- Academic Editor
†These authors contributed equally.
Objectives: To investigate the differences in functional brain
activity and connectivity between nurses working long-term shifts and fixed day
shift and explore their correlations with work-related psychological conditions.
Methods: Thirty-five nurses working long-term shifts and 35
nurses working fixed day shifts were recruited. After assessing work-related
psychological conditions, such as burnout and perceived stress of these two
groups of nurses, amplitude of low-frequency fluctuations (ALFF) and functional
connectivity (FC) analyses were performed to investigate the between-group
differences in brain functional activity and connectivity. Furthermore,
correlation analysis between the ALFF/FC metrics and psychological conditions was
conducted. Results: Compared with nurses working fixed day
shifts, nurses working long-term shifts showed higher levels of burnout,
perceived stress, and depression scores; lower z-transformed ALFF (zALFF) values
in the right dorsolateral prefrontal cortex (dlPFC), right superior parietal
lobule (SPL), and right anterior cingulate cortex (ACC); and higher zALFF values
in the right middle temporal gyrus (voxel-level p
Nursing is an essential component of clinical healthcare. High-quality and sustainable nursing is an important guarantee of clinical outcomes. Given the continuity and uncertainty of clinical work, hospitals generally adopt shift-based nursing. Namely, three groups of nurses take turns to provide nursing services for patients at specific periods through shifts [1], which is an effective approach for providing uninterrupted nursing services, alleviating manpower shortages, and improving nursing quality [2]. However, long-term irregular shifts tend to disrupt the biological rhythm of nurses, and are associated with symptoms of burnout, emotional disorders, and various physical and mental illnesses [3] such as anxiety and depression [4], dementia [5], and cardiovascular [6] and endocrine disorders [7]. A recent cross-sectional study from China showed that 58.1% of nurses working long-term shifts exhibited work-related disorders, manifesting as a higher incidence of mental health problems, sleep disorders, and burnout [8].
Neuroimaging techniques provide visual evidence that can help elucidate the impacts of shift work on the physical and mental health of workers. A recent neuroimaging study [9] enrolling 39 nurses demonstrated that long-term shift work might contribute to a change in gray matter morphology in the bilateral postcentral gyrus, right paracentral lobule, and left superior temporal gyrus. Moreover, gray matter alterations in the left postcentral gyrus are correlated with the degree of impact of sleep disturbance on depression in nurses. Another study [10] compared differences in the white matter structure of 61 shift workers and 31 non-shift workers, finding that the shift workers exhibited higher fractional anisotropy in the bilateral anterior cingulum, and that the increased fractional anisotropy in the right anterior cingulum was correlated with poor sleep quality in shift workers. These studies provided preliminary evidence that shift work influences the brain structural plasticity of workers. However, it remains unclear whether and how long-term shift work affects the functional brain activity patterns of nurses and what the relationship is between these patterns and work-related psychological conditions in nurses.
After assessing the burnout, perceived stress, anxiety, and depression conditions using self-reported questionnaires, we collected the brain functional magnetic resonance imaging (fMRI) data of 35 nurses working long-term shifts and 35 nurses working fixed day shifts, and then compared the regional spontaneous neuronal activity and inter-regional functional coupling patterns between these two groups using the amplitude of low-frequency fluctuations (ALFF) [11] and functional connectivity (FC) [12] methods. Correlation analysis between the ALFF/FC metrics and psychological conditions was then performed among these participants. The results were expected to provide visual evidence that would help to elucidate the impacts of long-term shift work on the psychological conditions and neuroplasticity of nurses.
Thirty-five nurses working long-term shifts and 35 nurses working fixed day shifts were recruited from three hospitals in Leshan City, Sichuan, China. Participants were identified for inclusion based on their self-reported information in the history taking. The inclusion criteria were as follows: (1) female, aged between 20 and 35 years old; (2) right-handed; (3) unmarried or married but not pregnant; (4) absence of endocrine, neurological, or psychiatric diseases, or any other primary illnesses; and (5) no contraindications for magnetic resonance imaging (MRI) scans. Furthermore, nurses working long-term shifts had to fulfill the following criteria: the work time alternated between days (8:00–16:00), evenings (16:00–23:00), and nights (23:00–8:00) for more than 1 year. Nurses working fixed day shifts needed to have been working on day (8:00–18:00) shifts only, for almost 1 year, with no experience of working shifts.
