- Academic Editor
Background: The acute changes that occur in the small-world topology of
the brain in concussion patients remain unclear. Here, we investigated acute
changes in the small-world organization of brain networks in concussion patients
and their influence on persistent post-concussion symptoms. Methods:
Eighteen concussion patients and eighteen age-matched controls were enrolled in
this study. All participants underwent computed tomography, magnetic resonance
imaging (MRI), susceptibility weighted imaging, and blood oxygen level-dependent
functional MRI. A complex network analysis method based on graph theory was used
to calculate the parameters of small-world networks under different degrees of
network sparsity. All subjects were evaluated using the Glasgow Coma Scale and
Rivermead Postconcussion Symptom Questionnaire. Results: Compared with
the controls, the normalized cluster coefficient (
Concussion (sometimes called brain concussion, BC) is an important subtype of
mild traumatic brain injury (mTBI), a condition in which the brain cortex is
suppressed due to transient dysfunction of the brainstem reticular system after
traumatic brain injury (TBI). Its main characteristics are short-term impaired
consciousness; short-term retrograde amnesia after waking up but no organic
damage, which is clinically easy to ignore due to its mild, short-duration
symptoms; and a self-limiting nature [1]. However, about 10–15% of patients
have symptoms for
The brain is an extensive, interactive, complex network with topological properties [4]. He et al. [5] successfully constructed the first human brain structural network in 2007 and found that the network has a “small-world” characteristic. Several subsequent studies have shown that brain networks have small-world properties and exhibit varying degrees of damage among different diseases [6, 7, 8, 9]. Zhou et al. [10] and Johnson et al. [11] reported that the default mode network (DMN) is the major functional network disrupted after concussion in the absence of structural deficits. Some researchers in the Salience Network found decreased functional connectivity after concussion [12, 13]. To date, there are few literatures reports on acute changes in the small-world properties of the brain in concussion patients.
In this study, a complex network analysis method was used to observe acute changes in the brain network topology of BC patients in the resting state, and the influence of these changes on PCS and possible mechanisms. The results of this study provide some insights into the central pathophysiology of BC patients with negative traditional imaging findings.
All of the participants or guardians provided informed written consent prior to
study enrollment. The study protocol was approved by the Human Research Ethics
Committee and Institutional Review Board of the First Affiliated Hospital of
Nanchang University. The project was based on the Clinical Research Center For
Medical Imaging In Jiangxi Province (No.20223BCG74001). All study procedures were
carried out in accordance with approved guidelines and the principles of the
Declaration of Helsinki. All BC patients were retrospectively selected from a TBI
database (n = 162) comprising multimodal magnetic resonance imaging (MRI)
results. The inclusion criteria for BC patients were as follows: met the
diagnostic criteria for mTBI of the American Congress of Rehabilitation Medicine;
presented within 24 h of injury with a Glasgow Coma Scale (GCS) score of 13–15;
negative CT, MRI, and SWI findings for brain tissue; age
MRI scanning was performed at our hospital using a 3.0-Tesla MRI system (Trio,
Siemens Healthcare, Erlangen, Germany). High-resolution T1-weighted anatomical
images were acquired using a sagittal magnetization-prepared rapid acquisition
gradient echo sequence for optimal gray-white matter contrast, with the following
parameter settings: repetition time (TR) = 1900 ms, echo time (TE) = 2.26 ms,
flip angle = 15°, the field of view (FOV) = 215 mm
Each patient underwent a detailed clinical interview and physical examination, including the GCS and Rivermead Postconcussion Symptoms Questionnaire (RPQ), on the day of the scan and after the acute phase of trauma. All demographic and clinical assessment results were compared between the two groups using SPSS 20.0 software (IBM Inc., Chicago, IL, USA).
