- Academic Editor
Background: The impact of the methylenetetrahydrofolate
reductase (MTHFR) C677T mutation on the relationship between plasma
homocysteine (Hcy) levels and stroke has been extensively studied and documented
in previous study. However, it remains unclear whether the MTHFR C677T
mutation can affect the response to Hcy lowering treatment in stroke patients
with hyperhomocysteinemia (HHcy). Understanding the impact of genetic factors on
treatment response can help optimize personalized treatment strategies for stroke
patients with HHcy. We aimed to investigate the potential association between the
MTHFR C677T gene polymorphisms and the effectiveness of Hcy lowering
treatment using vitamin therapy in stroke patients with HHcy.
Methods: The MTHFR C677T genotype polymorphisms were
identified using polymerase chain reaction-restriction fragment length
polymorphism, and the distribution of three genotypes in the MTHFR C677T
gene locus was compared. The treatment effects of Hcy lowering agents were
compared among patients with different genotypes.
Results: Among the 320 stroke
patients enrolled in the study, 258 (80.6%) were diagnosed with HHcy. Of these,
162 patients (Effective Group) responded well to the clinical Hcy lowering
treatment, while 96 patients (Invalid Group) failed to achieve sufficient
response even after taking combination supplements of folic acid, Vitamin B6, and
methylcobalamin for one month. Significant differences were observed in terms of
age (p
Due to the growing size and aging of the world’s population, the global incidence of stroke is alarmingly high, with approximately 16.9 million people experiencing a stroke each year [1]. Numerous conventional risk factors contribute to the onset and progression of stroke, such as diabetes mellitus, hypertension, atrial fibrillation, hyperlipidemia, and so on. These factors have been extensively studied, and their underlying mechanisms are well-established [2]. As a result, many primary prevention strategies have been developed and implemented to reduce stroke incidence, particularly in developed countries [3]. Additional effective stroke prevention strategies are still required to be developed to further decrease the global burden of stroke.
Nevertheless, there are several other potential risk factors that have not been thoroughly investigated, and homocysteine (Hcy) is one such non-traditional risk factor for stroke [2, 4]. Moreover, the predictive accuracy of the Framingham Stroke Risk Score could be improved by incorporating four biomarkers (including Hcy), based on the findings of Framingham’s offspring cohort [5]. Prior study has shown that elevated levels of Hcy was independently linked to the incidence of stroke in young individuals [6]. Hyperhomocysteinemia (HHcy) is currently recognized as a risk factor for cardiovascular and cerebrovascular diseases. However, its underlying mechanism is complex and not yet fully understood [7]. HHcy has been found in approximately 60.6% of stroke patients, and this is associated with low serum B12 level [8]. There is indeed a causal relationship between high plasma Hcy levels and ischemic stroke [4, 9]. Intracerebral hemorrhage constitutes 10–15% of all stokes cases and is associated with high morbidity and mortality rates. Elevated plasma Hcy levels can damage endothelial function, disrupt methylation reactions, increase oxidative stress, and alter the structure of proteins. These effects can indirectly contribute to the occurrence and development of atherosclerosis [10]. The elevated plasma Hcy levels could be influenced by various factors, including nutrition, diet, disease, medication, and heredity [11].
Methylenetetrahydrofolate reductase (MTHFR) is a crucial enzyme that regulates the conversion of N5, N10-methylene tetrahydrofolate to 5-methyltetrahydrofolate, a key step in folic acid metabolism. This process is essential for DNA methylation and repair, which are important for maintaining the integrity and stability of genetic material [12]. Recently, there has been increased interest in the TT genotypes of the MTHFR C677T polymorphism as a research topic [13]. Prior studies have shown that individuals with the C677T genotype of MTHFR gene have approximately twice the plasma Hcy levels compared to those with the normal genotype [13]. The impact of the MTHFR C677T mutation on the relationship between plasma Hcy levels and stroke is well-documented [14]. Nevertheless, it is still ambiguous whether MTHFR C677T mutation could influence the response to Hcy lowering treatment in stroke patients with HHcy.
Herein, the aim of our study was to explore the potential link between MTHFR C677T gene polymorphism and the risk of stroke, as well as to evaluate the effectiveness of Hcy lowering treatment in stroke patients with HHcy. Our findings could potentially help alleviate the social and economic burden associated with stroke by informing the development of effective prevention and treatment strategies for at-risk populations.
