- Academic Editor
†These authors contributed equally.
Background: Metformin has been shown to have potent analgesic effects; however, the underlying mechanism of synaptic plasticity mediating analgesia remained ambiguous. Methods: In this study, animal behavioral tests, whole-cell patch‑clamp recording, immunofluorescence staining, and network pharmacology techniques were applied to elucidate the mechanisms and potential targets of metformin-induced analgesia. Results: Single or consecutive injections of metformin significantly inhibited spinal nerve ligation (SNL)-induced neuropathic pain, and formalin-induced acute inflammatory pain. Network pharmacology analysis of metformin action targets in pain database-related targets revealed 25 targets, including five hub targets (nitric oxide synthase 1 (NOS1), NOS2, NOS3, epidermal growth factor receptor (EGFR), and plasminogen (PLG)). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis demonstrated that metformin-induced analgesia was markedly correlated with calcium signaling and synaptic transmission. Intrathecal injection of metformin significantly reversed nerve injury-induced c-Fos (neural activity biomarker) mRNA and protein expression in neuropathic rats by regulating NOS2 expression. In addition, whole-cell recordings of isolated spinal neurons demonstrated that metformin dose-dependently inhibited the enhanced frequency and amplitude of miniature excitatory synaptic currents (mEPSCs) but did not affect those of miniature inhibitory synaptic currents (mIPSCs) in neuropathic pain. Conclusions: This study further demonstrated that metformin might inhibit spinal glutamatergic transmission and abnormal nociceptive circuit transduction by monitoring synaptic transmission in pain. Results of this work provide an in-depth understanding of metformin analgesia via synaptic plasticity.
Pain is characterized by central and peripheral inflammation [1, 2]. Inflammation causes the release of inflammatory mediators, such as microglia-derived bradykinin, chemokines, nerve growth factor, pro-inflammatory cytokines, and prostaglandins, which might promote pain sensations by activating and sensitizing receptors on the terminal cell bodies of nociceptors, including G-protein–coupled receptors, ionotropic receptors, and tyrosine kinase receptors [2, 3, 4]. Inflammatory factors invade multiple ion channels in the presynaptic membrane, resulting in abnormal nociceptive circuits; animals exhibit an overreaction to external stimuli and hypersensitivity of neural circuits to nociceptive signals, leading to peripheral and central sensitization [5]. A series of calcium channel has been thoroughly investigated, including transient receptor potential cation channel subfamily V member 1 (TRPV1), T-type calcium, and transient receptor potential cation channel subfamily A member 1 (TRPA1) [6, 7]. The occurrence of pain is a consequence of neuronal adaptability in pain-coding pathways and circuits. Typically, the pain sensory balance is regulated by a delicate equilibrium between excitation and inhibition [8, 9]. Alterations in the pain circuits have been linked to the severity and persistence of inflammatory and neuropathic pain [10]. Current mainstream analgesic drugs, such as morphine, exert inhibitory effects by agonizing µ opioid receptor (µ-OR), resulting in reduced neuronal excitability and the inability to switch to nociceptive signals for analgesia and sedation [11]. Notably, the noxious circuitry exhibits a dynamic increase in excitability that involves the activation of chemical mediators, such as excitatory amino acids signals. This process also recruits intracellular signaling transduction cascades and induces long-lasting changes in synaptic transmission [12]. Thus, synaptic and circuit changes are the central mechanism of pharmacological analgesia.
Metformin (biguanide) is a disease-modifying drug indicated for type 2
diabetes. Recently, pre-clinical studies have shown that metformin has
antinociceptive effects and alleviates the pathological pain response via
regulation of the Adenosine monophosphate-activated protein kinase (AMPK)
signaling pathway in the dorsal root ganglion (DRG) of diabetic rats - mediated
by the phosphorylation of nuclear factor-kappaB (NF-
Therefore, the synaptic transmission of metformin may be a crucial or dominant factor for pain relief. In our study, intrathecal or intraperitoneal metformin produced potent analgesic phenotypes in rats with neuropathic- and formalin-induced pain. Network pharmacology hinted that the potential analgesic targets of metformin were related to synaptic transmission. In addition, immunofluorescence staining of the spinal cord indicated that metformin significantly reduced the expression of c-Fos following spinal nerve ligation (SNL). Thus, we found that the underlying synaptic plasticity mechanism of metformin in neuropathic pain was mediated by restoring NOS2 and c-Fos expression, subsequently inhibiting spinal glutamatergic transmission.