The Chinese version of the Maslach Burnout Inventory Human Service Survey (MBI-HSS) [13] developed by Maslach et al. [14] was applied to evaluate the occupational burnout of the participants. The MBI-HSS consists of 17 items, which belong to three dimensions: emotional exhaustion, depersonalization, and reduced personal accomplishment. MBI-HSS adopts a Likert scale of 0–6. The higher the score, the more severe the burnout of subjects. The Chinese version of the Perceived Stress Scale (PSS) [15] was used to evaluate the subjective perception of stress in the participants. PSS has 14 items and is scored on a Likert scale of 0–4. The higher the score, the greater the perceived stress of subjects. Furthermore, the Zung Self-Rating Anxiety Scale (SAS) [16] and the Zung Self-Rating Depression Scale (SDS) [17] were utilized to evaluate the anxiety and depression states of the subjects.
Statistical analysis of the demographic characteristics and psychological
conditions was performed using SPSS 24.0 (IBM Corp., Armonk, NY, USA). Continuous
variables were reported as mean
High-resolution T1-weighted structural images and blood-oxygen-level-dependent
images were acquired using a 3.0 T Philips MRI scanner (Philips Corp., Amsterdam,
Netherlands). The scanning parameters of the structural images were as follows:
repetition time/echo time = 9.1 ms/3.0 ms, slice thickness = 1 mm, field of view
= 240
fMRI data preprocessing, as well as ALFF and FC analyses, were performed using
DPABI 4.1 (http://rfmri.org/DPARSF) [18]. The preprocessing steps of the fMRI
data were as follows: (1) removal of the first ten time points of the scanning
sequence; (2) slice timing correction; (3) motion correction, excluding
participants with mean frame-wise displacement
Based on the preprocessed data, the ALFF values were calculated and then
transformed into z-scores to obtain the final z-transformed ALFF (zALFF) maps for
statistical analysis using DPABI. As the differences in age and work years
between nurses working long-term shifts and fixed day shifts were insignificant,
the two-sample t-test was used for intergroup comparisons of zALFF
values, with statistical significance thresholds set at voxel-level p
The regions identified through ALFF analysis that showed significant differences
between nurses working long-term shifts and fixed day shifts were selected as
regions of interest (ROIs). The mean time series of the ROIs were extracted using
DPABI, and the ROI-ROI FC analysis was conducted using Pearson
correlation analysis. The two-sample t-test was used for intergroup
comparisons of FC, with a statistical significance threshold of p
In addition, partial correlation analysis was performed between the ALFF/FC metrics showing significant between-group differences and the psychological conditions, including MBI-HSS, PSS, SAS, and SDS scores, for nurses working long-term shifts, nurses working fixed day shifts, as well as all of them, respectively. Age, working years, and mean frame-wise displacement were used as covariates in the correlation analyses.
