- Academic Editor
†These authors contributed equally.
Background: This study explored the specific relationship between
different lipid indicators and cognitive impairment and aimed to provide a
reference for implementing targeted lipid regulation measures to prevent and
alleviate cognitive impairment. Methods: We searched three databases
(PubMed, Embase, and Web of Science) for literature related to hyperlipidaemia,
lipid levels, and cognitive impairment, and used the Newcastle-Ottawa Scale to
evaluate the quality of the identified literature. A meta-analysis was performed
using RevMan 5.4, and the combined effect size ratio using a random-effects model
(odds ratio [OR] and 95% confidence interval [CI]) was used to evaluate the
association between dyslipidaemia and cognitive impairment. Results:
Among initially identified 2247 papers, we ultimately included 18 studies
involving a total of 758,074 patients. The results of the meta-analysis revealed
that patients with hyperlipidaemia had a 1.23-fold higher risk of cognitive
impairment than those with normal lipid levels (OR = 1.23, 95% CI: 1.04–1.47,
p = 0.02). Further subgroup analysis showed that elevated total
cholesterol (TC) levels increased the risk of cognitive impairment by 1.59-fold
(OR = 1.59, 95% CI: 1.27–2.01, p
Cognitive impairment encompasses dysfunction in various areas, including memory,
language, attention, and executive functioning. It is categorized into two
different stages based on severity: mild cognitive impairment (MCI) and dementia,
with Alzheimer’s disease (AD) being the most prevalent form of dementia [1, 2].
The US census reports an overall prevalence 11.3% for AD and 22.7% for MCI in
individuals aged
The primary risk factors for cognitive impairment include age, family background, cardiovascular disease, neurological disorders, and medications [1, 4, 8]. Dyslipidaemia is also closely associated with cognitive impairment [9]. Dyslipidaemia encompasses abnormalities in lipid levels, including triglycerides (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C), commonly referred to as hyperlipidaemia [10, 11]. Hyperlipidaemia can impair microvascular function, leading to memory deficits and reduced processing speed [12]. Hyperlipidaemia also increases the risk of cardiovascular diseases such as atherosclerosis [13], coronary heart disease [14], and cerebrovascular accidents [15] and leads to various degrees of cognitive impairment [16, 17, 18]. Dyslipidaemia is linked to cognitive impairment through the induction of an inflammatory response [19]. The interactions among dyslipidaemia, insulin resistance, and apolipoprotein E exacerbate the development of cognitive impairment [20]. Lipidomics can serve as a biomarker for early brain disease diagnosis, with various types of lipid dysregulation associated with neurodegenerative diseases [21]. Lipotoxicity is a metabolic disorder caused by lipid accumulation in non-adipose tissues, leading to cell dysfunction, lipid droplet formation, and cell death. Cerebral microvascular lipotoxicity can promote microglial activation and increased release of inflammatory factors, leading to neurodegeneration and cognitive impairment [22]. However, existing research on the relationship between lipid levels and cognitive impairment varies significantly and lacks consistent results. For example, Han [23] found an inverse relationship between high levels of HDL-C and cognitive impairment, whereas TG, TC, and LDL-C showed no such connection. Conversely, in a case-control study, higher TC and LDL-C levels, as well as lower HDL-C levels, as risk factors for AD [24].
In light of these findings, we hypothesized a correlation between dyslipidaemia and cognitive impairment. To investigate this relationship, we conducted a meta-analysis of published literature exploring the link between hyperlipidaemia, blood lipid levels, and cognitive impairment, aiming to provide evidence for mitigating cognitive decline.
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement Report and was registered on the PROSPERO platform (CRD42023395479). PRISMA checklist is shown in Supplementary Table 1.
Two researchers independently conducted searches in three databases: PubMed, Embase, and Web of Science, from the inception of databases to 1 March 2023. The search terms used were (‘hyperlipidaemias’ OR ‘TG’ OR ‘triglyceride’ OR ‘cholesterol’ OR ‘TC’ OR ‘hyperlipemia’ OR ‘low density lipoprotein cholesterol’ OR ‘LDL-C’) AND (‘cognitive dysfunction’ OR ‘dementia’ OR ‘Alzheimer disease’ OR ‘cognitive impairment’ OR ‘cognitive decline’) AND (‘case-control studies’ OR ‘cohort studies’) (Supplementary Table 2). A manual search was also conducted to identify relevant references in the literature.
