- Academic Editors
†These authors contributed equally.
Background: The flavonoid chrysin produces rapid and long-lasting
anxiolytic- and antidepressant-like effects in rats. However, it is not known
whether low and high doses of chrysin produce differential anti-immobility
effects through the Gamma-Aminobutyric Acid sub-type A (GABA
The flavonoid chrysin (5,7-dihydroxyflavone) is found in high concentrations in
propolis and honeybee, and particularly also in plants such as Passiflora
coerulea, Passiflora incarnata and Matricaria chamomilla [1].
It exerts antioxidant, anticancer and anti-inflammatory activity, as well as
important pharmacological effects in several disorders involving the central
nervous system, such as epilepsy, Parkinson’s disease, multiple sclerosis,
Alzheimer’s disease, depression and anxiety [1, 2]. Preclinical studies have
demonstrated anxiolytic-like effects of chrysin in different anxiety models in
mice and rats. For example, the injection of 1 mg/kg chrysin increases the time
spent by male mice [3] and rats [4] on the open arms of the elevated plus maze
(EPM). It also increases the time spent by male rats [5] and zebrafish [6] in the
illuminated compartment in the light/dark test (LDT). Similarly, the
administration of 2 mg/kg chrysin in ovariectomized [7] and cycling [8, 9] female
rats produces an anxiolytic-like effect in the EPM and LDT tests. The effect is
similar to that produced by anxiolytic drugs as diazepam [8] and some
neuro-steroids such as 17
In experimental anxiety models, daily administration for 14–28 days of 5–20
mg/kg chrysin reduced the total time of immobility in forced swim and tail
suspension tests [14, 15]. This was accompanied by higher consumption of sucrose,
suggesting an antidepressant-like effect. The effects were also associated with
an increased serotonin concentration in the prefrontal cortex, hippocampus and
nucleus accumbens, which could be blocked by pretreatment with
p-chlorophenylalanine, a selective inhibitor of the tryptophan hydroxylase enzyme
involved in serotonin biosynthesis [14, 15]. At the clinical level, abnormalities
in serotonin neurotransmission during depression can now be more reliably
evaluated due to advances in measurement techniques. However, there is still much
to learn about serotonin activity and its potential role in the causation of
illness [16]. Novel research approaches go beyond the serotonin hypothesis of
depression and have moved to molecular integration involving diverse receptors
and biochemical systems, some of which could become novel targets for
antidepressant drugs. For example, daily oral administration of 20 mg/kg chrysin
or of 10 mg/kg fluoxetine for 28 days increased the levels of the neurotrophins
Brain Derived Neurotrophic Factor (BDNF) and Nerve Growth Factor (NGF) compared
to controls in the hippocampus and prefrontal cortex of both non-stressed and
stressed mice [17]. This was associated with a decrease in depression-like
behavior [18, 19], and suggests the potential for developing new drugs from their
dipeptide mimetics [20]. Related to the above findings, the flavonoid chrysin,
similar to the neuro-steroid allopregnanolone, exerts anxiolytic- and
antidepressant-like effects through actions on GABAergic and serotonergic
systems. These actions are likely to be dependent on the doses administered and
the duration of treatment (21 to 28 days) [21, 22]. Therefore, we hypothesized
that low and high doses of chrysin may produce antidepressant-like effects
through different mechanisms of action. The goal of this study was to examine the
effects of 1 mg/kg and 5 mg/kg chrysin after 0, 1, 14, and 28 days of treatment,
and 48 h post-treatment (day 30) in the forced swim test. The results were
compared with those of fluoxetine (a serotonergic drug) and allopregnanolone (a
GABAergic drug) as pharmacological controls of antidepressant-like activity in
adult male rats. In addition, the involvement of the GABA
All experimental procedures were performed in strict accordance with the Guide for the Care and Use of Laboratory Animals published by the National Research Council [23] and the Mexican law for the use and care of laboratory animals [24]. All efforts were made to minimize animal discomfort and to reduce the number of animals, according to the 3R’s principles of preclinical research [25]. The experimental protocol received authorization from the Committee for the Use and Care of Laboratory Animals of the Biomedical Research Center from Universidad Veracruzana with the approval number CLCIB2023/2.