Blood oxygen level-dependent fMRI data were obtained using Onis 2.5
(http://www.onis-viewer.com) software. The brain was divided into 90 regions
according to the Anatomical Automated Labeling templates provided by the brain
network analysis and visualization software packages Gretna and BrainNet Viewer
(https://www.nitrc.org/projects/bnv/). The time series for each voxel was
calculated in each region and averaged. Pearson’s correlation coefficient between
two regions was calculated to construct a symmetrical correlation matrix, which
was then subjected to Fisher Z transformation for conversion to a binary
connectivity matrix. After obtaining the binary matrix, a binarized function of
the brain was constructed to connect the brain network, and the topological
properties of the network under different degrees of sparsity (i.e., network
density) were calculated including the clustering coefficient, shortest path
length, and
The clustering coefficient (C) reflects the local separation of the network and corresponds to the mean clustering coefficient for all nodes (i) therein. The number of edges (ei) near a node adjacent to an actual connection was divided by the maximum number of sides (k) that could be connected (ki [ki – 1]). Thus, the cluster coefficient of the node Ci is given by Ci = 2ei / ki [ki – 1]).
The shortest path length (L) reflects the whole brain integration effect and represents the best path in the network from one node (i) to another node (j). The shortest path length of each node was averaged to obtain L.
Notably, for the abovementioned parameters, the actual brain network topology
