- Academic Editor
Objective: Levodopa (L-DOPA) is the primary treatment for Parkinson’s
disease (PD). Nevertheless, the underlying mechanism of its action is not
entirely learned. This study aims to probe the action of L-DOPA on NLR pyrin
domain containing 3 (NLRP3) inflammasome activation and tyrosine hydroxylase (TH)
levels in the striatum (STR) and substantia nigra (SN) of mice with PD symptoms.
Methods: PD was simulated by administering
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; 25 mg/kg/d) to induce mice,
followed by L-DOPA (8 mg/kg/d) treatment. The behavioral performance of the mice
was assessed using the pole test, balance beam, and rotarod test. After
euthanasia with 120 mg/kg sodium pentobarbital, STR and SN were collected for
evaluation of protein level of TH, NLR pyrin domain containing 3 (NLRP3), ASC and Cleaved caspase-1 using Western
blot and mRNA levels of TH, inflammatory factors IL-1
Parkinson’s disease (PD), a progressive neurodegenerative disorder, poses the significant and increasing burden worldwide [1]. PD mainly affects individuals above 40 years old, with the incidence rising as the population ages [2]. PD is featured by debilitating motor symptoms, which can lead to progressive disability, impair daily activities, and significantly diminish quality of life [3, 4, 5]. The pathology of PD involves the progressive degeneration of dopamine (DA) neurons in the substantia nigra (SN) pars compacta and followed dopamine deficiency in the striatum (STR) [6]. Chronic inflammation has been considered as a contributor in PD progression [7], making neurogenic inflammation a prime target for research. Moreover, alterations in tyrosine hydroxylase (TH) levels have been implicated in PD pathogenesis [8], and targeting TH may compensate for functional deficits in PD [9]. Thus, investigating inflammation and TH alteration as potential therapeutic targets for PD treatment and prevention is promising.
Levodopa (L-DOPA) is currently the most capable agent for managing PD symptoms
[10]. Surprisingly, only a small fraction of L-DOPA is required to restore
dopaminergic neurotransmission in the STR [11], but the precise pharmacology of
L-DOPA remains incompletely understood. NLR pyrin domain containing 3 (NLRP3)
expression is elevated in DA neurons of PD patients and animal models [12]. The
NLRP3 inflammasome, a pivotal component of the innate immune system, has been
increasingly pitched in the PD pathogenesis [13, 14, 15]. Suppression of the NLRP3
inflammasome may play a momentous part in reducing
Given the current knowledge gap, it remains unclear whether L-DOPA improves behavioral deficits in PD by manipulating NLRP3 inflammasome activation and TH levels. Thus, this study is to probe whether L-DOPA improves behavioral deficits in mice with PD symptoms by suppressing NLRP3 inflammasome activation and increasing TH levels in the SN and STR.
The 24 male C57BL/6J mice, 8 weeks old and weighing between 24–31 g, were
acquired from Vital River Laboratory Animal Technology (Beijing, China). Then,
the acquired mice were bred in a pathogen-free room under controlled conditions
of 25
After a 1-week adaptation period, the mice were arranged into four groups randomly with six mice in each group: control group (intraperitoneally administration of an equal amount of normal saline), L-DOPA group (intraperitoneal injection of 8 mg/kg/d L-DOPA for 2 days [20]), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) group (intraperitoneal administration of 25 mg/kg/d MPTP from the 1st day to 7th day [21]), and MPTP + L-DOPA group (intraperitoneal injection of 25 mg/kg/d MPTP from the 1st day to 7th day and intraperitoneal injection of 8 mg/kg/d L-DOPA on day 6–7). MPTP hydrochloride (S4732) was obtained from Selleckchem (Shanghai, China), and L-DOPA (D9628) was acquired from Sigma-Aldrich (St. Louis, MO, USA). On the second day after medication, the mouse motor function was assessed using behavioral tests.
Each mouse was placed in a head-up position near the top of a 15-mm-diameter and 40-cm-long rough wooden pole. The time it took for each mouse to arrive the floor was collected. Prior to the formal tests, each mouse underwent a 3-day learning adaptation period with 3 training sessions per day.