Adult male Wistar rats (260–280 g) aged 2.5 months were used in the study. The
rats were bred in the vivarium of the Institute of Neuroethology of the
Universidad Veracruzana (Xalapa, Veracruz, Mexico) and weaned at 21 days
postnatal. They were housed in Plexiglas cages, at 4 rats per cage (44 cm width
The rats were randomly assigned to different groups using a free online program (https://random.org). The sample size per group (n = 8) was based on previous studies that found 7–8 rats per group [26, 27, 28] gave sufficient statistical power to detect antidepressant-like effects of the substances evaluated in the present study in the forced swim test (FST).
Solutions of chrysin, allopregnanolone, and fluoxetine (FLX) were prepared daily in
35% 2-hidroxypropyl-
Male rats were assigned to 5 independent groups, with 8 rats per group. The
vehicle group (VEH) received the 35% 2-hidroxypropyl-
Before pharmacological administration, all rats were subjected to a 5-min pre-test in the LAT, and subsequently to a 15-min pre-test in the FST. This was for habituation to novel situations, and to trigger the development of behavioral despair, respectively. Pre-test sessions were not considered in the statistical analysis. Twenty-four hours after the pre-tests (defined as day 0), rats were subjected to a 5-min test session in the LAT and subsequently in the FST in order to evaluate the baseline behavior activity. The pharmacological treatments were started after the test sessions on day 0. Behavioral effects were evaluated on the 1st, 14th, and 28th day of treatment, at 1 hour after drug injection. To assess the effect of treatment withdrawal, all rats were also tested at 48 hours after the last injection (day 30). The experimental design is shown in Fig. 1A.
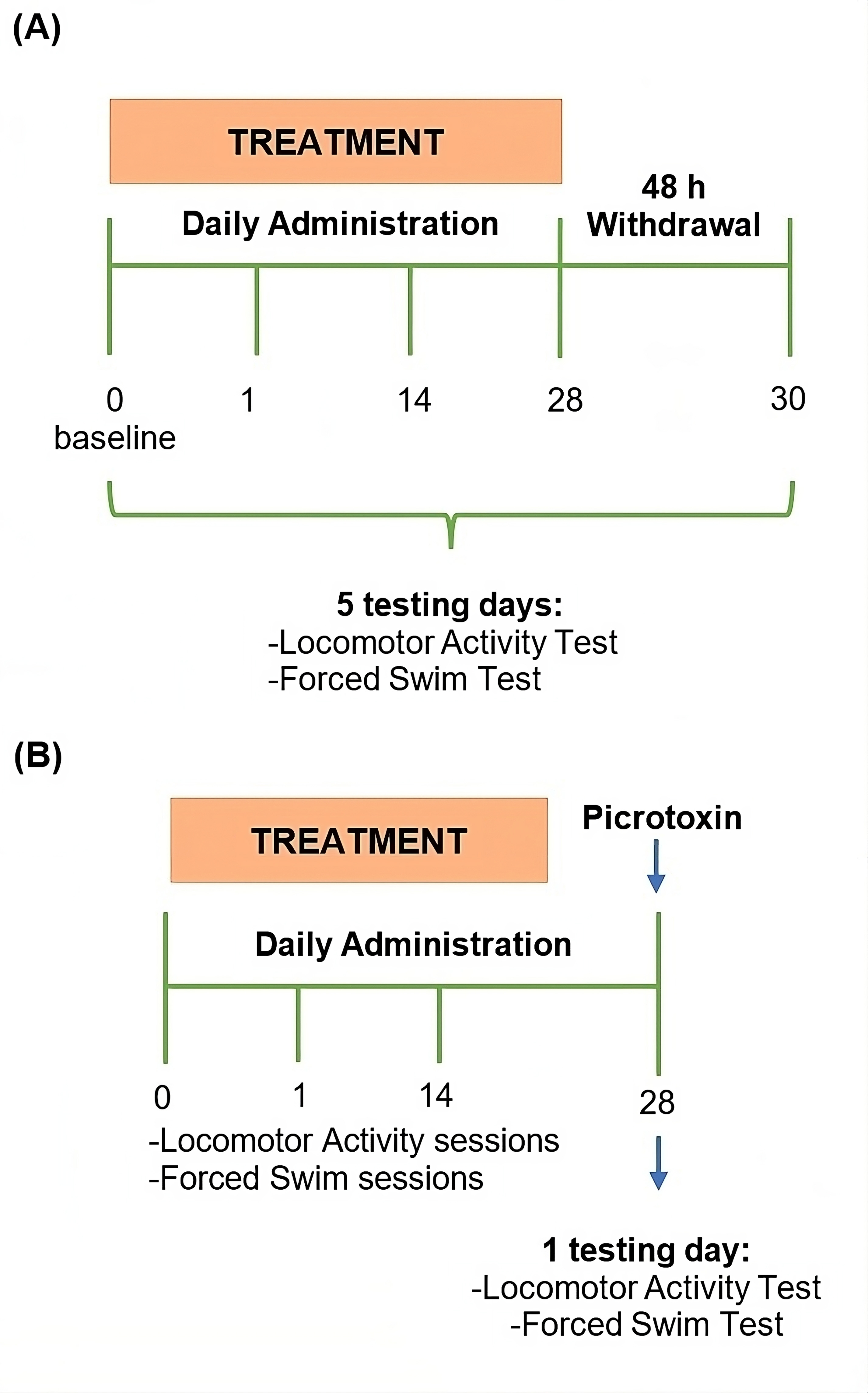 Fig. 1.
Fig. 1.Experimental design. (A) Experiment 1: Effect of chrysin on
depression-like behavior. (B) Experiment 2: Involvement of the GABA
The second experiment evaluated the role of the ion chloride channel of the
GABA
On the test days, rats were brought to the experimental room at 09:00 AM and
left for 1 h to acclimatize to the novel surroundings. Behavioral evaluations
were performed between 10:00 AM and 01:00 PM. A digital video camera (Sony
DCR-SR42, 40
Rats were placed individually in a Plexiglas cage (44 cm length