Articles were selected for this study based on the following inclusion criteria:
(1) patient age
Studies were independently selected from the retrieved literature by the two
researchers. In cases of disagreement, a third researcher determined study
inclusion. Data were extracted and recorded using Microsoft Excel (Microsoft
Office Excel 2016, Microsoft, Redmond, WA, USA). The key data extracted included
the first author, publication year, study type, patient sex, sample size, lipid
index, and lipid subgroup comparison. We also evaluated the literature quality
according to the NOS, as recommended by the Cochrane Collaboration Network, and
studies with scores
The primary outcome indicator in this study was the relationship between cognitive impairment and dyslipidaemia. The secondary endpoint was the variation in cognitive function under the impact of specific lipid indicators. Cognitive impairment was assessed employing tools such as the Mini-Mental State Examination, Diagnostic and Statistical Manual of Mental Disorders, or International Classification of Diseases (ICD) codes as diagnostic criteria for dementia and AD.
Statistical analysis was performed using RevMan 5.4 (RevMan 5.3, The Nordic
Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Considering
the different groups of lipid levels between the studies, the OR (Relative Risk
(RR), HR) values and 95% CI for the relationship between the highest vs. the
lowest concentrations of lipid levels with cognitive impairment in each study
were extracted for analysis, following reference to the relevant meta-analysis
[25]. For instance, in the study by Rantanen et al. [26], which
categorized the participants into four groups according to serum TC levels, and
ORs and 95% CI subgroups of cognitive impairment risk in the fourth quarter (Q4)
vs. the first quarter (Q1) were extracted for our meta-analysis. The magnitude of
heterogeneity between studies was assessed using the Cochrane Q test and I
Fig. 1 illustrates the detailed literature