Two nurses working long-term shifts were excluded due to excessive head
movements; therefore, 68 participants were included in the data analysis. There
was no statistically significant difference between the two groups regarding age
and years of work (p
| Group | Age (Years) | Working years | MBI-HSS | PSS | SAS | SDS |
| Group A | 27 |
4.3 |
63.55 |
31.18 |
52.75 |
51.32 |
| Group B | 26.6 |
3.97 |
47.34 |
24.69 |
46.64 |
44.45 |
| T-value | 0.371 | 0.699 | 7.415 | 4.199 | 1.986 | 2.542 |
| p-value | 0.712 | 0.487 | 0.051 | 0.013* |
Group A: Nurses working long-term shifts, Group B: Nurses working fixed day
shifts, *** p
The results of ALFF analysis showed that compared with nurses working fixed day
shifts, nurses working long-term shifts had significantly decreased spontaneous
functional activity (lower zALFF values) in the right dorsolateral prefrontal
cortex (dlPFC), right superior parietal lobule (SPL), and right anterior
cingulate cortex (ACC), and significantly increased functional activity in the
right middle temporal gyrus (MTG) (voxel-level p
| Brain regions | Cluster size | MNI coordinates (x, y, z) | T-value | Direction | ||
| R_dlPFC | 133 | 54 | 24 | 18 | –5.61 | |
| R_SPL | 91 | 21 | –60 | 63 | –5.13 | |
| R_ACC | 66 | 3 | 36 | 15 | –4.32 | |
| R_MTG | 125 | 54 | –36 | –3 | 4.86 | |
Abbreviations: dlPFC, dorsolateral prefrontal cortex; SPL, superior parietal
lobule; ACC, anterior cingulate cortex; MTG, middle temporal gyrus; R, right
hemisphere; MNI, Montreal Neurological Institute; ALFF, amplitude of low-frequency
fluctuations; zALFF, z-transformed ALFF;
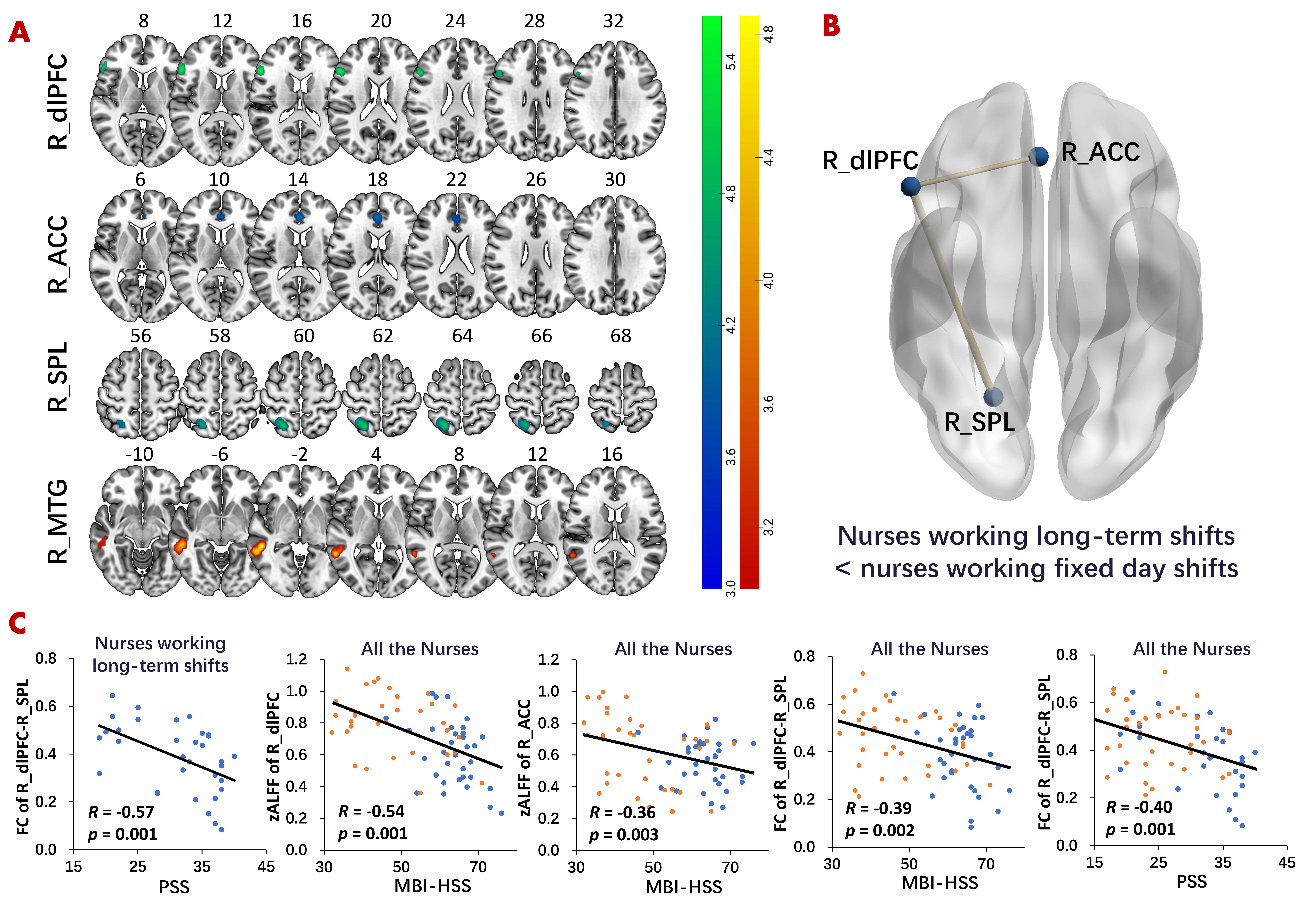 Fig. 1.