Adult male Wistar rats, weighing 120–150 g, were provided by SPF (Beijing, China)
Biotechnology Co., Ltd. All experimental procedures were approved by the National
Institutes of Health and the Center for Laboratory Animals of Shanghai Mental
Health Center and performed according to the guidelines of the National Research
Council for the care and use of laboratory animals. The animals were kept in
specific pathogen-free (SPF) conditions in a 12 h/12-h light/dark cycle at 22
°C–24 °C and 50%–60% relative humidity. All rats had free
access to food and water. Every effort was made to reduce suffering and the
number of rats used. Metformin (CAS:657-24-9, Mecklin, Shanghai, China) was
dissolved in artificial cerebrospinal fluid (ACSF; 1.2 mM MgCl
For intrathecal catheterization, polyethylene intrathecal catheters (PE-10: OD, 0.61 mm; ID, 0.28 mm, Advanced Biotechnologies Inc, South Dennis, MA, USA) were implanted into rats after inhalation of isoflurane anesthesia (R510-22, RWD, Shenzhen, Guangdong, China, 4%–5% induction, 1.5% maintenance). The catheter was inserted near the L5 spinal segmental level into the L5/L6 intervertebral subarachnoid space. Rats recovering from surgery were housed in individual cages for 14 days. Those that exhibited immediate bilateral hind-limb paralysis were chosen for the study following 10 µL of 4% lidocaine (CAS:137-58-6, Sigma, St Louis, MO, USA) injections. For intrathecal metformin administration, 10 µL of the metformin solution was injected slowly and then flushed with 15 µL of ACSF.
The SNL model was established based on Kim and Chung for neuropathic rats [20]. In brief, the rat was deeply anesthetized by inhalation. The L5 and L6 spinal nerves were isolated carefully and ligatured tightly with 4-0 silk thread. The wound was sutured after complete hemostasis was confirmed. The procedure for the sham group was identical to that of the SNL group, but ligation was not performed. Rats for subsequent experimental use were screened based on obvious unilateral allodynia in response to mechanical stimuli and the absence of motor deficits.
Rats were exposed to the testing environment for at least 30 min. Rats were placed in a plexiglass box on a metal grid to assess mechanical allodynia. The behavior test was carried out by using a 2290 CE electrical von Frey hair (IITC Life Science, Woodland Hills, CA, USA), ranging from 0.1 g to 90 g. The experimenter was blinded to the different treatment groups. The paw withdrawal thresholds (PWT) were defined as the force value when the rat’s contralateral or ipsilateral hind limb suddenly withdrew after being subjected to an increased upward force. The threshold was calculated by averaging the lowest force that evoked a withdrawal response over a 1 min interval [21, 22].
For thermal pain hypersensitivity tests, rats were kept in the testing apparatus for 30 min on a 30 °C heated glass floor. Thermal withdrawal latency was measured by a plantar test analgesic meter (II-390G, IITC Life Science, Woodland Hills, CA, USA). Each rat was tested over 3 sessions, each 5 min in length. To stimulate skin sensitization and tissue damage, the cutoff time was confined to 30 s. The average paw withdrawal latency (PWL) of the three tests was used for data analysis.
Before testing, rats were placed in the plexiglass chamber for 30 min to acclimatize to the experimental environment. Rats were gently restrained and injected subcutaneously with 50 µL of 5% formalin (221008, Yulu, Shangrao, Jiangxi, China) diluted in normal saline in the left hind paw. Rats were then immediately placed into the chamber and nociceptive behavior was observed with a mirror embedded in the rear part of the cage for better monitoring of animal behavior. Formalin-induced flinches were considered pain behavior and manually quantified by the experimenter for 1 min at 10 min intervals until 90 min; the experimenter was blind to the treatments [23]. Formalin-induced flinching was biphasic: phase I was mediated by noxious chemical stimulation immediately after formalin injection and lasted from 0–10 min, whereas phase II was mediated by a combination of peripheral inputs and formalin-induced central sensitization and began at 10 min and lasted until 90 min after formalin injection [24, 25, 26].