attributes of each subject were normalized (divided by the corresponding
attributes of 1000 random networks) to obtain the normalized cluster coefficients
(
The small world index (
Other indicators included whole brain efficiency, local efficiency, and node
betweenness. Differences in network graph theory metrics were determined by
calculating the area under the curve across all sparsities and using analysis of
variance and post-hoc analysis with age and sex as covariates and the false
discovery rate correction, q = 0.05. An independent two-sample t-test
was used for the comparisons. p
In this study, 18 patients (9 men and 9 women) with acute-stage BC who met the
requirements were selected from the TBI database; they were all right-handed, had
an average age of 36.8
| General information | BC | HCs | t | p |
| Age (y) | 36.8 |
36.4 |
0.086 | 0.932 |
| Sex (m/f) | 9/9 | 9/9 | - | - |
| Education (years) | 7.2 |
7.1 |
0.056 | 0.956 |
| Time since injury (days) | 3.6 |
- | - | - |
| GCS score | 14.9 |
15 |
–1.458 | 0.163 |
| RPQ score | 28.5 |
0 | 13.617 | 0.000* |
BC, brain concussion; HC, healthy control; GCS, Glasgow Coma Scale; RPQ,
Rivermead Postconcussion Symptom Questionnaire.
Age, education, illness duration, GCS, and RPQ data are shown as the mean
Using different correlation coefficients as thresholds to define network
sparsity, a 90
 Fig. 1.
Fig. 1.Functional correlation matrix in the healthy control (HC) group (left) and brain concussion (BC) group (right) under the minimum network sparsity (Dmin = 0.13).
After measuring the overall properties of brain functional networks under
