- Academic Editors
†These authors contributed equally.
Background: Tanshinone IIA (TSIIA) is an element of the effective
ingredients of Salvia miltiorrhiza Bunge (Labiatae), exhibits a
significant therapeutic effect in brain neuroprotection. The focus of this study
was the examination of synaptic plasticity of in Mg
Among the most prevalent severe brain diseases, epilepsy is characterized by recurrent, spontaneous seizures that are the result of hypersynchronous neuronal discharge [1]. As the fourth most common neurological disorder, it impacts over 70 million people worldwide [2]. More than 20 antiepileptic drugs, such as valproate, lamotrigine, carbamazepine, phenytoin and levetiracetam [3], are available as first-line treatments for epilepsy patients, but seizure control is not achieved in approximately one-third of patients [4]. The pathogenesis of epilepsy is complicated and diverse, involving a variety of factors such as signaling transduction, ion channels, inflammatory responses [5, 6, 7], synaptic transmission, gap junctions and the immunological system [8]. Therefore, the underlying causes of epilepsy and its potential therapies are extremely rewarding research areas.
Chinese herbal remedies have had considerable success in the treatment of
epileptic seizures and easing side effects associated with antiepileptic drugs.
Natural plants are used to obtain or synthesize traditional Chinese herbal
medicines. Tanshinone IIA (TSIIA) is an active component derived from
Salvia miltiorrhiza Bunge and exhibits multiple pharmacological
properties, including anti-atherosclerosis, anti-cancer, anti-inflammation,
antioxidation, anti-tumor, cardioprotection, neuroprotection, renoprotection and
hepatoprotection [9, 10, 11]. Recently, research into TSIIA on neuroprotection has
grown substantially. A study by Lin et al. [12] has shown that TSIIA
ameliorated the learning and memory deficits caused by Ab1-42 in rats with
mechanisms involving the extracellular signal-regulated protein kinase (ERK) and
glycogen synthase kinase-3b (GSK-3
The modification of neuronal connection strength which depends on activities, namely synaptic plasticity, is widely acknowledged as a crucial component of learning and memory [16]. The network functions of the brain are facilitated by the interaction of different types of synaptic plasticity [17, 18]. Furthermore, a variety of neurological and neuropsychiatric conditions, including Alzheimers, schizophrenia and epilepsy are accompanied by impairments in synaptic plasticity [15, 19]. Persistent status epilepticus or recurrent seizures can alter brain tissue, in particular the shape and function of the hippocampus, and damage synaptic plasticity in hippocampal neurons [20]. Consequently, understanding the mechanisms behind alterations in synaptic plasticity is crucial to the treatment of epilepsy.
Mg
Hippocampal neurons isolated from the brains of newborn Sprague-Dawley rats within 24 hours, purchased from Lanzhou University’s GLP Experimental Center (Lanzhou, Gansu, China) were used in this study. The Institutional Animal Care and Animal Ethics Committee of Lanzhou University Second Hospital authorized all experimental animal operations and sample collection (approval number: D2021-051).
Rats were decapitated and their brains were dissected out and separated for the
preparation of primary hippocampal neurons. 0.25% trypsin digestion solution
(meilunbio, Dalian, Liaoning, China, Cat# MA0234-Apr-26F) was used to digest the
hippocampal tissue for 15 minutes at 37 °C. To stop the digestion and
convert it into a single-cell suspension, an equivalent amount of pre-cooled
growing medium was added. This medium comprised three ingredients: Dulbecco’s
Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F12) (Shanghai Basalmedia
Technologies Co., Ltd., Shanghai, China, Cat# K210815), fetal bovine serum
(meilunbio, Cat# O0201A) and penicillin/streptomycin (100
Rat hippocampal neurons were cultured in vitro for seven days before
fixation for 20 minutes with 4% paraformaldehyde (meilunbio, Cat#
MA0192-Mar-23G) and permeabilization for 10 minutes with 0.3% Triton X-100
(Solarbio, Cat# T8200). Neurons were probed with 1:100 diluted
anti-microtubule-associated protein 2 (MAP2; 1:100 dilution; ABclonal
Biotechnology Co., Ltd., Wuhan, Hubei, China, Cat# 3560640007) stored overnight
in a 4 °C refrigerator after blocking with 5% bovine serum albumin
(Solarbio, China, Cat# 20210326) for 25 minutes at ambient temperature. The
secondary antibody Dylight 488-goat anti-rabbit IgG (1:100; Boster Biological
Technology Co., Ltd., Wuhan, Hubei, China, Cat# BST14B25C27) was then dark
applied to the cells for an hour at room temperature. 4,6-diamino-2-phenyl indole (DAPI, Boster Biological
Technology Co., Ltd., Cat# 15F03A76) was used to restain the nuclei for 15
minutes. Sections were then sealed using a fluorescence-quenching sealer (Boster
Biological Technology Co., Ltd., Cat# 14E07A09). To identify neurons,
fluorescence signals were observed using an ortho-fluorescence microscope
(Olymus, Tokyo, Japan, Cat# BX53+DP74). To calculate the percentage purity of
hippocampal neurons, the microtubule-associated protein (MAP2)-positive cell
count was divided by the DAPI-positive cell count
yields of hippocampal neurons in five randomly selected fields of vision at a
magnification of 200
A neuron-specific microtubule-associated protein called MAP2 is expressed in both adult and immature hippocampal neurons, making it possible to distinguish neurons [21]. The purity of hippocampal neurons was greater than 95% and sufficient for the subsequent experiments. Results of immunofluorescence staining identification are shown as 98% in Fig. 1.