The balance beam test involved a 1-meter-long, 0.5 cm wide, and positioned 40 cm high above the floor. To facilitate the rest of the mice, a dark box was kept at one end of the beam. Each mouse performed the balance beam test three times, with a minimum break of 15 minutes between each trial. The time taken by mice to traverse the balance beam was measured and noted.
Following 3 days of training and adaptation, each mouse underwent rotarod tests for 4 successive days. Three formal tests were conducted for each mouse using a rotarod device, with the rotation speed ranging from 5 rpm to 40 rpm over 5 min, with a minimum of 30 min between each trial. The time at which each mouse fell off the rotarod was recorded. If a mouse managed to stay on the rotarod device for more than 5 min, the time was recorded as 300 s. The results from independent replicate tests were averaged for analysis.
After the completion of the behavioral tests, all mice were sacrificed using 120
mg/kg sodium pentobarbital (cat#P3761-5G, Sigma-Aldrich, St. Louis, MO, USA). Subsequently, mouse brain tissues were isolated. The
brain tissues were then homogenized using a commercial Pro-Prep Protein
Extraction Solution (Beyotime, Shanghai, China). Fresh samples of the SN and STR
were collected and stored at –80 °C, from which total protein was
extracted. BCA reagent (Beyotime) was utilized to determine protein concentration
according to the manufacturer’s instructions. Subsequently, a 12% SDS-PAGE was
employed to isolate 40 µg of protein sample. The separated proteins were
transferred onto a polyvinylidene-difluoride (PVDF) membrane (0.22 µm) (Millipore, Billerica, MA, USA).
Thereafter, the membranes were blocked with non-fat milk (5%) for 2 h at room
temperature. Afterwards, the membrane was preserved at 4 °C with primary
antibodies overnight. Following the primary antibody incubation, appropriate
secondary antibodies at room temperature were utilized for 1 h. Then, ECL
detection reagents (Thermo Fisher Scientific, Rockford, IL, USA) were applied for
protein band visualization. Image J software
(NIH, Bethesda, MD, USA) were adopted to
quantify the signal intensity of the bands. The signal was normalized to that of
the internal control,
Total RNA extraction was conducted using TRIzol reagents (Takara, Tokyo, Japan) as per the manufacturer’s instructions. Subsequently, the RNA was reverse-transcribed into complementary DNA (cDNA). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed on a StepOne Plus system (ABI, Foster City, CA, USA) under the following cycling conditions: 95 °C for 5 min, accompanied by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. StepOne software version 2.3 was employed to analyze the results. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization. The primers sequences adopted for RT-qPCR are presented in Table 1.
| Primer | Sequence (5 |
| TH | Forward primer: CACTATGCCCACCCCCAG |
| Reverse primer: CGCCGTCCAATGAACCTT | |
| IL-1 |
Forward primer: TGCCACCTTTTGACAGTGATG |
| Reverse primer: TGATGTGCTGCTGCGAGATT | |
| IL-18 | Forward primer: ACTTTGGCCGACTTCACTGT |
| Reverse primer: GGGGTTCACT GGCACTTTGA | |
| GAPDH | Forward primer: TACCCGGACTGGATTCTACG |
| Reverse primer: AAGTTGGTGGGCTGTCAATC |
RT-qPCR, reverse transcription-quantitative polymerase chain reaction; TH, tyrosine hydroxylase; IL, interleukin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Data were analyzed and graphed using GraphPad Prism 8.01 software (GraphPad
Software, San Diego, CA, USA). Mean
PD was simulated by administering 25 mg/kg/d MPTP to induce mice for 7
successive days. Subsequently, mice with PD symptoms were treated with 8 mg/kg/d
L-DOPA for 2 days via intraperitoneal injection. On the 2nd day after completing
the drug treatment, the pole, balance beam, and rotarod tests were conducted.
There was no prominent difference in the latency to climb down the pole, cross
the balance beam, and fall from the rotating rod between the control group and
the L-DOPA group (Fig. 1A–C, all p
 Fig. 1.