screening process. In total, 2247 relevant papers were retrieved, including 714
in PubMed, 641 in Embase, and 892 in Web of Science. Endnote 20 software (Thomson
Corporation Inc., Stanford, CT, USA) was used to eliminate 609 duplicates.
Subsequently, 78 papers were obtained by reviewing titles and abstracts.
Ultimately, the meta-analysis included 18 studies after the final screening of
the full text and references. These studies comprised four case-control studies
[24, 27, 28, 29] and 14 cohort studies [26, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42]. Six papers were
published before 2010, and 12 papers were published after 2010. Eight countries
and 30 regions were included, with study sample sizes ranging from 117 to
469,466, totalling 758,074 individuals. A total of 25 data groups were included
in the analysis of the correlation between hyperlipidaemia and the risk of
cognitive impairment. Furthermore, five studies provided data on elevated TC
levels and the risk of cognitive impairment in populations aged
 Fig. 1.
Fig. 1.Flowchart of study selection for the meta-analysis. NOS, Newcastle-Ottawa Scale. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers).
| Study | Publication year | Type of research | Percentage of males (%) | Sample size | Ending indicators | Risk factors | Subgroup comparison |
| Suryadevara, V [27] | 2003 | Case-control study | 34% | 100 | Dementia | LDL-C | |
| 100 | Dementia | TC | |||||
| Notkola, IL [30] | 1998 | Cohort study | 100% | 444 | Dementia | TC | |
| Chen, H [24] | 2019 | Case-control study | 57.26% | 234 | AD | TC | |
| TG | |||||||
| LDL-C | |||||||
| Zambón, D [31] | 2010 | Cohort study | 45.30% | 117 | MCI | FH | Yes; No |
| Mundal, LJ [32] | 2022 | Cohort study | 46.90% | 73,233 | Dementia | FH | Yes; No |
| Rantanen, K [26] | 2014 | Cohort study | 100% | 1049 | AD | TC | Q1 vs. Q4 |
| Nordestgaard, LT [33] | 2021 | Cohort study | 36% | 125,757 | AD | TG | Q1 vs. Q4 |
| Reitz, C [34] | 2008 | Cohort study | 31.03% | 854 | MCI | LDL-C | Q1 vs. Q4 |