Fig. 1.Differences in brain activity and functional connectivity
between nurses working long-term shifts and fixed day shifts and the correlations
between ALFF/FC metrics and psychological conditions in nurses. (A) Results of
between-group comparison in ALFF analysis, the color bar indicates the
T-value of the clusters, warm colors indicate nurses working long-term
shifts
The results of the ROI-ROI FC analysis showed that compared with nurses working
fixed day shifts, nurses working long-term shifts had significantly decreased FC
between the right dlPFC and right SPL, as well as between the right dlPFC and
right ACC (p
The results of the correlation analysis are shown in Table 3 and Fig. 1C. As
shown in Fig. 1C, the FC value between the right dlPFC and right SPL was
negatively correlated with the PSS score in nurses working long-term shifts under
the threshold of FDR-corrected p
| MBI-HSS | PSS | SAS | SDS | |
| Nurses working long-term shifts (N = 33) | ||||
| zALFF of R_dlPFC | R = –0.41 | R = –0.14 | R = –0.12 | R = –0.09 |
| p = 0.02* | p = 0.46 | p = 0.53 | p = 0.64 | |
| zALFF of R_ACC | R = –0.11 | R = –0.12 | R = –0.15 | R = –0.14 |
| p = 0.55 | p = 0.54 | p = 0.43 | p = 0.46 | |
| zALFF of R_SPL | R = –0.01 | R = –0.05 | R = 0.06 | R = –0.07 |
| p = 0.95 | p = 0.79 | p = 0.74 | p = 0.71 | |
| zALFF of R_MTG | R = –0.12 | R = 0.17 | R = –0.13 | R = 0.05 |
| p = 0.52 | p = 0.37 | p = 0.50 | p = 0.80 | |
| FC of R_dlPFC-R_SPL | R = –0.37 | R = –0.57 | R = –0.21 | R = –0.03 |
| p = 0.03* | p = 0.001** | p = 0.26 | p = 0.86 | |
| FC of R_dlPFC-R_ACC | R = 0.19 | R = 0.22 | R = –0.03 | R = 0.09 |
| p = 0.32 | p = 0.23 | p = 0.87 | p = 0.63 | |
| Nurses working fixed day shifts (N = 35) | ||||
| zALFF of R_dlPFC | R = –0.36 | R = –0.07 | R = 0.27 | R = –0.04 |
| p = 0.04* | p = 0.71 | p = 0.14 | p = 0.81 | |
| zALFF of R_ACC | R = –0.28 | R = 0.30 | R = 0.33 | R = –0.28 |
| p = 0.11 | p = 0.09 | p = 0.07 | p = 0.13 | |
| zALFF of R_SPL | R = 0.06 | R = 0.03 | R = 0.08 | R = –0.11 |
| p = 0.74 | p = 0.89 | p = 0.66 | p = 0.54 | |
| zALFF of R_MTG | R = –0.07 | R = 0.38 | R = 0.08 | R = –0.10 |
| p = 0.72 | p = 0.03* | p = 0.65 | p = 0.60 | |
| FC of R_dlPFC-R_SPL | R = –0.29 | R = –0.11 | R = –0.36 | R = –0.05 |
| p = 0.11 | p = 0.53 | p = 0.04* | p = 0.80 | |
| FC of R_dlPFC-R_ACC | R = –0.10 | R = 0.03 | R = 0.06 | R = 0.02 |
| p = 0.59 | p = 0.88 | p = 0.75 | p = 0.92 | |
| All the nurses (N = 68) | ||||
| zALFF of R_dlPFC | R = –0.54 | R = –0.30 | R = –0.06 | R = –0.18 |
| p |
p = 0.02* | p = 0.62 | p = 0.16 | |
| zALFF of R_ACC | R = –0.36 | R = –0.05 | R = 0.01 | R = –0.23 |
| p = 0.003** | p = 0.70 | p = 0.91 | p = 0.07 | |
| zALFF of R_SPL | R = –0.20 | R = –0.17 | R = –0.03 | R = –0.13 |
| p = 0.11 | p = 0.17 | p = 0.81 | p = 0.29 | |
| zALFF of R_MTG | R = 0.05 | R = 0.25 | R = –0.02 | R = 0.05 |
| p = 0.70 | p = 0.04* | p = 0.85 | p = 0.71 | |
| FC of R_dlPFC-R_SPL | R = –0.39 | R = –0.40 | R = –0.30 | R = –0.12 |
| p = 0.002** | p = 0.001** | p = 0.01* | p = 0.34 | |
| FC of R_dlPFC-R_ACC | R = –0.20 | R = –0.03 | R = –0.04 | R = –0.07 |
| p = 0.10 | p = 0.81 | p = 0.75 | p = 0.60 | |
* p
This study investigated the differences in functional brain activity and connectivity between nurses working long-term shifts and fixed day shifts. The results demonstrated that compared with nurses working fixed day shifts, those who worked long-term shifts had reduced spontaneous activity and functional connectivity in the critical nodes of the right frontoparietal network, including the right dlPFC and SPL. Moreover, the FC of the right dlPFC-right SPL was significantly correlated with the perceived stress of nurses working long-term shifts.