This retrospective single-center study analyzed the clinical data of stroke patients with HHcy who were consecutively recruited from the Department of Neurology, Quanzhou First Hospital Affiliated to Fujian Medical University between May 2017 and December 2020. The inclusive criteria were as follows: (1) age of 18 years or older; (2) patients diagnosed with ischemic stroke based on the 2010 guidelines for the diagnosis and treatment of acute ischemic stroke in China [15]; or cerebral hemorrhage according to diagnostic criteria at the Fourth National Conference on Cerebrovascular Disease [16]; (3) patients with plasma Hcy levels of 15 µmol/L or higher. The exclusive criteria were as follows: (1) patients with subarachnoid hemorrhage or traumatic cerebral hemorrhage; (2) pregnant or lactating women; (3) patients with malignant tumors; and (4) incomplete follow-up data. All data collection, storage and processing were done in compliance with the Helsinki Declaration. All patients provided signed, informed consent and the study number (2017-022) was approved by the ethics committee of Quanzhou First Hospital Affiliated to Fujian Medical University.
To lower the plasma Hcy level, all patients were given a combination of vitamin supplements, which included folic acid tablets of 0.4 mg once daily, Vitamin B6 of 100 mg three times daily and methylcobalamin of 500 µg three times daily. The plasma Hcy levels were recorded at baseline and one month after treatment. Patients who did not achieve the normal range of plasma Hcy level or whose Hcy reduction amplitude was less than 20% were classified as the Invalid Group, indicating an insufficient response to the treatment. The patients who achieved the normal range of plasma Hcy level or whose Hcy reduction amplitude was greater than or equal to 20% were categorized as the Effective Group. Patients with other comorbidities, such as hypertension, diabetes, etc., were treated according to the corresponding standard therapeutic guidelines.
Upon admission, clinical data of all patients were collected, which included age, sex, medical history of hypertension, diabetes mellitus, or heart disease, disorder of lipid metabolism, and stroke type (hemorrhagic or ischemic). The baseline plasma Hcy level of each enrolled patient was measured in the morning. A fasting blood sample of approximately 5 mL was collected from the elbow vein (with heparin anticoagulation) and then centrifuged at 3000 rpm for 10 minutes. Furthermore, the plasma Hcy level was measured using the chemiluminescence immunoassay method with the Biochemical-Radiochemical Laboratories (BIO-RAD) automatic biochemical analyzer (S1000 PCR instrument, Bio-Rad C1000 PCR, Bio-Rad, Irvine, CA, USA) and Mindray reagent. The normal range for plasma Hcy levels is typically considered to be between 5 and 15 µmol/L [17]. Patients with plasma Hcy levels higher than 15 µmol/L were diagnosed with HHcy. The plasma Hcy levels of all enrolled patients were re-evaluated one month after receiving combination supplements of folic acid, Vitamin B6, and Vitamin B12.
Plasma Hcy levels were measured for all enrolled patients at the Laboratory Department. The detection of MTHFR C677T gene polymorphism was performed using the Baio MTHFR (C677T) gene detection kit (Shanghai BaiO Technology Co., Ltd., Shanghai, China) with a PCR instrument (BS-800M S1000TM Thermal cycler, Bio-Rad, Irvine, CA, USA). The standard protocol for genotyping all three known C677T polymorphic loci was polymerase chain reaction–restriction fragment length polymorphism analysis (PCR-RFLP) [18].
To assess the distribution of continuous data, the Kolmogorov-Smirnov test was
employed. Normally distributed continuous data were presented as mean
Among the 320 stroke patients with HHcy who received treatment at our hospital,
258 (80.6%) patients met the inclusive and exclusive criteria and included in
the final analysis. Sixty-two patients were excluded from the study due to
insufficient renal function (n = 17; 5.3%) and an unfinished whole treatment
course due to liver damage (n = 45; 14.1%). The majority of patients responded
to the clinical Hcy lowering treatment and were classified as the Effective
Group, with a return to the normal range or greater than 20% reduction in plasma
Hcy levels after completing the treatment course (n = 162; 62.8%). The remaining
96 patients (37.2%) were classified as the Invalid Group. There was a
significant difference in terms of age (p
| Variables | Invalid Group (n = 96) | Effective Group (n = 162) | p-value | |
| Age, years, mean |
48.2 |
58.6 |
||
| Gender, male | 81 (84.4) | 137 (84.6) | 0.967 | |
| Stroke type | 0.115 | |||
| Hemorrhagic | 53 (55.2) | 73 (45.1) | ||
| Ischemic | 43 (44.8) | 89 (54.9) | ||
| Hypertension | 87 (89.6) | 130 (80.2) | 0.034 | |
| Diabetes mellitus | 28 (29.2) | 60 (37.0) | 0.197 | |
| Dyslipidemia | 56 (58.3) | 117 (72.2) | 0.022 | |
| Hyperuricemia | 15 (15.6) | 10 (6.2) | 0.013 | |
| Genotype | ||||
| CC | 2 (2.1) | 13 (8.0) | ||
| CT | 14 (14.6) | 73 (45.1) | ||
| TT | 80 (83.3) | 76 (46.9) | ||
Data are n (%) unless otherwise indicated. SD, standard deviation.