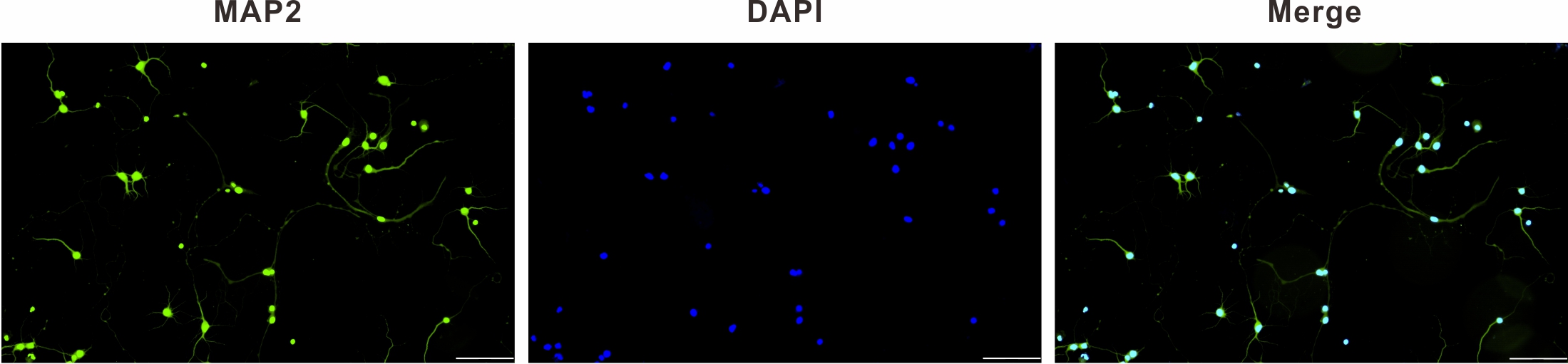 Fig. 1.
Fig. 1.MAP2 immunofluorescence identification of primary hippocampal
neurons (
Five groups of primary hippocampal neurons were selected at random: blank (Blank), model (Model), TSIIA (TSIIA, 20 µM), LY294002 (LY294002, 25 µM), and TSIIA+LY294002 (TSIIA+LY294002, 20 µM+25 µM). At nine days in vitro, the Blank group was added to the normal medium, and the other groups received additions of the magnesium-free external solution [22, 23, 24]. The above solutions were switched to a maintenance medium after three hours, and the maintenance medium of the drug groups was supplemented respectively with the corresponding concentrations of 20 µM TSIIA (Shanghai Yuanye Bio-Technology Co., Ltd., Shanghai, China, Cat# Y14M10C82864), 25 µM LY294002 (MedChemExpress LLC, Monmouth Junction, NJ, USA, Cat# 51598) and a combination of 20 µM TSIIA and 25 µM LY294002, and then incubated for further 24 hours.