different network sparsity conditions, cluster coefficients and shortest path
lengths were standardized to obtain standardized cluster coefficients
(
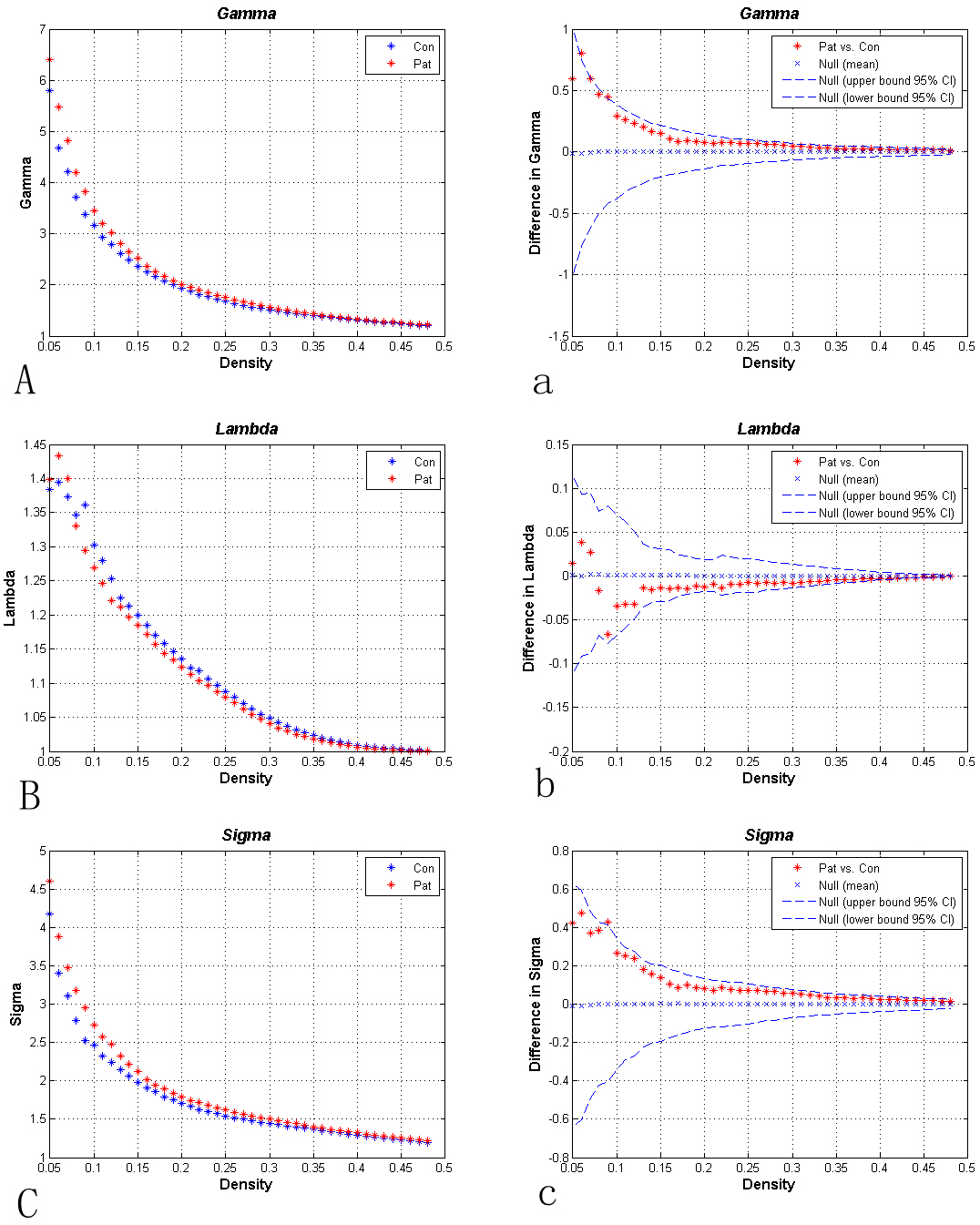 Fig. 2.
Fig. 2.Overall properties of brain networks under different sparsity
conditions. Plates (A–C) show the standardized cluster coefficients (A),
standardized shortest path length (B), and “small-world” index (
In the small-world network density range of the BC and HC groups, differences in brain function network effect parameters, including the whole brain effect (Eglobal) and local effect (Elocal), were not statistically significant under the same network sparsity conditions (Fig. 3).
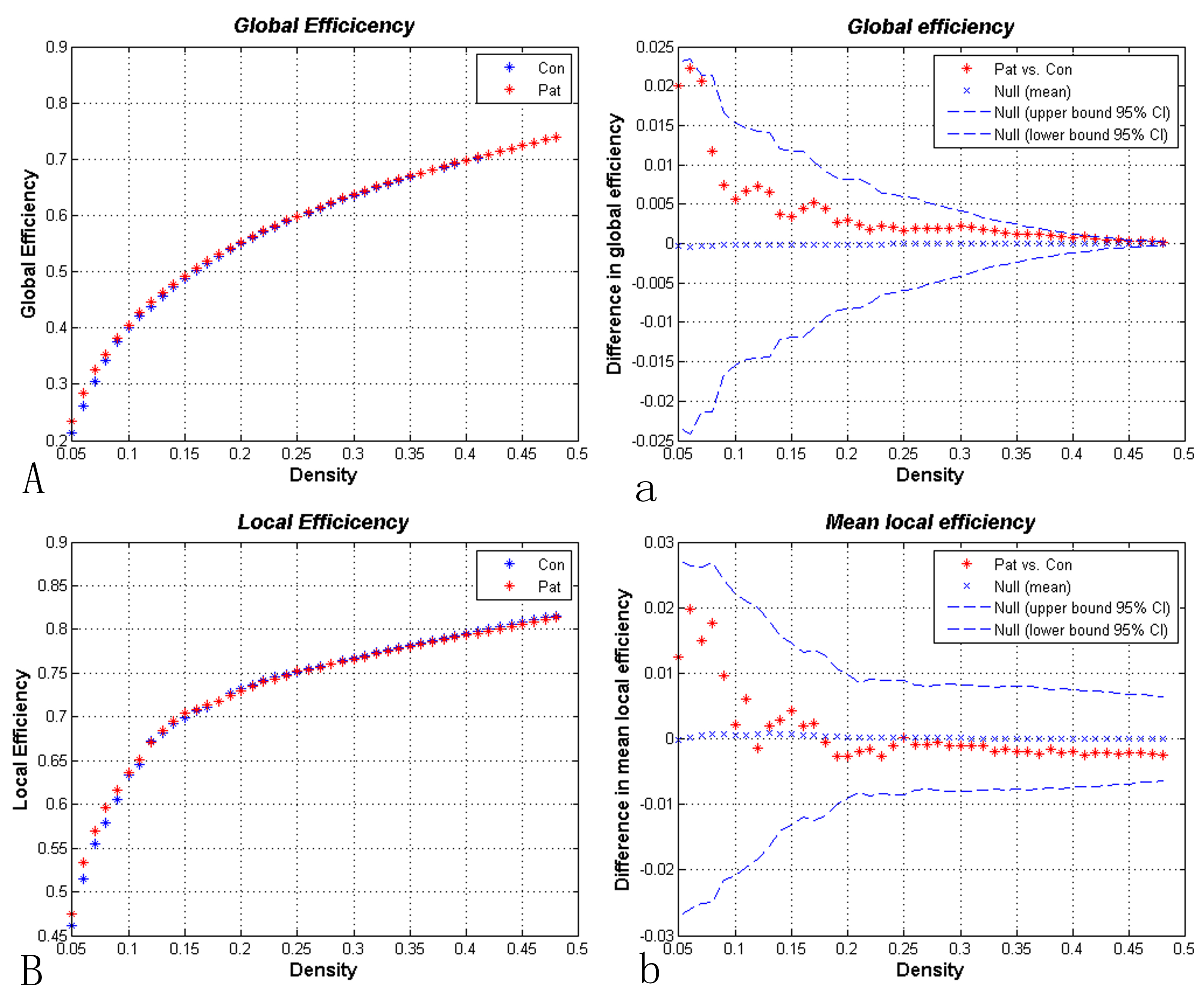 Fig. 3.
Fig. 3.Small-world network efficiency results obtained within the network sparsity range. Compared with the healthy control group, whole brain (A and a) and local measurements (B and b) were not significantly different in the brain concussion group.
Under the condition of Dmin = 0.13, node betweenness in the right intraorbital
superior frontal gyrus (ORBsupmed.R, AAL26, Anatomical Automatic Labeling), right globus pallidus (PAL, AAL76),
and Heschl’s gyrus (HG), also known as transverse temporal gyrus (AAL77 and
AAL78) in the BC patient group were significantly lower (p
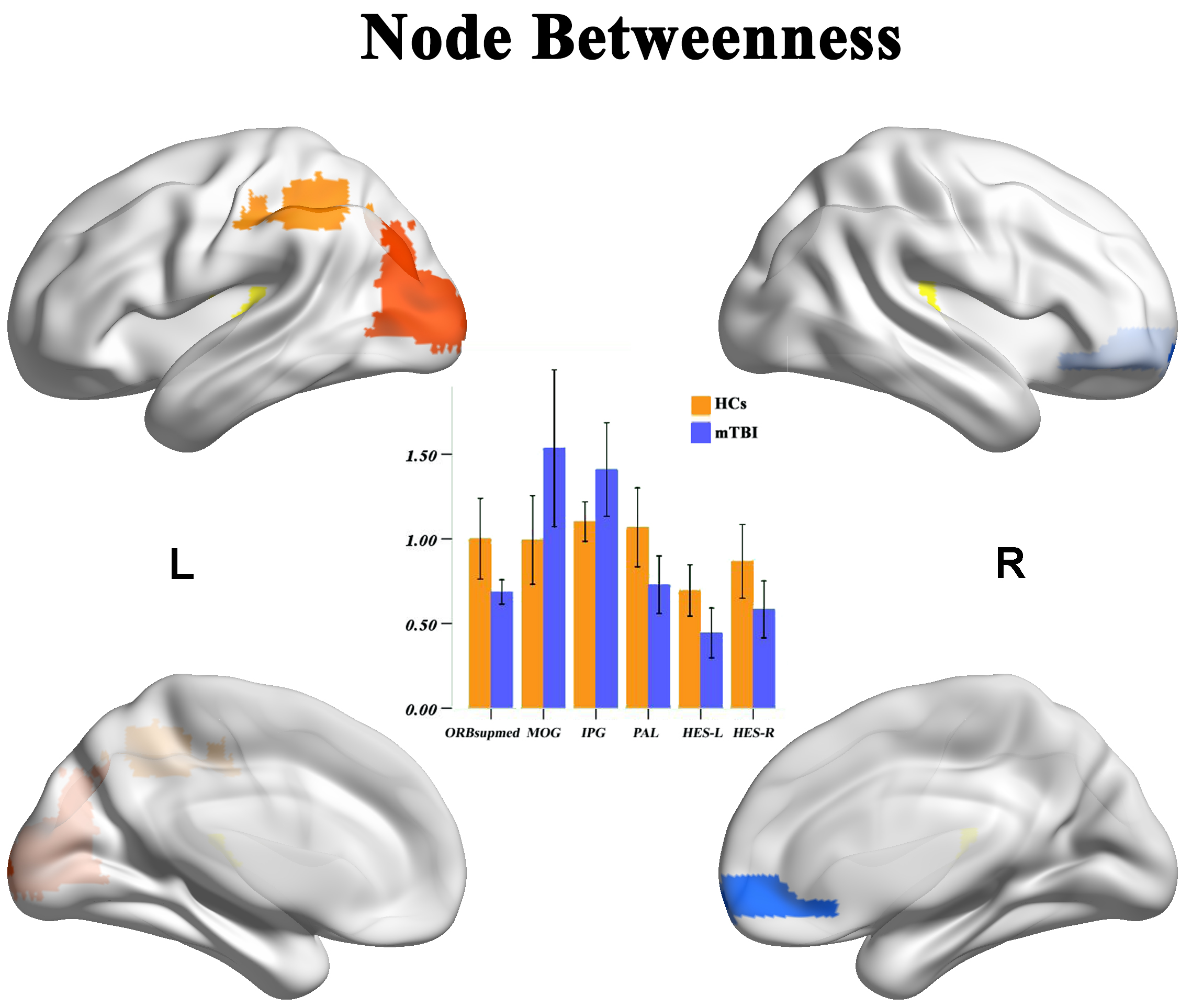 Fig. 4.
Fig. 4.Comparison of node betweenness under the minimum network
sparsity condition (Dmin = 0.13). Compared with the healthy control (HC) group,
node betweenness in the right intraorbital superior frontal gyrus (ORBsupmed.R,
right globus pallidus), and bilateral temporal traverse (Heschl’s gyrus) in the