The genotype frequencies of MTHFR C677T gene polymorphism were all in accordance with Hardy-Weinberg equilibrium (HWE) in stroke patients with HHcy. Among patients with an insufficient response (n = 96), there were 2 patients with CC genotype, 80 patients with TT genotype, and 14 patients with CT genotype, respectively. Of these patients, 12 patients had an unstable increase in Hcy levels (CC, n = 0; TT, n = 18; and CT, n = 2), and 47 patients had the Hcy reduction amplitude of less than 20% (CC, n = 2; TT, n = 62; CT, n = 12) (Fig. 1). Plasma Hcy levels at baseline and one month after treatment for each genotype are indicated in Table 2 and Fig. 2. The results suggested that there was a significant difference in plasma Hcy levels among patients with various genotype at baseline (p = 0.041) and one month after treatment (p = 0.004).
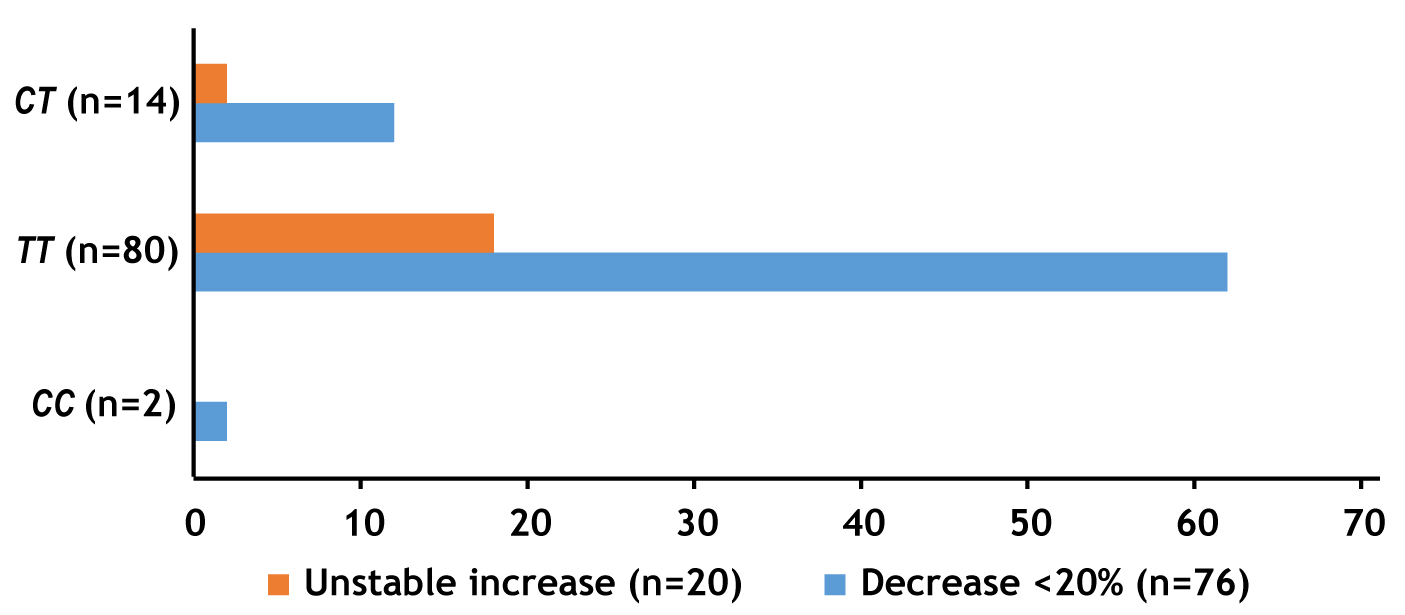 Fig. 1.
Fig. 1.Distribution of MTHFR C677T genotypes in patients of the Invalid Group (n = 96).