| TG | Q1 vs. Q4 | ||||||
| Reitz, C [35] | 2010 | Cohort study | 34.34% | 1130 | AD | LDL-C | Q1 vs. Q4 |
| Solomon, A [36] | 2007 | Cohort study | 61.69% | 1321 | Cognitive impairment | TC | Q1 vs. Q4 |
| Solomon, A [37] | 2009 | Cohort study | 45.86% | 9744 | Dementia | TC | Q1 vs. Q4 |
| Cohort study | TC | Q1 vs. Q3 | |||||
| Mielke, MM [38] | 2010 | Cohort study | 0% | 648 | Dementia | TC | |
| AD | TC | ||||||
| He, Q [28] | 2016 | Case-control study | 40.96% | 227 | MCI | LDL-C | Q1 vs. Q4 |
| Li, G [39] | 2005 | Cohort study | 40.45% | 2141 | AD | TG | Q1 vs. Q4 |
| Dementia | TG | Q1 vs. Q4 | |||||
| Gong, J [40] | 2022 | Cohort study | 45.77% | 469,466 | Dementia | TG | Q1 vs. Q4 |
| Ancelin, ML [41] | 2013 | Cohort study | 38.91% | 7053 | AD | TG | |
| Zhang, H [29] | 2021 | Case-control study | 58.35% | 497 | MCI | LDL-C | |
| Yang, Z [42] | 2022 | Cohort study | 50.81% | 63,959 | Dementia | TG | Q1 vs. Q5 |
AD, Alzheimer’s disease; MCI, mild cognitive impairment; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; FH, familial hypercholesterolaemia; Q1, first (lowest) quarter; Q3, third quarter; Q4, fourth quarter; Q5, fifth quarter.
The sensitivity analysis of elevated TC, TG, and LDL-C levels and their association with the risk of cognitive impairment revealed no substantial changes in the results after altering the model, indicating that the combined results from these studies remained stable and reliable (Table 2). Regarding the assessment of publication bias in the literature pertaining to hyperlipidaemia and the risk of cognitive impairment, the funnel plot displayed an asymmetric distribution of the included literature, with several articles falling outside the 95% CI (Supplementary Fig. 1).
| Study exposure indicators | Models | Consolidation model | Change model | ||
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Elevated TC | R | 1.59 (1.27–2.01) | 1.45 (1.27–1.64) | ||
| Elevated TG | F | 0.85 (0.79–0.92) | 0.85 (0.79–0.92) | ||