Hippocampal neurons in each group were permeabilized with 0.3% Triton X-100 after being exposed to 4% paraformaldehyde. The cells were then treated with 5% Bovine serum albumin (BSA) for 25 minutes to block them, before being incubated overnight with a primary antibody mixture consisting of MAP2, developmental regulation brain protein (Drebrin) (Santa Cruz Biotechnology, Dallas, TX, USA, Cat# B6535), 5% BSA and Phosphate Buffered Saline (PBS) in a ratio of 1:1:20:80, and then with a secondary antibody mixture consisting of Dylight 488-goat anti-rabbit IgG, Cy3-goat anti-mouse IgG (Boster Biological Technology Co., Ltd., China, Cat# BST16A25C16F31) and 5% BSA and PBS in a ratio of 1:1:20:80 for one hour. The nuclei were sealed with an anti-fluorescence blocker after a PBS rinse and 15 minutes of DAPI staining. Hippocampal neurons were examined and photographed utilizing a two-photon confocal laser scanning microscope (Carl Zeiss, Oberkochen, Bartenburg, Germany, Cat# Zeiss LSM880). Variations in neurite complexity, total length of hippocampal neurons, number of primary dendrites, and density of dendritic spines were analyzed using FIJI software (Version 2.9.0, National Institutes of Health, Bethesda, MD, USA). The average fluorescence density of each group of Drebrin treated neurons was calculated using Image J software (Version 1.52p, National Institutes of Health).
Immunofluorescence staining was carried out at nine days in vitro to
detect neurons using brain-derived neurotrophic factor (BDNF) antibody (1:100,
ABclonal Biotechnology Co., Ltd., China, Cat# 3507522015), and DAPI was used to
count all cells in the culture. Photographs were taken at a magnification of
200
Following their individual treatments, neurons were cleaved by using a cell
lysis buffer, containing radioimmunoprecipitation assay buffer,
phenylmethanesulfonyl fluoride and Phosphatase inhibitor cocktail I in a 98:1:1
ratio, to extract total protein, followed by Bicinchoninic acid (BCA) Protein
Assay Kit (Solarbio, China, Cat# PC0020) to detect protein concentration. To
denature the proteins, all samples were mixed with 5
GraphPad Prism 8.0.1 (GraphPad Software, San Diego, CA, USA) and SPSS Statistics
25.0 (IBM Corp., Armonk, NY, USA) were used to evaluate the obtained data, which
were expressed as x
FIJI software was used to examine the morphology of neurons to assess the state
of neuronal protrusions (Fig. 2A). After intervention with a magnesium-free
external solution, the protrusions and branches of the hippocampal neurons in
each experimental group were smaller and thicker than those in the Blank group,
and some were beaded. Sholl analysis was used to compare the complexity of
protrusions in each group (Fig. 2B, Table 1). Only the TSIIA+LY294002 group was
clearly distinguishable from the Blank group (p
 Fig. 2.
Fig. 2.Effect of TSIIA on protrusion complexity. (A) Cytoskeleton and
protrusion trajectory of hippocampal neurons in each group (magnification
| Distance from soma | Blank | Model | TSIIA | LY294002 | TSIIA+LY294002 |
| 10 | 8.40 |
4.80 |
7.00 |
5.20 |
3.80 |
| 20 | 11.40 |
5.40 |
8.80 |
4.00 |
3.60 |
| 30 | 10.80 |
3.80 |
7.80 |
3.80 |
4.20 |
| 40 | 8.60 |
2.60 |
8.20 |
2.40 |
4.00 |
| 50 | 8.60 |
2.25 |
7.40 |
2.00 |
4.00 |
| 60 | 6.20 |
1.75 |
5.40 |
2.50 |
2.00 |
| 70 | 5.80 |
1.25 |
4.80 |
2.00 |
2.40 |
| 80 | 4.20 |
1.25 |
4.20 |
1.75 |
1.00 |
| 90 | 4.40 |
1.25 |
3.20 |
2.50 |
1.00 |
| 100 | 3.40 |
1.50 |
2.80 |
2.00 |
1.00 |
| 110 | 2.20 |
1.50 |
1.75 |
2.50 |
1.00 |
| 120 | 2.00 |
2.00 |
2.00 |
2.00 |
1.00 |
| 130 | 1.00 |
1.00 |
2.00 |
2.00 |
1.00 |
| 140 | 1.00 |
1.00 |
3.00 |
2.00 |
1.00 |
| 150 | 1.00 |
1.00 |
2.00 |
1.00 | |
| 160 | 1.00 |
1.00 |
1.00 |
1.00 | |
| 170 | 1.00 |
1.00 |
|||
| 180 | 1.00 |
Results are presented as mean
The “Simple Neurite Tracer” in the FIJI software was used to measure the total
length of neurite protrusions (Fig. 3A). The total protrusion length of the
experimental groups decreased after intervention with the magnesium-free external
solution, with significant differences among the Model, LY294002, TSIIA+LY294002
and the Blank groups (p
 Fig. 3.
Fig. 3.Total length and the primary dendrites count of hippocampal
neuron protrusions in each group. (A) The total length of hippocampal neuron
protrusions in each group.