Fig. 1.L-DOPA improves behavioral deficits of MPTP-led mice with PD
symptoms. (A) The pole test. (B) The balance beam test. (C) The rotarod test. N
= 6. Data were shown as mean
Recent studies have indicated a decrease in TH levels in the STR of mice with PD
symptoms [22]. RT-qPCR and Western blot analyses were conducted so as to explore
the role of L-DOPA on TH expression. The results displayed no obvious difference
in TH mRNA/protein levels between the control group and L-DOPA group
(Fig. 2A,B, all p
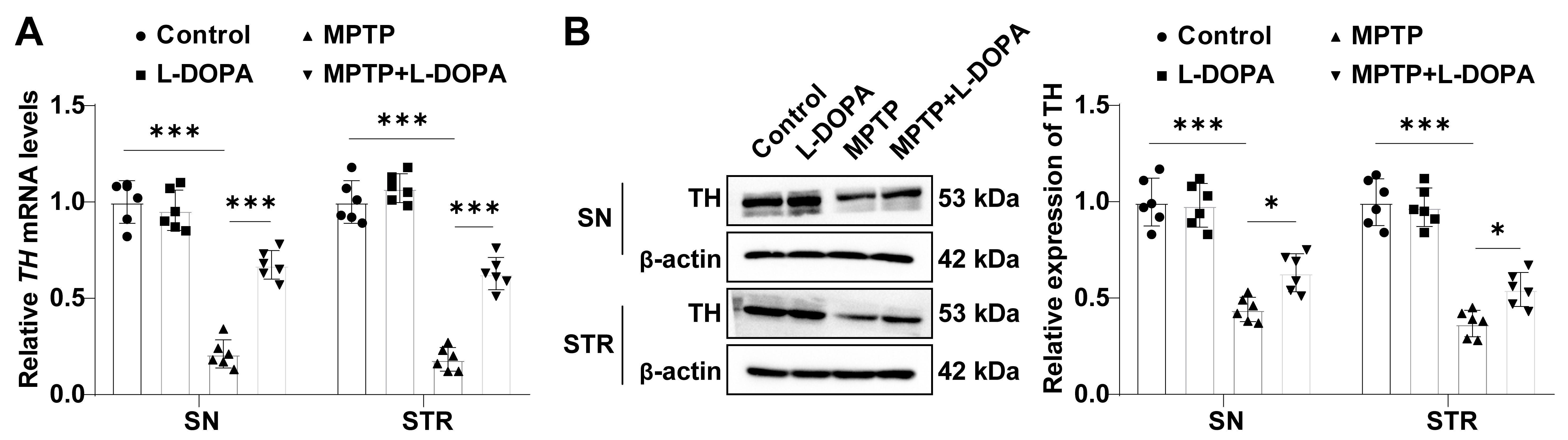 Fig. 2.
Fig. 2.L-DOPA elevates TH levels in the SN/STR of MPTP-led mice with PD
symptoms. (A) The mRNA expression of TH measured by RT-qPCR. (B) TH
protein level estimated by western blot. N = 6. Data were shown as mean