| Elevated LDL-C | R | 1.45 (0.74–2.83) | 0.28 | 1.03 (0.84–1.28) | 0.75 |
R, random-effects model; F, fixed-effects model; OR, odds ratio; CI, confidence interval.
The meta-analysis, incorporating data from the 18 publications on
hyperlipidaemia and the risk of cognitive impairment, revealed that individuals
with hyperlipidaemia faced a 23% increased risk of developing cognitive
impairment, which was a statistically significant difference. However, the
heterogeneity test demonstrated a substantial degree of heterogeneity among the
studies (I
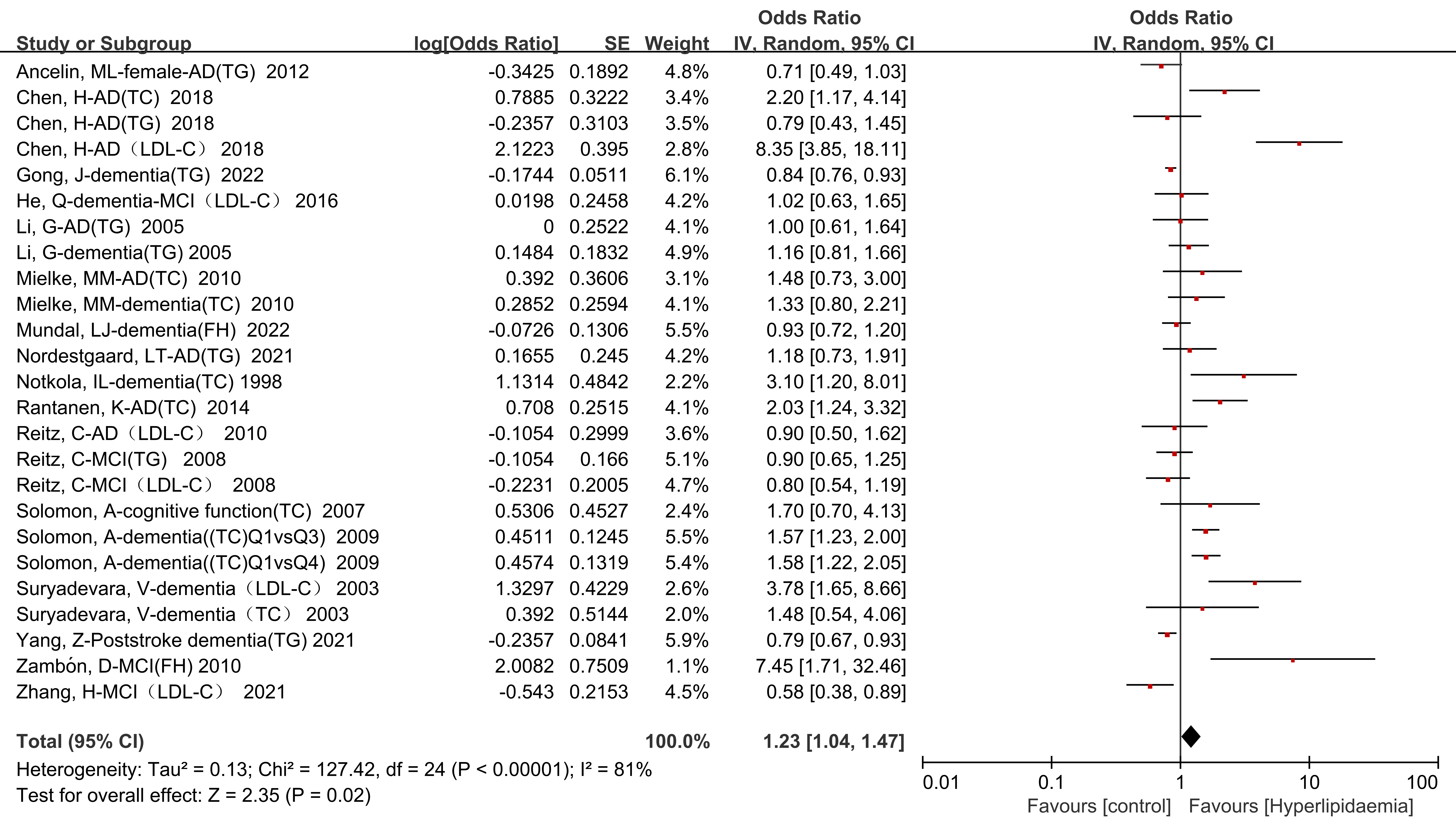 Fig. 2.
Fig. 2.Forest plot of meta-analysis results depicting the relationship
between hyperlipidaemia and cognitive impairment. SE, standard error; Tau
Nine of the included studies (n = 86,890) provided specific data regarding the
impact of TC levels on the risk of developing cognitive impairment. The results
from the meta-analysis revealed that individuals with increased TC levels had a
1.59-fold increased risk of developing cognitive impairment after follow-up
compared with normal TC levels (OR = 1.59, 95% CI: 1.27–2.01, p
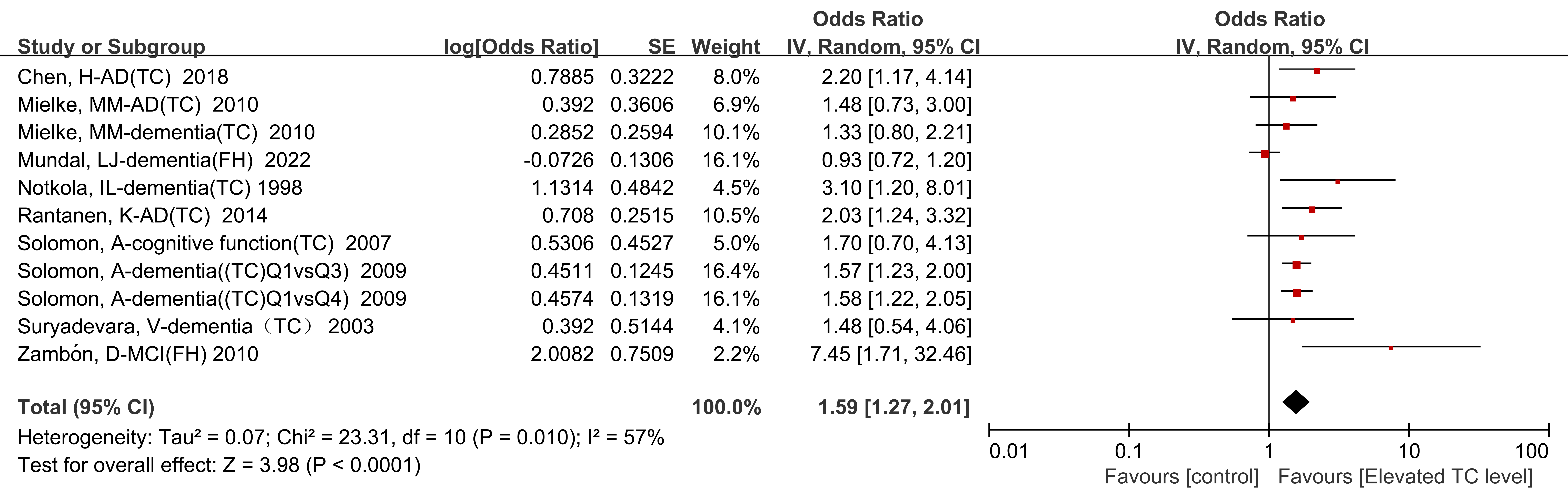 Fig. 3.
Fig. 3.Forest plot of meta-analysis results depicting the relationship between elevated TC level and the risk of cognitive impairment.
Among the studies, five and six studies provided ORs for elevated TC levels in
relation to the risk of cognitive disability in individuals aged
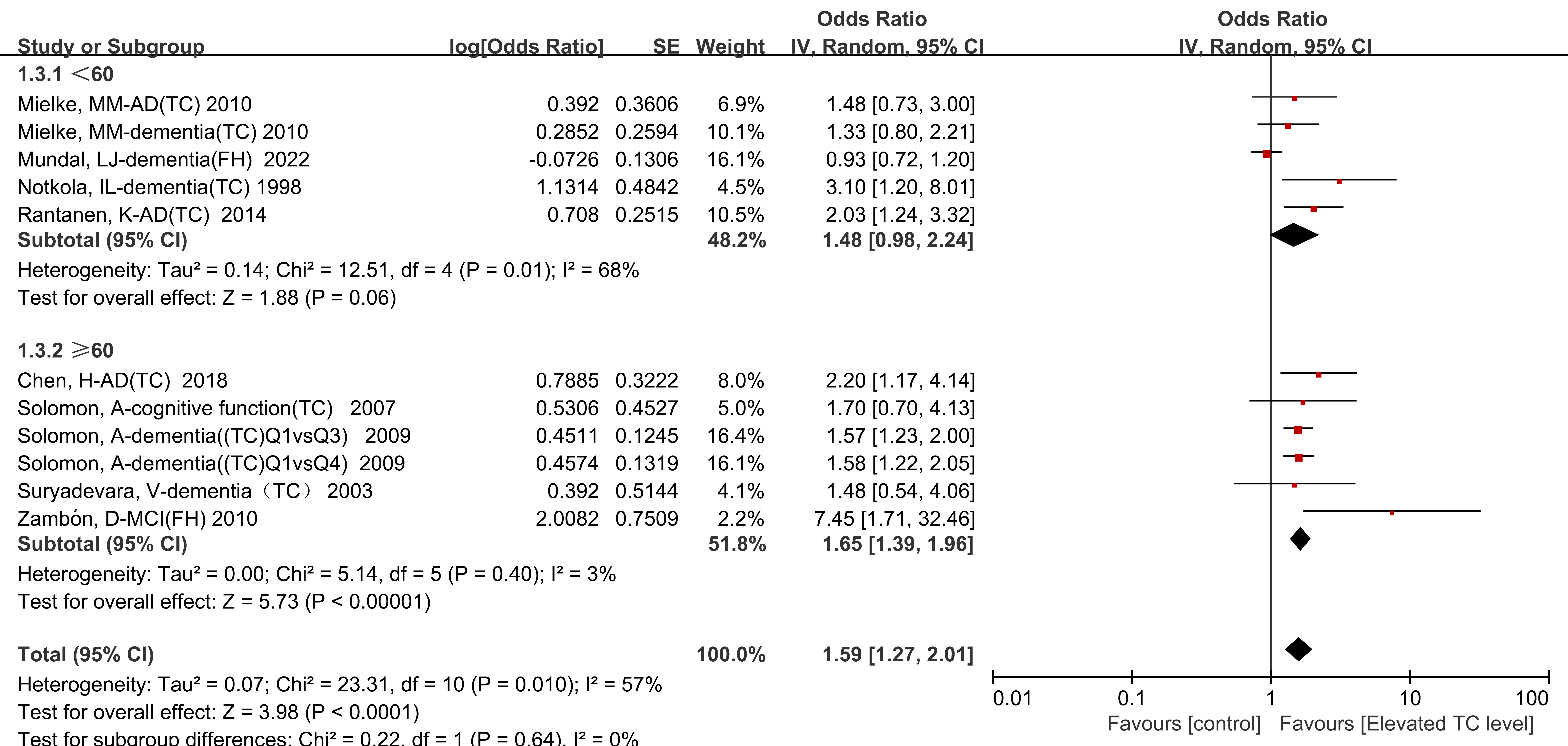 Fig. 4.
Fig. 4.Forest plot of elevated TC levels and cognitive impairment by age.
In this analysis, four studies provided data on the association between elevated
TC levels and cognitive impairment in patient populations comprising
 Fig. 5.
Fig. 5.Forest plot of elevated TC levels and cognitive impairment by sex ratio.
In this analysis, eight studies provided data on TG levels and the risk of
developing cognitive impairment. The results of the meta-analysis showed a