In the FST, rats were individually forced to swim in a rectangular pool (50 cm
Data from experiment 1 were analyzed using two-way mixed analysis of variance,
with treatment (between subjects) and days of treatment (within subjects) as
factors. Data from experiment 2 were analyzed using one-way analysis of variance
(one-way ANOVA), with treatment as a single factor. Data were transformed to
satisfy the assumption for the normality test and equal variance test. The level
of significance was set at p
Analysis of the number of crossings did not reveal any significant differences
according to treatment [F (4,140) = 0.655, p = 0.627] or to the
interaction of factors [F (16,140) = 1.028, p = 0.431] (Fig. 2A).
However, significant differences were found according to the number of days of
treatment [F (4,140) = 160.780, p
 Fig. 2.
Fig. 2.Locomotor activity test. No differences between treatment
groups were observed for the number of crossings (A), or time spent rearing (B).
The C1, C5, and ALLO groups maintained grooming behavior from day 1 to 28 of
treatment (C), while FLX showed a “U shape”, with increased behavior from day
14 to 28 compared to the VEH group. At day 30 (48 h after treatment withdrawal),
only C5 and FLX showed more time spent on grooming compared to the VEH group.
*p
The time spent on grooming was significantly different according to treatment [F
(4,140) = 36.368, p
The latency to first immobility was significantly different between treatments
[F (4,140) = 187.700, p
 Fig. 3.
Fig. 3.Forced swim test. Effects of chronic treatment on latency to
first immobility (A), and total time of immobility (B). C5, ALLO and FLX showed
gradually increased latency to the first immobility, and decreased total time of
immobility. Only C1 returned to the baseline level of immobility time 48 h after
the last treatment. *p
The involvement of GABA
As shown in Table 1, no significant differences between treatments were observed for the number of crossings [H (9) = 11.104, p = 0.269] or for the time spent rearing [F (9,70) = 1.099, p = 0.375].
| Crossings (n) | Rearing (s) | |
| VEH | 18.0 |
14.0 |
| P | 15.2 |
15.4 |
| C1 | 16.0 |
16.4 |
| C1+P | 17.8 |
17.3 |
| C5 | 21.6 |
14.7 |
| C5+P | 17.0 |
19.5 |
| ALLO | 18.2 |
19.5 |
| ALLO+P | 20.5 |
18.6 |
| FLX | 16.5 |
15.4 |
| FLX+P | 14.2 |
16.0 |
No significant differences in crossings or rearing were observed between treatments.