Statistical analysis of primary dendrites in each group (Fig. 3B), Model,
LY294002, and TSIIA+LY294002 showed these groups all exhibited considerably fewer
primary dendrites than those in the Blank group 6.13
Among the dendritic spine morphologies on the secondary dendrites of hippocampal
neurons in each group (Fig. 4A), the Model and LY294002 groups mostly had stubby,
filopodia-shaped dendritic spines, whereas the Blank, TSIIA and TSIIA+LY294002
groups mostly had thin mushroom-shaped spines. Data (Fig. 4B) showed the
dendritic spine density was significantly less in the Model, LY294002 and
TSIIA+LY294002 groups than in the Blank group (p
 Fig. 4.
Fig. 4.Dendritic spine density of hippocampal neurons in each group.
(A) Hippocampal neurons in each group were immunofluorescently stained for MAP2
(scale bar: 20 µm), and the secondary dendrites in the white dashed box
were locally magnified (scale bar: 5 µm). (B) Statistics of dendritic spine
density of hippocampal neurons in each group.
Drebrin has an essential influence on the growth and development of dendritic
spines. According to the findings of immunofluorescence staining (Fig. 5A),
Drebrin protein was predominantly expressed in the primary dendrites and
cytoplasm of hippocampal neurons, and its fluorescence intensity gradually
decreased with the extension of neuronal protrusions. When the average
fluorescence intensity of Drebrin protein in each group was counted (Fig. 5B), we
discovered that the expression of Drebrin within the Model, LY294002 and
TSIIA+LY294002 groups was significantly lower when compared to the Blank group
(p
 Fig. 5.
Fig. 5.Drebrin protein expression on hippocampal neurons in
each group. (A) Plots of immunofluorescence staining results of Drebrin in
hippocampal neurons in each group (magnification
Extensively expressed in the brain and nervous system, BDNF is involved in
promoting neuronal processes such as axonal and dendritic growth as well as
synapse formation. Immunostaining results (Fig. 6) showed that the Model,
LY294002 and TSIIA+LY294002 groups had significantly lower levels of BDNF
expression than the Blank group (Blank 0.98
 Fig. 6.
Fig. 6.Intracellular BDNF staining in hippocampal neurons. (A)
Immunofluorescence staining plots of intracellular BDNF in hippocampal neurons of
each group (magnification
The Western Blot findings are illustrated in Fig. 7A, analyzed in Fig. 7B–E and
Table 2. BDNF protein expression analyzed with Image J software showed (Fig. 7B)
that the expression of BDNF protein within the Model, LY294002 and TSIIA+LY294002
groups was significantly decreased (p
 Fig. 7.
Fig. 7.Expression of BDNF, SYN, PSD-95, p-Akt and Akt proteins in each
group of hippocampal neurons. (A) Western blot results of BDNF, SYN, PSD-95,
p-Akt, Akt and GAPDH proteins in each group of hippocampal neuronal cells. (B–E)
Relative expression of BDNF/GAPDH, SYN/GAPDH, PSD-95/GAPDH and p-Akt/Akt in each
group of hippocampal neurons.
| Proteins | Blank | Model | TSIIA | LY294002 | TSIIA+LY294002 |
| BDNF | 0.29 |
0.22 |
0.29 |
0.21 |
0.15 |
| SYN | 0.48 |
0.35 |
0.58 |
0.36 |
0.27 |
| PSD-95 | 0.89 |
0.57 |
0.78 |
0.71 |
0.59 |
| p-Akt | 0.70 |
0.43 |
0.70 |
0.41 |
0.42 |
Results are presented as mean
When assessing the biological functions of synapses, researchers frequently look
at the expression levels of synaptophysin (SYN) and postsynaptic density 95
(PSD-95). Results demonstrated (Fig. 7C,D) that SYN and PSD-95 proteins
expression in the Model, LY294002 and TSIIA+LY294002 groups were significantly
decreased as compared to the Blank group (p
According to the p-Akt/Akt protein expression data results (Fig. 7E), the
expression of this protein was significantly lower within the Model, LY294002 and
TSIIA+LY294002 groups when compared to the Blank group (p
Treatment of neuronal cells with magnesium-free extracellular fluid for three hours resulted in convulsive cellular discharges and induced a model of spontaneous recurrent epileptiform discharge [22, 24]. The persistent epileptic seizures showed morphological features of apoptosis [25, 26]. In the present study, after three hours of induction of hippocampal neurons by magnesium-free extracelluar fluid, some cells floated in the medium leading to a sharp decrease in the number of hippocampal neurons, which indicates that the hippocampal neuronal epilepsy model due to this induction procedure not only alters changes in the synaptic plasticity of neurons, but also leads to apoptotic cell death.