Growing evidence suggests a crucial role of inflammatory response and NLRP3
inflammasome activation in PD development [13, 15, 23]. Western blot analysis
revealed no significant difference in the levels of NLRP3, ASC, and Cleaved
caspase-1 between control mice and L-DOPA-treated mice in the SN and STR (all
p
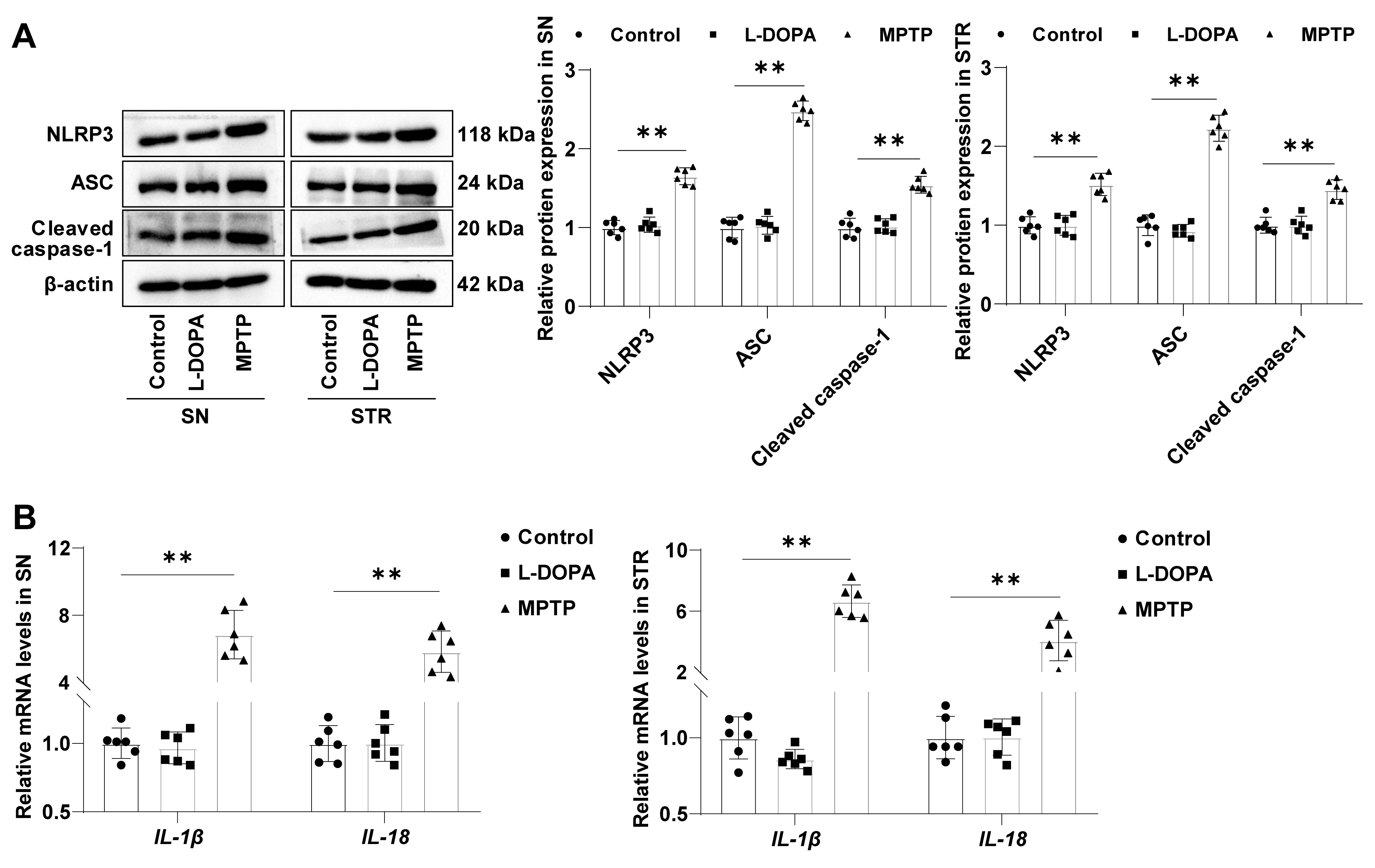 Fig. 3.
Fig. 3.NLRP3 inflammasome is activated in the SN/STR of MPTP-led mice
with PD symptoms. (A) NLRP3/ASC/Cleaved caspase-1 level assessed by Western
blot. (B) IL-1
To probe the role of L-DOPA on NLRP3 inflammasome activation, protein expression
levels of NLRP3/ASC/Cleaved caspase-1, and mRNA levels of
IL-1
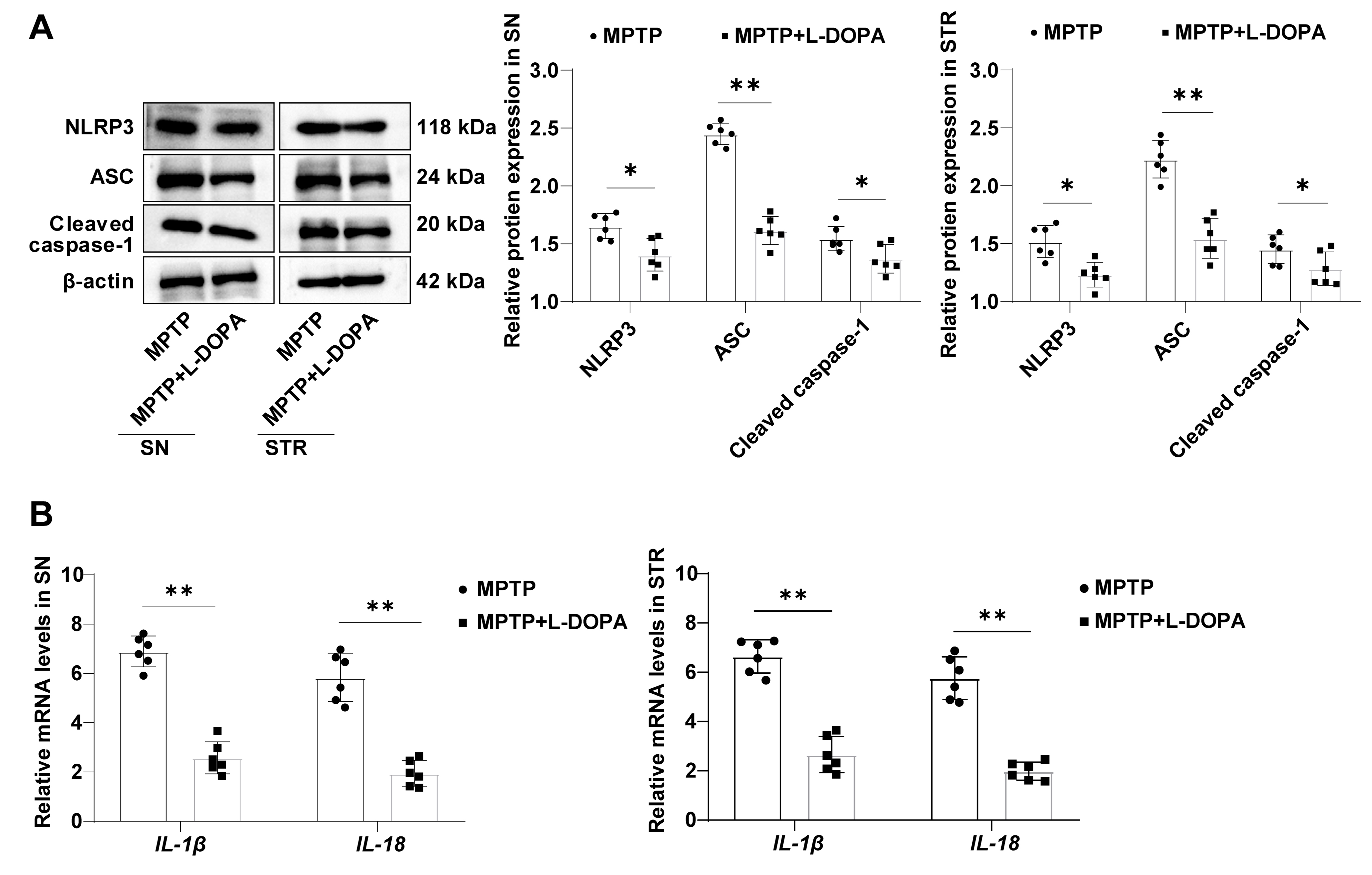 Fig. 4.
Fig. 4.L-DOPA curbs NLRP3 inflammasome activation in the SN/STR of
MPTP-led mice with PD symptoms. (A) NLRP3/ASC/Cleaved caspase-1 levels measured
by Western blot. (B) IL-1
The use of L-DOPA has been well-established for several decades to treat PD, as it effectively enhance dopaminergic signaling and alleviates movement deficits in the the disease’s early stage [24]. However, the exact mechanism of action by which L-DOPA exerts its therapeutic effects remains to be fully elucidates [25]. The purpose of this study was to delve into the hypothesis that L-DOPA improves behavioral deficits in mice with PD symptoms by suppressing NLRP3 inflammasome activation and increasing TH expression in the SN and STR. The results of this study demonstrated that L-DOPA treatment effectively inhibited NLRP3 inflammasome activation and boosted TH levels in the SN and STR of MPTP-induced mice with PD symptoms.