After the mechanical allodynia behavioral test, an electrophysiological test was
performed on spinal cord slices obtained from rats at 14 days post surgery. In
brief, the rats were deeply anesthetized via isoflurane inhalation. The lumber
enlargements of L3–L5 were quickly separated and immersed for 90 s in cold
high-sucrose ACSF with a mixture of 95% O
To record glutamatergic transmission, a bath application of 0.5
µM tetrodotoxin (TTX; Aladdin, Shanghai, China), 1 µM
strychnine (Sigma, St Louis, MO, USA), and 100 µM PTX (Sigma, St
Louis, MO, USA) was used to inhibit sodium channels, glycine, and GABA receptors.
The recording was conducted using a borosilicate glass recording electrode
(BF150-86-10, Sutter, Sacramento, CA, USA), which was filled with a K
Isoflurane inhalation was used to induce deep anesthesia. About 100 mL of normal saline was perfused intracardially, followed by 100 mL of 4% paraformaldehyde (PFA) (w/v) (BL539A, Biosharp, Hefei, Anhui, China) in phosphate-buffered saline (PBS, pH 7.4). Spinal lumbar enlargements were immediately removed and post-fixed for 12 h in the same fixative at 4 °C before being dehydrated in sucrose solutions dissolved in PBS, with concentrations ranging from 10% to 30%. Tissue was mounted in an optimal cutting temperature (OCT) compound and sliced into 30 µm frozen sections using a Leica Microsystems (CM1950, Leica, Wetzlar, Germany). The frozen sections were incubated in 10% goat serum (v/v) (Sigma, St Louis, MO, USA) and 0.5% Triton X-100 (v/v) (CAS: 9002-93-1, Sangon, Shanghai, China) in PBS for 1 h at room temperature before being incubated with the rabbit anti-c-Fos (1:1000; 226008, SYSY, Gottingen, Germany) and mouse anti-NeuN antibodies (1:300; ab128886, Abcam, Boston, MA, USA) for an additional 24 hours at 4 °C. The sections were washed three times in PBST for 5 min each and then incubated with the appropriate secondary antibodies (conjugated with Alexa Fluor 488 or 594; 1:1000; A-10631, A-21205, Invitrogen, Carlsbad, CA, USA) for 2 h at room temperature. Finally, the sections were mounted on slides before being dried, cleared, and cover slipped. Immunofluorescence images were captured with a confocal scanning laser microscope (Fluo View FV1000, Olympus, Tokyo, Japan).
Spinal cord tissue was extracted and homogenized with a Radio-immunoprecipitation assay (RIPA) lysis buffer (Beyotime, Shanghai, China) to determine the expression level of pain-related proteins. Protein concentrations were measured with a BCA protein detection kit (Beyotime, Shanghai, China). The proteins were separated on 8–20% Precast-Gels (Beyotime, Shanghai, China), followed by electro-transference to polyvinylidene difluoride membrane (PVDF) (Sigma, St Louis, MO, USA) membranes. The membranes were then blocked with 5% skim milk for 1 h at room temperature. After being blocked, the membranes were incubated with primary antibodies for c-Fos (1:1000, ab190289, Abcam, Cambridge, UK) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:1000, Cat No. 60004-1-Ig, Proteintech, Chicago, IL, USA) at 4 °C overnight. Subsequently, they were incubated with corresponding HRP-conjugated secondary antibody (1:1000, 31402, ThermoFisher, Waltham, MA, USA) for 1 h at room temperature. Proteins were detected and quantified using the iBright FL1000 Imaging System (Invitrogen, Carlsbad, CA, USA).
The lumbar enlargements of the spinal dorsal were quickly
separated for RNA extraction after rats were sacrificed by decapitation. Total
RNA was extracted with Trizol reagent (9108, Takara, Shiga, Japan) and converted
into cDNA using the PrimeScript RT reagent kit (RR036A, Takara, Shiga, Japan).
