1 REGEnLIFE, 75008 Paris, France
2 FRconsulting, 34800 Clermont l'Hérault, France
3 Maintain Aging Research Team, CERPOP, Université de Toulouse, Inserm, Université Paul Sabatier, 31000, Toulouse, France
4 Gérontopôle, Department of Geriatrics, Toulouse University Hospital, 31000, Toulouse, France
5 Faculty of Medicine, University of Montpellier, 34000 Montpellier, France
Abstract
Recently, novel non-pharmacological interventions, such as photobiomodulation
(PBM) therapy, have shown promise for the treatment of Alzheimer’s disease (AD).
This article outlines the translation from the preclinical to clinical stages of
an innovative brain–gut PBM therapy in a mouse model of AD, a pilot clinical
trial involving mild-to-moderate AD patients, and a continuing pivotal clinical
trial with a similar patient population. In a mouse model of AD
(A
Keywords
- Alzheimer's disease
- neuro degenerescence
- memory
- neuroinflammation
- amyloid
- phosphorylated tau
- photobiomodulation
- electromagnetic
- magnetic
- photonics
- oxidative stress
Alzheimer’s disease (AD) is an irreversible neurodegenerative disease for which new therapeutic strategies are urgently needed [1]. In recent years, novel non-pharmacological interventions, such as transcranial photobiomodulation (PBM) therapy, transcranial magnetic stimulation, transcranial electromagnetic treatment, transcranial direct or alternating current stimulation, and deep brain stimulation, have been developed to treat neurological and psychiatric disorders, and these interventions have demonstrated potential for application in the treatment of AD [2, 3, 4, 5, 6, 7, 8, 9]. PBM, a safe and non-invasive therapy that utilizes red or near-infrared (NIR) light to stimulate specific mitochondrial functions [10], has known benefits, including improved tissue healing, cell survival promotion, and reduction of inflammation and oxidative stress [11, 12].
The assumption of various mechanisms in AD, such as mitochondrial dysfunction [13] or inflammatory processes [14], supports the use of transcranial PBM therapy that has been used in various clinical trials demonstrating improvement in cognitive performance in persons with mild cognitive impairment (MCI) [15], improvement in the quality of life and self-independence of patients with dementia [16], and better cognitive performance of patients diagnosed with mild-to-moderate AD [17]. Furthermore, there is increasing evidence that the brain–gut axis plays an important role in neurodegenerative diseases [18], and we investigated abdominal PBM stimulation in association with the classical transcranial application. To the best of our knowledge, this type of study has not been reported elsewhere.
This article describes the preclinical to clinical translation of an innovative technology based on brain–gut PBM therapy (REGEnLIFE RGn500, RGn530, and RGn600 devices) that combines photonic and magnetic emissions in a mouse model of AD, a pilot clinical trial, and an ongoing pivotal clinical trial involving mild-to-moderate AD patients.
The neuro-magneto-photonic treatment used in our studies combines PBM with a static magnetic field. Low static magnetic field (SMF) stimulation has shown promise in animal models, and transcranial magnetic stimulation with a high magnetic field has been investigated as a non-invasive therapeutic tool to treat neurological and psychiatric diseases [9]. SMF stimulation is thought to induce a long-term change in the excitability and connectivity of the stimulated brain networks by modulating membrane excitability [19, 20, 21], and its application to human subjects is safe [22]. For those reasons, we decided to combine light with a magnetic field in our therapeutic devices.