BC group were significantly lower, whereas node betweenness in the left inferior
parietal lobule (IPL.L) and left median occipital gyrus (MOG) were significantly
higher (p
The normalized cluster coefficients of the ORBsupmed.R (AAL26) and left posterior cingulate gyrus (PCG, AAL35) in the BC group were significantly higher (p
 Fig. 5.
Fig. 5.Under the minimum network sparsity condition (Dmin = 0.13),
clustering coefficients (plates A and a), and local node effects (plates B and b)
were compared between the groups for each brain region. Compared with the
healthy control group, the standardized cluster coefficients of the right
intraorbital superior frontal gyrus (ORBsupmed) and left posterior cingulate
gyrus (PCG) were significantly higher in the patient group, whereas the
normalized cluster coefficients of the right triangular inferior frontal gyrus
(IFGtriang) and left inferior parietal lobules (IPLs) were significantly lower
(p
The human brain is a complex, internally connected system, with a highly diverse set of important topological attributes such as small-world attributes, low efficiency, and high connectivity of hub nodes [14, 15]. The small-world network model, also known as the Watt–Strogatz model [16], combines the characteristics of shortest path length and high cluster coefficients and provides a powerful means for interpreting brain network models. The combination of high cluster coefficients and the shortest path length reflects two important attributes for the management of the human brain: functional separation and functional integration.