The specific primer sequences used for cDNA amplification were as follows: NOS1,
forward 5
First, we searched for potential targets of metformin. The PubChem database (https://pubchem.ncbi.nlm.nih.gov) contains chemical information such as molecular formula, chemical structure, biological activities, and other identifiers. The SwissTargetPrediction database (http://www.swisstargetprediction.ch) was employed to predict the most possible protein targets of small molecules. We uploaded the structure of metformin from PubChem to acquire its putative targets. Second, we searched the potential pathogenic targets on the GeneCards database (https://www.genecards.org), Online Mendelian Inheritance in Man (OMIM) database (https://www.omim.org), DisGeNET database (https://www.disgenet.org), and Therapeutic Target Database (TTD) database (https://db.idrblab.net/ttd) using “pain” as the keyword. A pain potential target databank was created after deleting duplicate targets. The targets of metformin were compared with the pain databank to obtain the intersection targets. These intersection targets were the potential targets of metformin for pain treatment. Third, the overlapped targets were displayed as a Venn figure (Venny 2.1.0, CNB, Madrid, Spain), and the protein-protein interaction (PPI) network of targets shared by metformin and pain was acquired from the STRING database (https://string-db.org/, version 11.5). The PPI network was uploaded to Cytoscape 3.9.1 (https://cytoscape.org/) to search hub targets according to the topological features “Betweenness centrality”, “closeness centrality”, and “degree” [27, 28]. Finally, to clarify the biological function of shared targets between metformin and pain, an enrichment analysis of pathways from the Kyoto Encyclopedia of Genes and Genomes (KEGG) was performed with “clusterProfier” package in the RStudio 4.2.2 (https://rstudio.com) [29].
The data were expressed as means
To assess the analgesic effects following metformin treatments, we applied an
SNL and formalin-induced hypersensitivity model. An intrathecal injection of 30
or 100 µg (dissolved in 10 µL of ACSF) of metformin was administered
to two groups of neuropathic pain rats. The PWT to mechanical or thermal stimuli
was measured before and after injections at 1, 2, and 4 h. Intrathecal injections
of 30 or 100 µg of metformin and ACSF did not change contralateral hind
paws in sham rats over 4 h. In contrast, metformin via intrathecal delivery
prolonged the mechanical and thermal response in ipsilateral hind paws in a dose-
and time-dependent manner (Fig. 1A, mechanical allodynia F
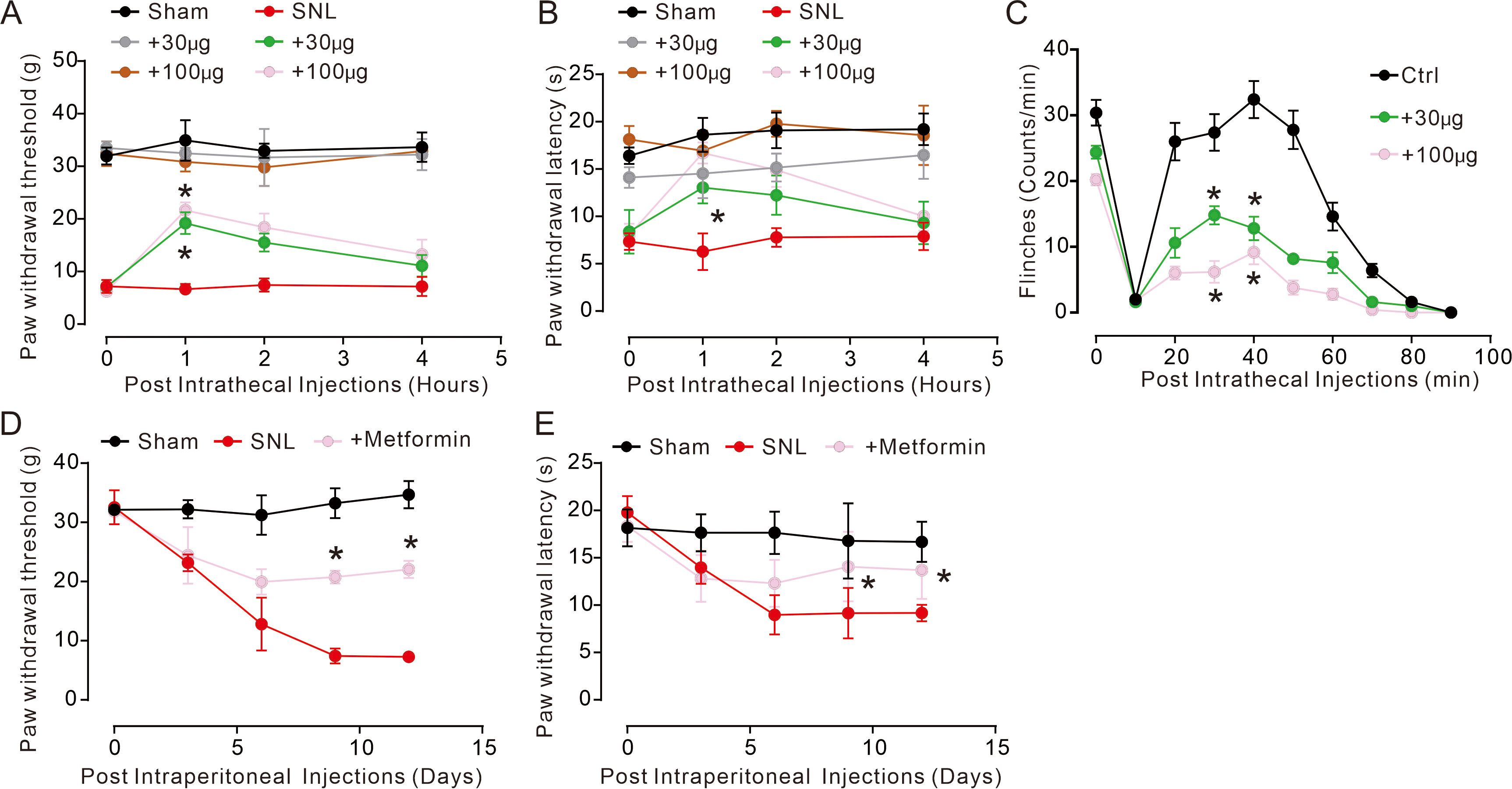 Fig. 1.