However, analysis of the time spent grooming revealed significant differences
between the treatments [H (9) = 56.530, p
 Fig. 4.
Fig. 4.Locomotor activity test. Involvement of the GABA
Significant differences in the latency to first immobility were observed between
treatments [F (9,70) = 80.249, p
 Fig. 5.
Fig. 5.Forced swim test. Involvement of the GABA
The present study compared the effects of low and high doses of chrysin on
depression-like behavior in adult male rats with those of fluoxetine and
allopregnanolone. In addition, we investigated the mechanism involving the
GABA
The evaluation of crossings with the LAT allows the detection of changes in general locomotor activity caused by treatment. It is well known that stimulants of the central nervous system reduce immobility times [37], but the LAT can discriminate motor effects from motivational effects. This can occur with a substance that has antidepressant activity, which reduces immobility in the FST without significant changes in locomotion [26, 28, 35]. For this reason, we and others run the LAT before the FST [45]. When longitudinal studies are carried out using LAT, reductions in crossings may occur even when anti-immobility effects are exerted by the substance with anti-despair properties [27]. Therefore, in the present study it is possible to disregard any motor influence on behavior detected by the FST. This demonstrates that chrysin, similar to allopregnanolone and fluoxetine, produces an antidepressant-like effect associated with motivation of the animals, rather than with a motor component.
Also in relation to the LAT, the time spent vertically exploring the environment (rearing) did not change with chrysin treatment, but differences were observed in grooming. Rearing and grooming are behavioral indicators of the emotional state of rats when exposed to novel environments [46]. Rearing is a measure of the active exploration carried out by the rat on the environment in which it is located. This can vary depending on the experimental situation. Some studies have reported that rearing increases after acute administration of drugs such as benzodiazepines, e.g., diazepam [32, 47, 48], or substances with anxiolytic potential such as neuro-steroids, e.g., progesterone or allopregnanolone, or flavonoids such as chrysin [7, 28]. However, other studies have reported no changes with the same treatments [4, 6, 9, 49]. There are no reports of increases in rearing after long-term administration of the abovementioned treatments. Indeed, no changes in this exploratory behavior are often reported [4, 27, 50], as found in the present study.
Grooming behavior is an indicator of animal motivation [51]. It may increase in
mildly stressful situations, but is drastically reduced under conditions of
severe stress [27, 52, 53]. Reduction of grooming time is prevented by anxiolytics,
antidepressants and neuro-steroids, which re-establish this behavior to levels
similar to those found in undisturbed animals [36, 53, 54, 55]. As expected, our
results showed that grooming decreased during the course of treatment compared to
the baseline value in the VEH group. However, all treatments maintained a value
similar to baseline from day 1 of administration, except for fluoxetine. This
treatment showed a “U-shaped” effect, with an increase from day 14, and similar
values to the baseline at day 28. Interestingly, 48 hours after the last
administration, only the C5 and FLX-treated groups maintained grooming behavior
at levels similar to baseline. This may reflect increased motivation consistent
with reduced total immobility time, and is possibly also associated with changes
in brain plasticity produced by prolonged treatment with antidepressants [53]. It
is worth noting this effect was not observed with low dose chrysin, thus
supporting the hypothesis that low doses exert “anti-stress” actions through
effects on the GABA
In the FST, low and high doses of chrysin produced antidepressant-like effects similar to fluoxetine and allopregnanolone. Picrotoxin prevented the effects of low dose chrysin, but not of the higher dose, suggesting a differential mechanism for chrysin action depending of the dose. The mechanism for higher doses could involve the activation of other neurotransmitter systems (e.g., serotonin) and neurotrophic factors, as identified in previous studies with chronic administration of chrysin [17, 29, 56]. The FST has been validated as a behavioral model and reveals substances with antidepressant activity because they produce longer latency and shorter immobility time, similar to clinically effective antidepressant drugs [26, 37, 57, 58, 59]. However, the terms “depression-like” and “despair” may be incorrect and FST behavior in general may represent a copying behavior [60, 61]. Neural networks involved in the stress sessions of FST are heterogeneously complex and are linked by relevant factors to depression and to other conditions related to stress. The present work used naive rats to study the response to chrysin, and the findings with allopregnanolone and fluoxetine support the robust antidepressant-like effects observed with chrysin. In particular, 14 days of administration with high dose chrysin increased the latency to the first immobility, similar to the antidepressant drug fluoxetine and the neuro-steroid allopregnanolone. Moreover, the effect was maintained 48 h after ending the treatment, suggesting there was a long-term increase in motivation associated with changes in brain neuroplasticity, as occurs after treatment with antidepressant substances [29, 62].