The device used in our preclinical studies is the RGn500 device (REGEnLIFE,
Paris, France), which provides tri-photonic stimulation using light sources in
the red and NIR spectrums, including lasers and light-emitting diodes (LEDs). The
device contains an NIR laser (
The medical device RGn530 has been used for brain–gut PBM therapy in clinical
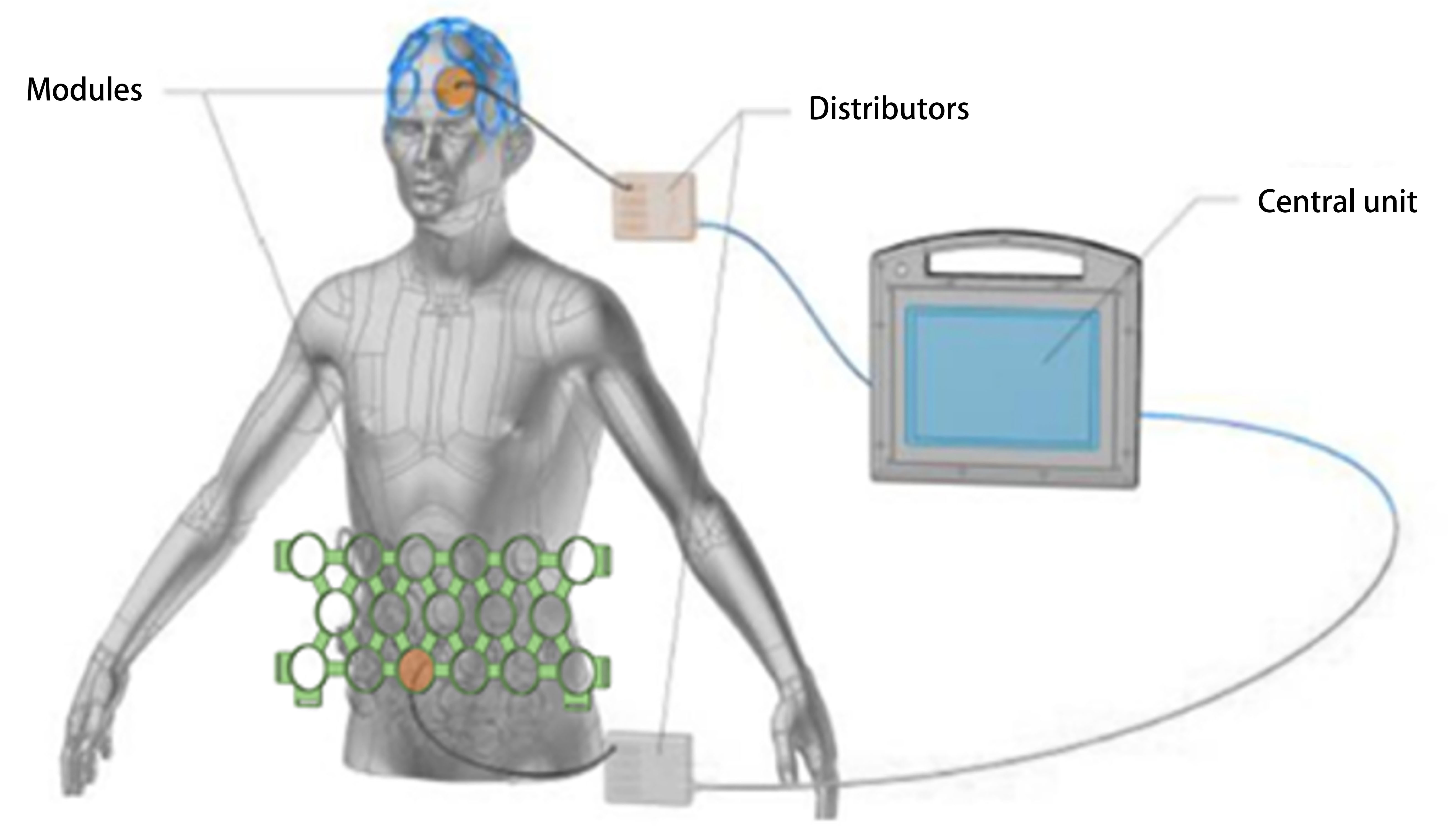
studies. As shown in Fig. 1, it consists of a modular helmet and an abdominal
belt, each of which is includes NIR lasers (
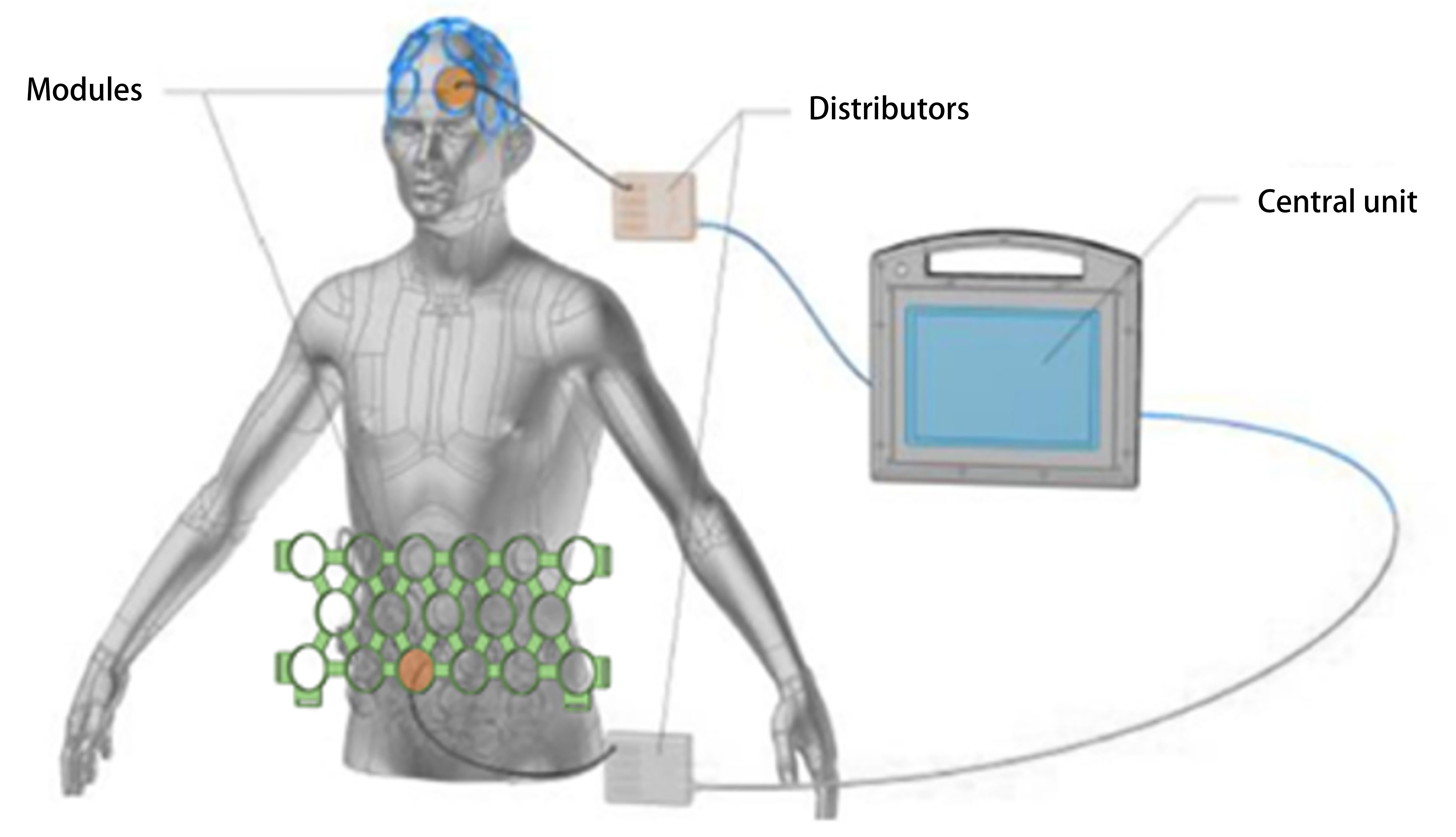 Fig. 1.
Fig. 1.Photobiomodulation (PBM) device. Main components of the PBM device (REGEnLIFE RGn530/RGn600), including the central unit, helmet, abdominal panel integrated modules, and two distributors that centralize the connections. Reprinted from Journal of Alzheimer’s disease, Vol. 90. Guillaume Blivet, Aroaa Relano-Gines, Mélanieb Wachtel, Jacques Touchon. A Randomized, Double-Blind, and Sham-Controlled Trial of an Innovative Brain-Gut Photobiomodulation Therapy: Safety and Patient Compliance. Pages No. 811-822, Copyright (2022), with permission from IOS Press. The publication is available at IOS Press through https://www.iospress.com// [DOI:10.3233/JAD-220467].
An initial preclinical study focused on a mouse model of AD,
A
A significant disruption in memory performance was observed eight days after the
A
Considering the possibility of an abscopal effect from such stimulation based on developing evidence of brain–gut interactions, the treatment was applied to two sites: the head and abdomen.