Our complex network analysis based on graph theory revealed that both the BC
patient and normal control groups had efficient small-world attributes in
whole-brain functional networks (
We observed no significant differences in the whole brain or meant local efficiency between the BC and HC groups. However, there were significant differences in the local efficiency of some brain regions, suggesting compensatory changes in the brain network of concussion patients. This was consistent with Yan’s study [25] but was inconsistent with Churchill’s study [26]; the slight differences may be explained by differences in patient inclusion criteria and too few samples. Regarding the right medial orbitofrontal cortex, left posterior cingulate gyrus, and left sub-parietal lobules (i.e., the core area of the DMN), an increase in the local efficiency was seen in the former two and a reduction was seen in the latter, suggesting functional separation of the DMN. Functional separation of the DMN was consistent with some smaller research results [10, 27]. A sleep deprivation study by Gujar et al. [28] revealed the bidirectional separation of DMN function; combined with the results of this study, we speculate that the function of some brain areas of the DMN after BC injury may be impaired, while other brain regions provide complementary functionality. Alternatively, different brain regions within the DMN may control different subfunctions, where compensation by one side due to functional impairment of the other side may result in the separation of DMN function [10]. The specific mechanism requires further study.
This study also found that compared with the HC group, the nature of brain network nodes in BC patients was consistently altered, which was mainly reflected in abnormal changes in emotional circuits. The cluster coefficient and average local effect of the upper frontal node on the right orbit were increased, while the median number of nodes was reduced, in the BC group. The former two findings reflect the enhancement of node betweenness, which indicates that the influence of nodes in the functional loop was weakened. The intraorbital prefrontal gyrus is associated with the prefrontal lobe system and abnormal emotional processing. Abnormal changes in the frontal gyrus contribute to emotional numbness, mental alertness, and psychological avoidance in patients with post-traumatic stress disorder [29]. The superior frontal triangle and the superior frontal insular gyrus are part of the frontal gyrus; a change in the nature of their nodes may contribute to the abnormal brain emotional circuits seen in BC patients. The PAL is part of the limbic system and is involved in emotional processing. The reduction in the number of nodes in the left PAL seen in this study may play a role in the negative mood of BC patients. Functional separation of the nodes in the core area of the DMN was also seen, as stated above. The number of nodes mediating the hearing-related brain region (bilateral temporal transverse gyrus) is reduced, whereas the number in the vision-related brain region (left occipital midgeal gyrus) is increased, in BC patients, which may explain their noise sensitivity. The left occipital midgeal gyrus is associated with the visual cortex, and plays a role in mild cognitive impairment and early manifestations of AD [19]. These abnormalities may explain some of the symptoms of BC, but their clinical relevance has yet to be explored.
The clinical scoring scale of patients in this study had few categories and lacked a follow-up study. The range of education level and age in this patient group was large and the number of cases was relatively small, which was not enough to group patients with different levels of education and age. This study did not study the correlation between topology attribute parameters and clinical scales. We expect this to be further studied in the future.
In patients with concussion, random abnormalities may be seen in the overall and local properties of the resting brain functional network. Functional abnormalities in the default network and changes in the emotional circuit in the resting state are particularly significant. We speculate that these abnormalities may lead to cognitive dysfunction and emotional changes in BC patients.
BC, brain concussion; HC, healthy control; GCS, Glasgow Coma Scale; RPQ, Rivermead Postconcussion Symptom Questionnaire; MRI, magnetic resonance imaging; mTBI, mild traumatic brain injury; PCS, post-concussion symptoms; CT, computed tomography; SWI, susceptibility weighted imaging; DMN, default mode network.
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
HHA and GJX designed the research study. HMK and YC performed the research. GJX provided help and advice on research. JLH and JL analyzed the data. NZ contributed analytic tools and finalized the manuscript, HMK, NZ and JLH participated in the writing of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All of the participants or guardians provided informed written consent prior to study enrollment. The study protocol was approved by the Human Research Ethics Committee and Institutional Review Board of the First Affiliated Hospital of Nanchang University (No: (2022)CDYFYYLK(09-043)). All study procedures were carried out in accordance with approved guidelines and the principles of the Declaration of Helsinki.
We would like to thank two professional editors for helping polishing the English language.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
