the early stages of PD
in both the brains of patients and animal models [25]. In the present study, ICA
decreased the expression of -synuclein in the striatum of 13-month-old
A53T mice, which might partially explain the beneficial effects of ICA on PD.
Besides A53T mutant -synuclein, the over-expression of wild-type
-synuclein is also a risk factor for PD [26, 27]. Thus, we used SH-SY5Y
cells, a human dopaminergic cell line, to overexpress wild-type
-synuclein through gene transfection. The results showed ICA has
inhibitory effects on the overexpression of -synuclein, which might be
the underlying mechanism ameliorating motor deficits in PD mice.
The phosphorylation and abnormal aggregation of -synuclein show
important impacts on -synuclein-related pathology in PD and
synucleinopathies [28]. A previous study on aging monkey brains showed that
oligomerization and phosphorylation of -synuclein progressively
increased with age in the striatum and hippocampus of the mice [29].
Oligomerization or the polymer form of -synuclein indicate its
aggregation, and the aggregation of -synuclein appears to be a key
predictor of neuronal loss and a pivotal event in the pathogenesis of
synucleinopathies and PD [30, 31]. In this study, ICA decreased the expression level
of -synuclein in 13-month-old A53T mice and the aggregation of
overexpressed -synuclein in SH-SY5Y cells, indicating that ICA
inhibited the aggregation of -synuclein.
Phosphorylation at Ser129 is one of the main pathological modifications of
-synuclein in sporadic and familial Lewy body disease [5]. Members of
the PLK family have been reported to phosphorylate -synuclein, with
PLK2 phosphorylating -synuclein at Ser129 in the central nervous system
[6, 7]. Moreover, PLK2 modulates -synuclein aggregation in mammalian
cells and yeast [6]. The inhibition of PLK2 represents a promising direction for
developing novel therapeutics for synucleinopathies [32]. In the present study,
ICA decreased the phosphorylation level of -synuclein and the
expression of PLK2, indicating the possible effects of ICA on
-synuclein accumulation and toxicity.
The UPS can eliminate unfolded or misfolded proteins through several enzymatic
reactions involving ubiquitin (Ub) protein ligases (E3), Ub-activating enzymes
(E1), and Ub-conjugating enzymes (E2), which contribute to the degradation of
-synuclein [8, 33]. Although the degradation mechanism of
-synuclein in neurons is unclear, agents targeting degradation are
considered a promising strategy for synucleinopathy treatment [34, 35]. Parkin is
a well-known Ub E3 ligase that attaches a polyubiquitin chain to proteins to
target them for UPS degradation and plays a key role in the degradation of
-synuclein [9]. The expression level of parkin is decreased in
-synuclein overexpression models and contributes to
-synuclein degradation, dysfunction, and neurotoxicity [10, 11]. Thus,
increasing parkin expression has been reported to show a protective effect by
promoting the proteasomal clearance of -synuclein [36, 37]. In the
present study, we found that ICA increased the expression level of parkin in A53T
Tg mice, which might partially explain the beneficial effects of ICA on
-synuclein degradation in synucleinopathies.
5. Conclusions
In conclusion, we used A53T mutant -synuclein transgenic mice and
SH-SY5Y cells transfected with wild-type -synuclein to examine the
pharmacological effects of ICA on -synuclein-related pathology in
synucleinopathies, including PD, and the mechanisms involved. We found that the
intragastric treatment of ICA for 3 months significantly improved motor function
and coordination in A53T Tg mice at 8 and 13 months of age. ICA alleviated
-synuclein pathology by decreasing the expression, Ser129
phosphorylation, and aggregation of -synuclein in the striatum of A53T
Tg mice and -synuclein-overexpressing cells. The underlying mechanisms
include an ICA-induced decrease in the expression of PLK2 and an increase in the
expression level of the UPS-associated protein parkin.
Availability of Data and Materials
The datasets used and/or analyzed during the current study are available from
the corresponding author upon reasonable request.
Author Contributions
CS, LZ and LL designed the research study. CS, XZ and DM conducted experiments.
CS and DM analyzed the data. All authors contributed to editorial changes in the
manuscript. All authors read and approved the final manuscript. All authors have
participated sufficiently in the work and agreed to be accountable for all
aspects of the work.
Ethics Approval and Consent to Participate
Animal studies were approved by the Bioethics Committee of Xuanwu Hospital of
Capital Medical University (approval number: 20120912) and were performed in
accordance with the National Institutes of Health Guide for Care and Use of
Laboratory Animals. The study was conducted in compliance with the ARRIVE
guidelines. All methods were performed in accordance with relevant guidelines and
regulations.
Acknowledgment
We thank Professor Hong Ma from the Beijing Institute of Technology for gifting
the -synuclein-transfected SH-SY5Y cell line. We thank Li Zhang and
Yali Li for their technical assistance.
Funding
This study was supported by the National Natural Science Foundation of China
(81673406, 82104419), R&D Program of Beijing Municipal Education Commission
(KM202210025017), Incubation Program Beijing Postdoctoral Sustentation Fund of
China (2013ZZ-25), Cultivation Fund of Hospital Management Center in Beijing
(PZ2022006), Beijing Hospitals Authority Ascent Plan (DFL20190803) and Science
and Technology Think Tank Youth Talent Program (20220615ZZ07110074).
Conflict of Interest
The authors declare no conflict of interest.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5.