Changing the intensity and effectiveness of synaptic transmission at
pre-existing synapses in response to variations in activity is known as synaptic
plasticity, which is a fundamental characteristic of neurons [27], that includes
functional and structural plasticity. Changes in functional plasticity are the
biological underpinning of learning and memory, whereas structural plasticity is
a key mechanism for maintaining synaptic strength within a dynamic range suited
for the bidirectional modulation of neuronal excitability [28]. Here, the
structural plasticity of the hippocampus was investigated. When counting
protrusion complexity, total protrusion length and the number of primary
dendrites, it was found significant changes in the morphology of hippocampal
neurons within 24 hours of culture with Mg
Small, slender, specialized protrusions of neuronal dendrites called dendritic
spines mostly exhibit excitatory synapses [29]; their morphology and density
serve crucial functions in synaptic plasticity and changes in the shape and
quantity of dendritic spines regularly impact synaptic growth, persistence, and
plasticity in both biological and pathological circumstances [30, 31]. Dendritic
spines are composed of four morphologies, including thin, filopodial, mushroom
and stubby rows, the latter two being the mature forms [32, 33]. Hippocampal
neurons with Mg
Drebrin, a actin cytoskeletal regulator in neurons, is criucial for neurite production, synaptic plasticity, and neuronal migration [34]. BDNF is involved in an assortment of neurophysiological processes, including developmental processes, modulation of neurons, glia and synaptogenesis, neuroprotection, and control of short- and long-term synaptic interactions that affect cognitive and memory mechanisms [35]. The findings of this study demonstrated that Drebrin protein expression levels in hippocampal neurons decreased when seizures occurred and that its expression level was significantly increased by TSIIA treatment. In the case of BDNF expression levels, both immunofluorescence staining and Western blot results showed generally consistent results, indicating that TSIIA may increase the amount of BDNF expression in hippocampal neurons with abnormal discharge.
SYN and PSD-95, two synapse-associated proteins, are crucial indicators of synaptic plasticity in the brain [36, 37]. As demonstrated by Western blot examination, the discharge of epileptic hippocampal neurons had a substantial impact on SYN and PSD-95 expression, illustrating that synaptic plasticity was impaired. After TSIIA intervention, their expression levels were considerably increased, which demonstrated that TSIIA may regulate SYN and PSD-95 expression to improve synaptic plasticity in epileptic hippocampal neurons.
Activation of the PI3K/Akt pathway promotes adult central neuron regeneration
and maintains synaptic plasticity [38, 39]. Hippocampal neuronal protrusion
complexity, total protrusion length, number of primary dendrites, and dendritic
spine density did not differ substantially from the Model group following the
administration of LY294002 alone. Simultaneously, Drebrin, BDNF, SYN and PSD-95
expression levels tended to increase, especially PSD-95, suggesting that blockade
of the PI3K/Akt signaling pathway made the Mg
The current investigation provides support for TSIIA usage in a model of neuronal epilepsy brought on by magnesium-free extracellular fluid. By the PI3K/Akt signaling pathway, TSIIA controls the synaptic biological functions of hippocampal neurons, reduces synaptic plasticity damage, and elongates the total length of axon and dendrite growth by elevating the expression levels of the Drebrin, SYN and PSD-95 proteins, all of which have unmistakable neuroprotective effects.
TSIIA, tanshinone IIA; MAP2, Microtubule-associated protein2; BDNF, Brain-derived neurotrophic factor; SYN, synaptophysin; PSD-95, postsynaptic density 95; Drebrin, Developmental regulation brain protein; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; BCA, bicinchoninic acid; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
HJ designed the research study and supervised the project. MM and XH performed the research, analyzed the data, and wrote the manuscript. CJ, NX, LZ, and LW provided help and advice on experimental operations and data analyses. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The Institutional Animal Care and Animal Ethics Committee of Lanzhou University Second Hospital authorized all experimental animal operations and sample collection (approval number: D2021-051).
We would like to thank all the participants for their contributions and the reviewers for their constructive comments.
This research was funded by the National Natural Science Foundation of China (Grant number 82160840), Cuiying Scientific and Technological Innovation Program of Lanzhou University Second Hospital (Grant number CY2023-QN-B11), and Gansu Province Science Foundation for Youths (Grant number 23JRRA1007).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