significantly negatively between elevated TG levels and cognitive impairment (OR
= 0.85, 95% CI: 0.79–0.92, p
 Fig. 6.
Fig. 6.Meta-analysis graph of elevated TG levels and risk of cognitive impairment.
In this analysis, six datasets were included, with three studies [24, 27, 29]
reporting an association between elevated LDL-C levels and cognitive impairment,
and three other studies [28, 34, 35] reporting no such correlation. The results
of the meta-analysis showed no significant correlation between elevated LDL-C
level and the risk of cognitive impairment (OR = 1.45, 95% CI: 0.74–2.83,
p = 0.28, as shown in Supplementary Fig. 2). However,
significant inter-study heterogeneity was observed (I
| Subgroup factors | No. of studies | OR (RE) | 95% CI | Heterogeneity test | Overall effect | Interaction p-value | |||
| Q | p | I |
Z-value | p-value | |||||
| Summary | 6 | 1.45 | 0.74–2.83 | 46.38 | 89% | 1.08 | 0.28 | ||
| Year of publication | |||||||||
| 3 | 1.3 | 0.58–2.89 | 11.24 | 0.004 | 82% | 0.64 | 0.52 | 0.77 | |
| 3 | 1.63 | 0.45–5.98 | 35.13 | 94% | 0.74 | 0.46 | |||
| Study Type | |||||||||
| Case-control study | 4 | 1.21 | 0.92–1.60 | 43.26 | 93% | 1.36 | 0.17 | 0.08 | |
| Cohort study | 2 | 0.83 | 0.60–1.15 | 0.11 | 0.74 | 0% | 1.12 | 0.26 | |
| Male ratio | |||||||||
| 2 | 1.07 | 0.74–1.55 | 35.1 | 97% | 0.36 | 0.72 | 0.83 | ||
| 4 | 1.02 | 0.79–1.32 | 11.24 | 0.01 | 73% | 0.14 | 0.89 | ||
RE, relative error.
The aggregate analysis in this study included a total of 18 studies related to lipid levels and cognitive disorders. The results demonstrated a 1.23-fold increased risk of developing cognitive impairment in individuals with hyperlipidaemia. Further subgroup analysis showed that elevated TC levels were associated with an increased risk of cognitive impairment, whereas increased LDL-C levels were not. Additionally, high TG levels were found to be protective against cognitive impairment. Furthermore, older or male patients with elevated TC levels had a high risk of developing cognitive impairment.