Fig. 1.Intrathecal administration of metformin attenuated neuropathic-
and formalin-induced pain. (A,B) Intrathecal delivery of metformin exhibited
mechanically antiallodynic and thermal hyperalgesia effects in SNL-induced
neuropathic pain rats. (C) Intrathecal delivery of metformin significantly
reduced flinch counts and exhibited analgesic effects in formalin-induced
inflammatory pain rats (n = 5–6 per group). *p
Furthermore, we assessed the antinociceptive effects of metformin in
formalin-induced acute inflammatory pain. The rats displayed flinching behaviors
following 5% formalin injection into the left hind paws. These responses were
separated into phase I and phase II, and counted with the frequencies of flinching
behaviors (Fig. 1C, F
Network pharmacology was used to determine the underlying mechanism mediated by metformin treatment to further assess the underlying target and signaling pathways. Through the online Venn diagram tool, we found 25 shared targets for metformin to treat pain (Fig. 2A). The targets were imported into the STRING database to construct and identify the PPI network and then visualized by Cytoscape. Subsequently, we collected the hub targets, including nitric oxide synthase 1 (NOS1), NOS2, NOS3, epidermal growth factor receptor (EGFR), and plasminogen (PLG), by the Centiscape 2.2 (https://apps.cytoscape.org/apps/centiscape) plugin in Cytoscape based on three topological features: degree, betweenness centrality, and closeness centrality (Fig. 2B). Gene Ontology (GO) and KEGG pathway enrichment analyses of the 25 shared targets were performed using RStudio. The top 10 remarkably enriched biological processes (BP), molecular functions (MF), cellular components (CC), and KEGG signaling pathways involved in the shared targets were identified. The BP of the targets included postsynaptic signal transduction, regulation of hemostasis, and biosynthetic processes (Fig. 2C). The MF comprised neurotransmitter receptor activity and binding, mitogen-activated protein (MAP) kinase kinase kinase activity, and calcium-dependent protein binding (Fig. 2D). The CC consisted of synaptic cleft, neuronal cell body and spine, dendritic spine, and membrane raft (Fig. 2E). The underlying target genes of metformin in pain were specifically and predominantly enriched in the Relaxin signaling pathway, the cyclic guanosine monophosphate (cGMP)-protein kinase G (PKG) signaling pathway, neuroactive ligand-receptor interaction, calcium signaling pathways, neuroactive ligand-receptor interaction, and cholinergic synapses (Fig. 2F). These findings suggest that the main bioactive components of metformin had different action targets in synaptic transmission pathways.