 Fig. 2.
Fig. 2.Boxplots showing the distribution of homocysteine (Hcy) levels before and after 1-month of treatment in patients with CC (n = 15), CT (n = 87), and TT (n = 156) genotypes.
| CC (n = 15) | CT (n = 87) | TT(n = 156) | p-value | |
| Hcy levels before treatment, µmol/L | 19.57 |
19.21 |
21.51 |
0.041 |
| Hcy levels after 1-month of treatment, µmol/L | 14.19 |
13.07 |
19.89 |
0.004 |
| p-value |
0.013 | 0.070 |
ANOVA, analysis of variance; LSD, least significant difference.
The unadjusted model showed that age, medical history of hypertension, dyslipidemia, and hyperuricemia were associated with an increased risk of insufficient Hcy lowering treatment effect, with all these factors having a p-value of less than 0.05. These factors were adjusted for in the multivariate regression analysis. The multivariate regression analysis also showed that the Tallele was independently associated with an insufficient Hcy lowering treatment effect (OR, 1.327; 95% CI, 1.114–1.580; p = 0.0015). Furthermore, in the codominant model, the TT genotype was also independently associated with insufficient response (OR, 1.645; 95% CI, 1.093–2.476; p = 0.017), which was consistent with the findings observed in the recessive model (TT versus CC + CT; OR, 1.529; 95% CI, 1.145–2.042; p = 0.004). However, no relationship between CT + TT genotypes and poor treatment effect was observed in the dominate model (Table 3).
| Effective Group | Invalid Group | Adjusted OR |
95% CI | p-value | |||
| n (%) | n (%) | ||||||
| Allele | |||||||
| C | 99 (30.6) | 18 (9.4) | 1.00 | Reference | - | ||
| T | 225 (69.4) | 174 (90.6) | 1.327 | 1.114–1.580 | 0.0015 | ||
| Model type | |||||||
| Codominant | |||||||
| CC | 13 (8.0) | 2 (2.1) | 1.00 | Reference | - | ||
| CT | 73 (45.1) | 14 (14.6) | 0.742 | 0.411–1.339 | 0.322 | ||
| TT | 76 (46.9) | 80 (83.3) | 1.645 | 1.093–2.476 | 0.017 | ||
| Dominant | |||||||
| CC | 13 (8.0) | 2 (2.1) | 1.00 | Reference | - | ||
| CT + TT | 149 (92.0) | 94 (97.9) | 0.826 | 0.652–1.046 | 0.113 | ||
| Recessive | |||||||
| CC + CT | 86 (53.1) | 16 (16.7) | 1.00 | Reference | - | ||
| TT | 76 (46.9) | 80 (83.3) | 1.529 | 1.145–2.042 | 0.004 | ||
OR, odd rations; CI, confidence interval.
Stroke can result in long-term disability, making it crucial to prioritize early detection and active management of potential risk factors. This can effectively prevent the occurrence of stroke [3]. The role of genetic factors in the pathogenesis of stroke has been confirmed through associations between specific gene variants and the risk of stroke occurrence. However, conflicting results [19, 20] have made it difficult to determine the effects of these polymorphisms on the risk of stroke development. This ambiguity may be due to the heterogeneity of cerebral infarction [20, 21].
HHcy has been identified as an independent risk factor for cerebrovascular and
cardiovascular atherosclerotic occlusive diseases [22]. It is a relatively new
risk factor for ischemic stroke, with approximately 60% of stroke patients
exhibiting HHcy, which may be associated with lower levels of serum B12 [23].
Additionally, there is a positive correlation between plasma Hcy levels and
ischemic stroke [24, 25]. The National Health and Nutrition Survey conducted a
survey in 1999–2004, analyzing the effects of high Hcy (
A Meta-analysis revealed that the TT genotype of MTHFR C677T gene polymorphism had a greater influence on plasma Hcy levels in Asian countries than in non-Asian countries [27]. Our study revealed that the frequency of the TT genotype in stroke patients was as high as 60.5%, which was higher than that reported in the Caucasian population [28]. Our study did not find a significant difference in the frequency of the TT genotype between patients with ischemic stroke and those with hemorrhagic stroke. However, the proportion of patients with insufficient treatment effects was significantly higher in those with the TT genotype (83.3%) compared to those with the CC and CT genotype (46.9%). This finding was consistent with the results of a prospective study conducted in a similar ethnic background and disease context [24]. Our study also emphasized the significance of early detection of plasma Hcy levels in the Chinese population with the TT genotype of the MTHFR gene polymorphism. Early detection can aid in identifying individuals who are at a higher risk of developing HHcy and experiencing suboptimal treatment outcomes.