Elevated TC levels increased the risk of cognitive impairment by 1.59-fold in
the present meta-analysis, consistent with the findings reported by An et
al. [43] and Chen et al. [24]. Previous meta-analyses have
also shown that increased TC concentrations lead to an increased incidence of AD
[44]. Hypercholesterolaemia exacerbates the impairment of cognitive function by
inducing neuroinflammation [45] and the levels of inflammatory factors,
interleukin (IL)-6 and tumour necrosis factor (TNF)-
The results of our meta-analysis also revealed an age-sex disparity in the interaction between elevated TC levels and cognitive impairment risk. Zhao et al. [54] also reported a positive association between high TC levels and cognitive impairment in older men. In a study assessing the dietary intake of older Chinese individuals, daily cholesterol consumption was higher in men than in women caused by differences in dietary habits and preferences. Older individuals showed the highest percentage of excess cholesterol intake, which is directly correlated with TC levels. High dietary consumption of cholesterol increases the risk of borderline elevated cholesterol levels and hypercholesterolaemia in older men [55, 56]. Ma et al. [57] demonstrated that hypercholesterolaemia is involved in the development of atherosclerosis and exacerbates cognitive deficits by impairing vascular function. The higher the serum cholesterol concentration in older people, the more significant the degree of cognitive decline.
The association between elevated TG levels and cognitive disorders remains controversial. A 20-year cohort study by Power et al. [58] showed reduced executive performance, attention duration, and processing speed in patients with increased TG levels. The cognitive-impairing effects of elevated TG levels are primarily caused by impaired vascular endothelial function. The more severe the impairment of microvascular endothelial function, the worse the executive and verbal memory abilities of the patient [59]. Takaeko et al. [60] observed that patients with low TG levels had higher vasodilation and better vascular endothelial function compared with patients with high TG levels. The results of our meta-analysis showed that increased TG concentrations were negatively associated with cognitive impairment, consistent with the findings reported by Zhao et al. [54], Lv et al. [61], and others. However, owing to the limited number of included studies, only two studies [40, 42] showed a significant correlation between TG levels and cognitive impairment; the remaining studies showed no obvious statistical significance. Therefore, further clinical trials are required to verify the association between high TG levels and cognitive impairment.
Elevated serum LDL-C levels increase the risk of AD [62, 63]. LDL promotes the
release of inflammatory mediators TNF-
The study has several limitations. First, the results were highly heterogeneous and limited by the characteristics of the observational studies. Second, the criteria for categorizing blood lipid subgroups differed among studies, which inevitably influenced the research results when determining the OR (RR) value of the ratio of the highest and lowest blood lipid concentrations. Furthermore, some related studies have suggested that elevated HDL-C levels are positively correlated with cognitive function [70]. However, we did not conduct a meta-analysis of HDL-C because the included studies did not provide data on the correlation between HDL-C levels and cognitive impairment.
In conclusion, the results of our meta-analysis suggest that hyperlipidaemia increases the risk of cognitive impairment. Elevated serum TC levels exacerbate the development of cognitive disabilities and are more likely to be observed in older male patients. TG levels were negatively correlated with the risk of impaired cognition, whereas serum LDL-C levels were not significantly associated with cognitive impairment. Understanding the interaction between blood lipid levels and cognition can provide valuable insights for tailoring specific blood lipid regulation strategies for high-risk individuals with lipid disorders, potentially reducing the incidence of cognitive impairment. To strengthen our understanding and support the prevention and treatment of conditions related to cognitive function, further comprehensive clinical studies investigating the interplay between lipids and cognition are necessary.
AD, Alzheimer’s disease; MCI, mild cognitive impairment; CI, confidence interval; HDL-C, High-density lipoprotein cholesterol; ICD, International Classification of Diseases; LDL-C, Low-density lipoprotein cholesterol; NO, nitric oxide; NOS, Newcastle-Ottawa Scale; OR, odds ratio; RE, relative error; HR, relative hazard; TC, total cholesterol; TG, triglyceride.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
XXD, DYZ, YZ and HXZ designed the research study. YZ and HXZ performed the research. YZ, HXZ, JC and YTZ completed data selection and extraction. YZ and HXZ analyzed the data. XXD, DYZ, YZ and HXZ contributed to the writing of the article. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