 Fig. 2.
Fig. 2.GO and KEGG enrichment analyses on metformin. (A) Venn diagram of targets for metformin to treat pain. (B) A PPI network of metformin to treat pain. (C) The top 10 significantly enriched terms in biological process (BP). (D) The top 10 significantly enriched terms in molecular function (MF). (E) The top 10 significantly enriched terms in cellular component (CC). (F) The top 10 significantly enriched terms in KEGG. GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PPI, protein-protein interaction; BP, biological process; MF, molecular function; CC, cellular component.
Network pharmacology highlighted that metformin significantly remolded the
painful information mediated by complex circuits associated with excitatory and
inhibitory networks. The protooncogene c-Fos is a gene expression-regulated
nuclear protein with elevated expression in neurons following neuronal
activation. The increase in c-Fos protein was stimulated by neurotransmitters on
membrane receptors under physiological stimuli, especially in pain states in
response to mechanical stimulus. To further illustrate neuronal activity after
metformin treatment in neuropathic rats, we used double immunofluorescence
staining to identify c-Fos expression levels (Fig. 3A–C). The results showed
that c-Fos expression significantly increased in neuropathic rats, and
intrathecal injections of metformin abolished c-Fos expression in the spinal cord
(Fig. 3A–D, immunofluorescence staining: F
 Fig. 3.
Fig. 3.Dual fluorescence labeling of c-Fos with NeuN among three
different groups. (A) c-Fos with NeuN co-labeling in the sham group. (B) c-Fos
with NeuN co-labeling in the SNL group. (C) c-Fos with NeuN co-labeling in the
metformin-treated group. Images were captured in laminae I–III of spinal cord (n
= 6–8 animals). Scale bar: 100 µm (
We demonstrated that metformin inhibited nerve injury-induced neuronal activity,
but the underlying plastic mechanism remains unclear. Electrophysiological
recording is the most dynamic method used to study neural plasticity [30].
Behavioral tests suggested that metformin may significantly lower nociceptive
behaviors in the ipsilateral side after SNL (Fig. 1A). Thus, the mechanism for
neural plasticity was assessed after nerve injury. Both the frequency and
amplitude of mEPSCs in the spinal lamina II significantly increased during
neuropathic pain, and this finding was consistent with our prior studies [31].
Frequency reflected the release of presynaptic glutamate, whereas amplitude was
associated with postsynaptic receptors. Bath application of 10–100 µM
metformin ameliorated the increase in mEPSCs amplitude and frequency in a
dosage-dependent manner (Fig. 4A–E, frequency: F
 Fig. 4.
Fig. 4.Metformin attenuated glutamatergic transmission in the
spinal dorsal horn of the ipsilateral hind paws in pain states. (A) Sample
traces of mEPSCs. (B,C) Cumulative distribution of frequency and amplitude in
mEPSCs. (D,E) Statistical analysis of frequency and amplitude in mEPSCs. (F)
Sample traces of mIPSCs. (G,H) Cumulative distribution of frequency and amplitude
in mIPSCs. (I,J) Statistical analysis of frequency and amplitude in mIPSCs. Data
were presented as the means
In the present study, we observed that intrathecal injection of metformin exhibited remarkable analgesic effects in a dose-dependent manner, which significantly inhibited SNL-induced chronic pain and formalin-induced acute pain. Using whole-cell recording and immunofluorescence, we demonstrated that metformin significantly inhibited spinal nociceptive transduction by alleviating glutamatergic transmission, thereby attenuating chronic and acute pain in rats. Through network pharmacology, we discovered the analgesic targets of metformin by overlapping its predicted targets with pain targets and revealed the signal transduction mechanism of metformin analgesia through calcium signaling and cGMP-PKG signaling. Intracellular calcium ions are essential for initiating nerve injury and related signaling molecule transduction. Our results further demonstrated that metformin significantly reversed AMPK-mediated nerve injury. Thus, metformin exerted an important neuroprotective effect by regulating intracellular calcium ion levels and reversing neuropathic pain caused by spinal nerve ligation. We demonstrated that metformin produced analgesic behaviors by inhibiting spinal glutamatergic transmission, calcium signaling, and cGMP-PKG signaling in nociceptive hypersensitivity.