As expected, the total immobility time decreased with both low and high doses of
chrysin, but important differences were noted. Low dose chrysin produced a rapid
(day 1) anti-immobility effect, but was no longer effective 48 h after the last
administration. This finding suggests that 1 mg/kg chrysin may act through
ionotropic receptors to exert a reduction in immobility that is unrelated to
antidepressant-like effects. Instead, it may be an “anti-stress” effect that
allows the rat to quickly deal with the urgent situation, as previously reported
for allopregnanolone [30]. Low dose chrysin may act in a manner similar to the
acute administration of neuro-steroids such as progesterone or allopregnanolone.
These reduce immobility through actions on ionotropic receptors, exhibiting rapid
(30 min) but short-lasting (6 h) activity after injection [53]. In contrast,
chronic application of 5 mg/kg chrysin may instead involve neuronal plasticity
changes that take time to establish, as reported in previous studies [63, 64].
This flavonoid continues to produce anti-immobility effects 48 h after the
suspension of 28-day treatment. Such long-term effects are similar to those seen
with antidepressant drugs such as fluoxetine, which require 2–3 weeks for their
effects to occur [26], as seen here with the FLX group. This effect could be
related to chrysin-induced changes in the level of metabotropic serotonergic
receptors such as 5-hydroxytryptamine subtype 1A (5-HT
Many flavonoids also act in a biphasic manner by enhancing the actions of GABA
at low concentrations, and inhibiting it at high concentrations [72]. In
particular, chrysin has agonist actions on the GABA receptor, producing
anxiolytic effects similar to diazepam, that disappear with the administration of
GABAergic antagonists [3]. In the present study, the absence of an
antidepressant-like effect with high dose chrysin on day 1 may be due to the
overstimulation of GABA
A limitation of the present study was that antagonism through a non-competitive
mechanism using picrotoxin was only measured after 28 days of treatment. It would
also be preferable to study the antagonism at days 1 and 14 of treatment. In
addition, it would be preferable to antagonize the recognition site in the
GABA
The anti-immobility effects of low dose chrysin are time-dependent and different to those of high dose chrysin. Low dose chrysin produced a rapid onset, anti-immobility effect in the FST. In contrast, high dose chrysin produced a delayed but sustained anti-immobility effect during chronic treatment and 48 h after withdrawal, similar to the antidepressant fluoxetine. The mechanism underlying the antidepressant-like effects of low dose chrysin is GABAergic, while the antidepressant-like effects of high dose chrysin may be via other neurotransmission and neuromodulation systems likely related to serotonergic actions. Possible applications of chrysin for the treatment of comorbid anxiety-depression will first require the completion of relevant preclinical and clinical studies with this flavonoid.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
JFRL designed the research study. AKLV and OJOV performed the research. BBM provided help and advice on technical elements. GGR and JFRL analyzed the data. GGR, BBM, AKLV, OJOV and JFRL wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The experimental protocol received authorization from the Committee for the Use and Care of Laboratory Animals of the Biomedical Research Center from Universidad Veracruzana with the approval number CLCIB2023/2.
To SIREI project no. DGI: 26650202346 to J.F.R-L. and CONAHCYT (CVU 121508, 285449, 121609, 863438, 635392).
This research was funded by SEP-PROMEP (grant no. 103.5/05/1955, UVER-PTC-155), High Quality Postgraduate Academic Strengthening Program (I010/458/2013, C-703/2013), CONAHCYT (grant 1840).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