Our results demonstrated that nurses working long-term shifts had higher MBI-HSS, PSS, and SDS scores than those nurses who work regular day shifts, which is consistent with the findings of previous studies [4, 20, 21]. For example, Booker et al. [4] found that shift work might lead to sleep disorders and symptoms of depression and anxiety. Another perspective study [21] demonstrated that after 1 year of shift work, workers experienced an impairment in their mental health, which manifested as burnout, depression, and anxiety, as well as sleep disturbances. Moreover, sleep disorders mediated the impacts of shift work on the mental health of participants. These highly consistent findings could be plausibly explained by a concept in chronobiology, that is, rhythm disruption. The irregular or reduced sleep duration in shift work leads to reduced sensitivity to peripheral cortisol receptors [22], decreased production of melatonin [23], and decreased secretion of monoamine neurotransmitters [24], which further contributes to the development of psychological disorders.
Our results support the altered activity patterns in the right frontoparietal network in nurses working long-term shifts, which manifested as lower spontaneous activity and weaker connectivity than nurses who worked fixed day shifts. The frontoparietal network is composed of the dlPFC and the parietal cortex, which includes the SPL, superior parietal sulcus, and lateral parietal cortex. According to the lateralization of their functions, the frontoparietal network is divided into left and right networks. The right frontoparietal network is mainly responsible for working memory, endogenous perception, emotion perception, and inhibitory control. In contrast, the left frontoparietal network mainly involves speech, semantic recognition, and cognition [25, 26]. Numerous neuroimaging studies have shown that the altered activity of the right frontoparietal network might be closely related to impaired working memory, attention deficits, and abnormal psychological states [27, 28, 29, 30]. A recent neuroimaging meta-analysis also showed that clinical subjects with emotion regulation disorders exhibited reduced activity in the right frontoparietal network and compensatory activation of the emotion-related brain regions, such as the ACC [31]. The results of the current study suggest that nurses working long-term shifts exhibited not only higher levels of burnout, heavier psychological stress, and more pronounced depression symptoms, but also lower spontaneous activity and weaker connectivity in the right dlPFC and right SPL. Our correlation analysis showed significant correlations between the FC of the right dlPFC-right SPL and both the MBI-HSS and PSS scores of nurses. The negative correlation coefficient indicates that nurses having lower frequency activity/synchrony of the right dlPFC have higher levels of burnout and perceived stress. It is worth noting that correlation between ALFF/FC metrics and MBI-HSS/PSS scores is not specific for shift-working nurses. It is, however, the case that shift-working nurses show generally higher values in scores, but lower ALFF/FC. Hence, the association between ALFF/FC and symptoms is possibly not specific for shift work, but in shift-working nurses the level of both is more severe. These findings suggest that the inhibition of functional activity of the right, rather than left, frontoparietal network in nurses working long-term shifts may imply a work-related impairment in their emotional perception and regulation.