Neural activity is a critical mechanism in the nervous system for maintaining functions and behavioral performance. Metformin significantly inhibited excitatory synaptic transmission, but did not affect inhibitory synaptic transmission. Our previous study demonstrated that analgesia could be achieved by simply interfering with excitatory synaptic transmission [31]. Glutamate transmission is central to changes in the nociceptive circuit, leading to neuronal excitotoxicity and loss via a trans-synaptic mechanism [32]. In addition, intrathecal injection of metformin was insufficient to reverse hypersensitivity in neuropathic rats, as shown in Fig. 1A. In brief, the immunohistochemical and electrophysiological whole-cell recording results showed that metformin had crucial neuroprotective effects.
Network pharmacology is currently the most critical and mature method for drug
target research. In our study, we discovered that EGFR and NOS may be the targets
of metformin analgesia. The study demonstrated that altered tetrahydrobiopterin
(BH4) production following sensory neuron injury contributes to pain sensitivity
and duration, regulating Gch1 expression in sensory neurons. A molecular link
exists between BH4 and pain perception via EGFR/Kirsten ras sarcoma virus (KRAS),
and the pharmacological modulation of BH4 has significant analgesic effects via
EGFR/KRAS [33]. Clinical trials have revealed that EGFR inhibitors have positive
analgesic effects in patients suffering from neuropathic pain [34]. NO activates
soluble guanylate cyclase activity in response to the production of cGMP. cGMP
then activates cGMP-dependent protein kinase (PKG), which causes multiple targets
to be activated, including the ATP-sensitive K
The activation of NO/cGMP signaling in pain states clearly causes a series of
intracellular molecular activities, as well as reactive astrogliosis processes
and microglial polarization, both of which contribute to chronic pain sensations.
The natriuretic peptide commonly binds to membrane guanylyl cyclase in DRG
neurons - generating and further activating the cGMP/PKG pathway, the release of
microglial-mediated inflammatory factors, and the development of chronic pain
[35]. Inflammatory factors significantly promote glial cell-induced alteration in
spinal synaptic plasticity in the early stages of nerve injury. Microglia have
the potential to induce signal transduction and synaptic plasticity in neural
circuits by modifying synaptic proteins via the complement ligand pathway [36].
In whole-cell recording mode, perfusion of TNF-
Spinal nerve ligation caused changes in neural circuits, whereby depolarization led to the opening of calcium channels. Large amounts of calcium ions diffused into the presynaptic neuron, allowing synaptic vesicles to enter circulation. These vesicles carried neurotransmitters to merge with the synaptic membrane, which released into the synaptic cleft and finally diffused into the postsynaptic neuron (Fig. 3). Metformin significantly reversed calcium ion efflux, intracellular second messenger-mediated AMPK phosphorylation levels, and NOS2 gene expression. This led to inhibited fusion of presynaptic glutamatergic vesicles with presynaptic membranes in the dorsal horn of the spinal cord, preventing glutamatergic neurotransmission and mechanically stimulated c-Fos expression. Therefore, metformin may regulate the synaptic vesicle cycle by inhibiting calcium channels and glutamatergic synaptic transmission, providing analgesia (Fig. 5).
 Fig. 5.
Fig. 5.Schematic showing the role of metformin in the spinal excitatory synaptic transmission and pain hypersensitivity in neuropathic pain.
All data supporting the conclusion of the article are included in this article.
YM, YMM and LM designed the research study. DXD, XJW and UA performed the research. DW analyzed the data. XL and RML provided help and advice on visualization. DXD wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All animal experimental protocols and procedures in this study were reviewed and approved by the Animal Care and Welfare Committee of Shanghai Mental Health Center (A2018081).
We thank all members who were involved in this research.
This research was funded by the Program of Characteristic Disciplines of Shanghai Mental Health Center (2017-yjxk-06), and Shanghai Mental Health Center (2021-YJ11 and 2023-YJ02).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