The mice were manually restrained by holding their bodies down, and the photonic
emitters were applied 1 cm from the shaved skin of the head and/or center of the
abdomen. Three applications were tested: the top of the head as a transcranial
PBM through an SMF (1 cm
Daily application of RGn500 to both the head (at a pulse frequency of 10 Hz) and
the abdomen (at a pulse frequency of 1000 Hz) for 10 min produced a
neuroprotective effect against the detrimental effects of the
A
A complementary study implementing a similar device, the preclinical RGn530, has
made it possible to explore the role of gut microbiota dysbiosis in AD with this
new biophotonic-based therapeutic strategy [25]. A
A double-blind, randomized, mono-centered, sham-controlled pilot clinical trial [26] involving 53 individuals with mild-to-moderate AD was conducted. These patients were randomly assigned, with 27 in the brain–gut PBM group and 26 in the sham group. All participants underwent 40 treatment sessions, each lasting 25 minutes, over an 8-week period, followed by a 4-week observation period.
The brain–gut PBM therapy proved to be safe in regard to the number of recorded adverse events (AEs) (44% of the patients), which were balanced between the PBM and sham groups. The AEs were mainly mild, and no serious AEs were reported. The majority of the patients (92.5%) were highly compliant, which confirms the feasibility of the PBM treatment. Compared to the sham patients, after two months of treatment, the PBM-treated patients had lower Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) comprehension sub-scores, higher forward verbal spans, and lower Trail Making Test (TMT) Part B (TMT-B) execution times, which suggest an improvement in cognitive functions measured at baseline that was still present two months after the end of the treatment.
This first study provided important information for the design of the pivotal clinical trial that is currently ongoing to evaluate the cognitive benefits of the novel improved medical device RGn600 for brain–gut PBM therapy in a larger sample of AD patients [27]. The RGn600 is an improved version of the RGn530 medical device manufactured by REGEnLIFE and consists of a helmet and an abdominal belt that combine tri-photonic technology from red to near-infrared wavelengths from lasers, LEDs, and static magnetic stimulation.
In June 2023, a multicenter clinical trial, featuring a double-blind, randomized, sham-controlled pivotal clinical trial, commenced at Toulouse University Hospital. The study has enrolled a total of 108 individuals diagnosed with AD based on National Institute on Aging – Alzheimer’s Associatio (NIA-AA) clinical criteria. These participants were randomly divided into a treatment group (n = 54) and a sham group (n = 54). These individuals will receive 84 treatment or sham sessions over a period of 26 weeks, each lasting 20 minutes, followed by a 26-week monitoring phase post-treatment.
The primary objective of this ongoing pivotal clinical trial is to assess the cognitive progression of patients following 26 weeks of brain–gut PBM therapy, as assessed by the ADAS-Cog global score. Secondary endpoints include the investigation of neuropsychological functions, autonomy, overall clinical response, quality of life (AD and inflammation biomarkers), and an analysis of blood and fecal (microbiota) biomarkers, as well as the exploration of the medical and economic implications. In addition, the safety of this brain–gut PBM therapy will be rigorously evaluated.
The trial’s initial patients visit occurred in July 2023, and analyses will be conducted across different sets, including the full-analysis set, per-protocol populations, and treated set.
Improvement of memory performance and biochemical markers associated with AD
were observed through brain-gut exposure to PBM therapy in the mouse
A
The modifications observed in bacterial abundance in the mouse
A
The initial pilot clinical study showcased the tolerability, acceptability, and practicality of the innovative PBM-centered treatment for individuals with mild-to-moderate AD. This safe and non-invasive therapeutic solution is a feasible and appealing alternative to conventional AD management approaches and, furthermore, has the advantage of potential administration within patients’ homes.
The pivotal clinical trial is poised to demonstrate that REGEnLIFE’s RGn600 brain–gut PBM therapy is a safe, well-tolerated, and effective disease-modifying treatment for mild-to-moderate AD patients. In addition, it is expected to demonstrate both medical and economic benefits [44].
Transcranial PBM therapy has recently emerged as a potential clinical treatment and cognitive enhancement method for various neurodegenerative pathologies, which could also increase the potential of pharmacological therapies. The association of abdominal application to transcranial PBM could provide optimal efficacy to the treatment of AD by mobilizing multiple mechanisms in synergy with clinical drug development.
FJR and GB performed the literature searches, designed and wrote the paper, and contributed to the editorial changes in the manuscript. JD and JT contributed to its analysis, its critical review, and its final version approval. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
Guillaume Blivet is an employee of REGEnLIFE and owns equity. Jacques Touchon is a consultant for REGEnLIFE. François J. Roman is the director of FR Consulting. The authors declare no conflict of interest and the writing were not influenced by this relationship.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.