Furthermore, sleeping disorder studies have provided further evidence for understanding the right frontoparietal network and psychological disorders of shift-working nurses. For instance, one study detected that long-term sleep deprivation could seriously affect emotion regulation, cognitive attention, and working memory [32, 33]. In another study, subjects in a state of sleep deprivation exhibited reduced activity in the right frontoparietal network and unstable mutual inhibition between the frontoparietal network and the default mode network when performing attention control tasks [34]. Long-term and high-density shift working is highly likely to cause chronic sleep deprivation, which could impair the working memory and emotion regulation of nurses [35]. The plasticity of the right frontoparietal network and increased burnout and perceived stress of nurses working long-term shifts may therefore attribute to their long-term chronic sleep deprivation.
In addition to the right frontoparietal network, our study also detected decreased spontaneous activity in the right ACC in nurses who work long-term shifts. The ACC is an essential component of the limbic system and a key node for emotion perception and stress control [36, 37]. Previous studies have shown that patients with emotional disorders, such as anxiety and depression, generally exhibit functional and structural abnormalities in the ACC [38, 39, 40]. Results from animal experiments have also demonstrated that the content of ACC-derived neurotrophic factors is closely related to the negative emotions and behaviours of rats [41, 42]. In our study, we observed a significant increase in psychological stress and depression scores and decreased spontaneous activity in the ACC in nurses who work long-term shifts, which validated the correlations between the ACC and stress and depression from a clinical perspective. Except for emotion regulation, as part of the old cortex, the ACC is closely related with vigilance in humans [43]. Piantoni et al. [44] found that acute sleep deprivation caused a decrease in vigilance and task performance in healthy subjects, which could be explained by the altered functional connectivity patterns of the ACC. Nurses who work long-term shifts always suffer from chronic sleep deprivation and decreased vigilance and reaction [45, 46]. The decreased functional activity of the ACC in nurses working long-term shifts may therefore also be associated with their vigilance impairment. However, since this study did not directly assess the vigilance and reactions of participants, further task-based neuroimaging studies are needed to explore the relationship between the decreased functional activity in the ACC and vigilance.
Our study had some limitations. First, this was a cross-sectional study that investigated the differences in functional brain activity and connectivity patterns of nurses working long-term shifts versus nurses working fixed day shifts. The progressive impacts of long-term shift work on the brain of nurses need to be further investigated in a longitudinal study. Second, this was a resting-state fMRI study, and the findings were unable to identify the causal relationship between functional brain activity and alterations in work-related psychological conditions. Follow-up studies could apply a task design to further explore the relationships between brain activity associated with long-term shift work and impairments in cognition and working memory. Third, the inclusion criteria for nurses working fixed day shifts were no experience of shift work for almost 1 year. However, a recent study suggested that former night shift workers may also manifest structural and functional neural plasticity [47]. Therefore, further validation of the current findings in nurses who have not experienced shift work is needed.
In conclusion, this current study using ALFF and FC methods indicated that nurses working long-term shifts had lower functional activity and weaker functional connectivity in the right frontoparietal network than nurses working fixed day shifts. The altered functional activity and connectivity patterns in the right frontoparietal network were correlated with occupational burnout and perceived stress in nurses. The results provide new insights into the impacts of long-term shift work on the mental health of nurses from a functional neuroimaging perspective and provide a potential and quantifiable indicator for future research regarding the mitigation of the hazards of shift work.
The main data supporting our findings can be found within the manuscript. The raw data can be requested from the corresponding author.
KQ and YZ contributed to the conception and design of the study. YD and XW performed data collection and analysis. YD wrote the first draft of the manuscript. KQ revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Leshan Hospital of Traditional Chinese Medicine (Approval number: 2019-10-003).
The authors would like to express their sincere gratitude to all those who provided valuable guidance and support throughout the research. Additionally, we would like to thank our colleagues for their invaluable assistance with data collection and analysis.
This work was supported by Sichuan Science and Technology Project (NO. 19RKX0261) and the Key Science and Technology Innovation Project of Leshan Vocational and Technical College (NO. 2019020).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