Behavioral symptoms are a hallmark of PD, and their severity can greatly impact the patients’ life quality [26]. Patients may experience impaired hand function, difficulties in walking, and an enhanced risk of falls in advanced disease [27]. The use of MPTP-induced mice to simulate PD in this study closely recapitulates the motor symptoms observed in human PD patients [28]. Our findings revealed that mice with PD symptoms exhibited reduced performance in the pole test and balance beam test, as well as decreased duration on the rotarod, which are indicative of motor deficits. However, following L-DOPA treatment, mice with PD symptoms showed improved performance in these behavioral tests. Consistent with prior studies, these findings have unveiled the ability of L-DOPA to reverse behavioral deficits in mice with PD symptoms, such as the Pitx3 mouse model, which specifically exhibits prenatal loss of dopaminergic neurons in the nigrostriatal pathway [29]. Additionally, the administration of L-DOPA-loaded crystallization nanoparticles has shown promising effects in ameliorating behavioral deficiencies in mice with PD symptoms [30]. Collectively, our results provide further support for the beneficial effects of L-DOPA in improving behavioral deficits in PD.
PD is characterized by the progressive degradation of the nigrostriatal system, which plays a vital role in the modulation of motor function and motor memory [29]. Before the occurence of typical PD motor symptoms, the nigrostriatal system has long-term latent degradation, with up to 50–60% of dopamine neuron bodies degeneration [31]. In PD, there is a dopamine (DA) deficiency in the STR, and the enzyme TH is accountable for the DA synthesis from tyrosine in DA neurons [8]. Therefore, the loss of TH in the STR partcipated in the PD pathogenesis [32]. Our results presented a reduction in TH expression in the SN and STR of MPTP-induced mice with PD symptoms, but this decrease was reversed after L-DOPA treatment. This is consistent with previous studies demonstrating that L-DOPA treatment can enhance TH levels in the STR of mice with PD symptoms [33]. Furthermore, combination treatment of L-DOPA with embelin has been shown to restore TH protein levels in rotenone-induced mice with PD symptoms [34]. Taken together, our findings provide evidence that L-DOPA could upregulate TH expression in the SN and STR of mice with PD symptoms.
NLRP3 inflammasome bear a part in neuroinflammation in various diseases
[35, 36, 37]. In PD, the activation of inflammasome signaling and the innate immune
system play a significant role in the neuroinflammatory processes [38]. Among the
inflammasomes, NLRP3 inflammasome has been strongly associated with PD
development [12]. When activated, NLRP3 interacts with ASC to generate the
inflammasome complex, which then brings about caspase-1 activation and the
production of inflammatory cytokines, for instance, IL-1
The findings of this study highlight the potential of L-DOPA in improving the
behavioral deficits observed in mice with PD symptoms, potentially through the
repression of NLRP3 inflammasome activation and the augmentation of TH expression
in the SN and STR. However, it is important to acknowledge the limitations of
this study. due to constraints in time, resources, and funding, our investigation
focused solely on examining TH expression, changes in NLRP3
inflammasome-associated proteins, and IL-1
Moreover, our study primarily explored the involvement of L-DOPA in modulation of NLRP3 and TH expression without delving into the intricate mechanisms underlying its actions. Future research should aim to elucidate the detailed functional mechanisms through which L-DOPA exerts its effects in mice with PD symptoms. By conducting further investigations, we can gain a deeper understanding of how L-DOPA impacts the molecular pathways associated with NLRP3 inflammasome activation and TH expression. Due to time, financial and manpower constraints, this study only briefly performed behavioral experiments in mice with PD symptoms. We only detected the levels of NLRP3 inflammasome and TH in the STR and SN, and did not involve neuroimaging studies for the time being, which we will further improve in subsequent experiments. Collectively, while this study provides scientific insights into the potential of L-DOPA in ameliorating behavioral deficits in mice with PD symptoms by targeting NLRP3 inflammasome activation and TH expression, future studies should expand upon these findings. Utilizing an NLRP3 inflammasome agonist and investigating the underlying mechanisms of L-DOPA in greater detail will contribute to a more comprehensive understanding of its therapeutic effects in PD.
This study highlighted that L-DOPA improves the behavioral deficits in mice with PD symptoms possibly by suppressing NLRP3 inflammasome activation and increasing TH levels in the STR/SN. These findings provide scientific basis into the therapeutic mechanism of L-DOPA in PD.
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
XC designed the research study. XC and ZW performed the research. XC and YF provided help and advice on experiments. WY analyzed the data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Institute of Biotechnology, Shanxi University (approval number: 2022037).
Not applicable.
This research received no external funding.
Xi Chen, Zhao Wang and Weihua Yang are come from Changxing Pharmaceutical Co., Ltd. The authors have no conflicts of interest to declare.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