Vitamin B, specifically mecobalamin, has been proven to effectively lower plasma Hcy levels. The standard clinical practice is to supplement with a combination of Vitamin B9 (folic acid), Vitamin B12, and Vitamin B6 [24]. This treatment regimen has been found to reduce the risk of stroke occurrence, and is widely recommended as a preventive measure against stroke [3]. Prior study indicated that the dosage of 0.8 mg folic acid per day exerted sufficient effect on lowering plasma Hcy level [29].
However, there was still insufficient evidence to support the notion that long-term intake and higher doses of folic acid can enhance the treatment effects. Additionally, the safety of such practices still requires careful consideration. Triple combinations of folic acid tablets, Vitamin B6, and mecobalamin have been utilized in the treatment of patients with HHcy. Chinese guidelines for the secondary prevention of ischemic stroke and transient ischemic attack recommend the supplementation of folic acid, Vitamin B6, and Vitamin B12 in recent ischemic stroke or transient ischemic attack patients, or those with elevated plasma Hcy level [15]. Furthermore, the guidelines also suggested that the supplementation of the three compound vitamins can effectively lower Hcy levels and significantly reduce the risk of stroke occurrence [3].
In our study, the majority of patients with CT and CC
genotypes experienced a satisfactory treatment outcome following standard
combination therapy. After one month of vitamin combination supplements, there
was limited effect in lowering Hcy levels in patients with the TT
genotype. That is to say, patients who were more likely to be influenced by
hereditary factors did not show an obvious response to treatment, which may
require prolonged treatment cycles. A previous study also reported that even
though folic acid and Vitamin B12 levels were normal in HHcy patients with the
MTHFR C677T TT genotype, they could still benefit from folic acid
supplementation [30]. However, there is still a lack of studies on how to modify
treatment in a few patients with increased folic acid and Vitamin B12 levels.
Additionally, co-morbidities associated with oxidative stress may act
synergistically with genetic polymorphisms that limit the anti-oxidative stress
capacity of MTHFR (and cystathionine
An increased plasma Hcy level can be influenced by various factors, including diet, heredity, and drugs [32]. Our study evaluated the treatment effects of stroke patients with HHcy and demonstrated that the TT genotype of the MTHFR C677T gene contributes to the genetic susceptibility of stroke. This genotype is more common in the Chinese population, which may further increase the risk of stroke occurrence. Therefore, primary hospitals could conduct genotype detection to assist in stratifying risk factors. It is recommended to administrate standardized folic acid in combination with Vitamin B to patients with HHcy, particular those with MTHFR C677T TT genotype. Plasma Hcy level should be screened as age increases, and targeted treatment to lower Hcy levels should be initiated as early as possible.
The present study has some potential limitations that should be acknowledged. Firstly, it is a retrospective study conducted at a single center with a limited sample size. Therefore, our findings need to be validated in larger multicenter prospective trials. Secondly, we only investigated the MTHFR gene’s role in regulating Hcy levels, and other genes that may affect Hcy levels were not examined. Additionally, long-term follow-up beyond one month is necessary to explore the treatment duration’s impact. Thirdly, future studies should consider how to manage abnormal Hcy elevation in HHcy patients with the TT genotype, including but not limited to dosage modification, alternative agents, or various administration methods.
Our findings indicated that the TT genotype and T allele of the MTHFR C677T gene polymorphism were independently associated with insufficient Hcy lowering treatment effects in stroke patients with HHcy.
MTHFR, methylenetetrahydrofolate reductase; Hcy, homocysteine; HHcy, hyperhomocysteinemia; PCR-RFLP, polymerase chain reaction–restriction fragment length polymorphism analysis; SD, standard deviation; OR, odd rations.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ZL and ZZ prepared the conception and design of the study, participated in the acquisition and analysis of data. ZL and ZZ wrote the original draft of the manuscript and critically revised the manuscript. MH, QY, CL, BH and JW participated in the acquisition and analysis of data, critically revised and edited the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All data collection, storage and processing were done in compliance with the Helsinki Declaration. All patients provided signed, informed consent and the study number (2017-022) was approved by the ethics committee of Quanzhou First Hospital Affiliated to Fujian Medical University.
We thank all patients and their families for participating in this study.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
