- Academic Editor
Background: Motor neuron diseases (MNDs) are progressive neurodegenerative disorders characterized by motor impairment and non-motor symptoms. The involvement of the thalamus in MNDs, especially in conditions such as amyotrophic lateral sclerosis (ALS), and its interaction with frontotemporal dementia (FTD), has garnered increasing research interest. This systematic review analyzed magnetic resonance imaging (MRI) studies that focused on thalamic alterations in MNDs to understand the significance of these changes and their correlation with clinical outcomes. Methods: Following PRISMA 2020 guidelines, the PubMed and Scopus databases were searched from inception to June 2023 for studies related to MRI findings in the thalamus of patients with MNDs. Eligible studies included adult patients diagnosed with ALS or other forms of MND who underwent brain MRI, with outcomes related to thalamic alterations. Studies were evaluated for risk of bias using the Newcastle-Ottawa scale. Results: A total of 52 studies (including 3009 MND patients and 2181 healthy controls) used various MRI techniques, including volumetric analysis, diffusion tensor imaging, and functional MRI, to measure thalamic volume, connectivity, and other alterations. This review confirmed significant thalamic changes in MNDs, such as atrophy and microstructural degradation, which are associated with disease severity, progression, and functional disability. Thalamic involvement varies across different MND subtypes and is influenced by the presence of cognitive impairment and mutations in genes including chromosome 9 open reading frame 72 (C9orf72). The synthesis of findings across studies indicates that thalamic pathology is a prevalent early biomarker of MNDs that contributes to motor and cognitive deficits. The thalamus is a promising target for monitoring as its dysfunction underpins a variety of clinical symptoms in MNDs. Conclusions: Thalamic alterations provide valuable insights into the pathophysiology and progression of MNDs. Multimodal MRI techniques are potent tools for detecting dynamic thalamic changes, indicating structural integrity, connectivity disruption, and metabolic activity.
Motor neuron diseases (MNDs) are a group of neurodegenerative disorders characterized by progressive neurodegeneration of motor neurons that lead to muscle weakness and disability, including amyotrophic lateral sclerosis (ALS), primary lateral sclerosis (PLS), spinal muscular atrophy (SMA), and others [1, 2, 3, 4, 5]. ALS is the most common and severe form of MND and can affect both upper and lower motor neurons (UMNs and LMNs), with a median survival of 3–5 years after diagnosis [6, 7, 8]. MNDs are not only characterized by motor impairment but also by non-motor symptoms such as cognitive and behavioral alterations [9, 10, 11].
In recent years, the thalamus has been increasingly recognized as a crucial structure involved in the pathophysiology of MNDs [1, 12]. The thalamus serves as a relay center for motor, sensory, and cognitive information, and thalamic dysfunction contributes to a variety of motor and non-motor symptoms in MNDs, including muscle weakness, spasticity, and cognitive decline [13, 14, 15]. Recently, Ghaderi et al. [11] (2023) conducted a systematic review of memory-related impairments in ALS, emphasizing the role of the thalamus in cognitive functions and processes. The thalamus consists of multiple nuclei that modulate distinct neural circuits through connections with the cortex and subcortex [16, 17]. In MND, specific thalamic nuclei implicated in motor, sensory, cognitive, and limbic processing undergo significant degeneration [18, 19].
Another important aspect of thalamic pathology in MNDs is the interaction with frontotemporal dementia (FTD), a common comorbidity of MNDs [20, 21]. FTD covers a group of neurodegenerative diseases affecting the frontal and temporal lobes of the brain, leading to progressive changes in behavior, language, and executive function [22, 23]. FTD is classified into three main subtypes: behavioral variant FTD (bvFTD), primary progressive aphasia of the semantic variant (svPPA), and non-fluent/analogue variant PPA (nfvPPA) [24, 25, 26]. Each FTD variant is associated with distinct patterns of cortical atrophy and cognitive deficits as well as different degrees of thalamic involvement. In MNDs, particularly ALS, a significant subset of patients develop FTD-like cognitive and behavioral impairments, further complicating the clinical presentation and prognosis [27, 28]. Moreover, thalamic changes may reflect the underlying molecular pathology of FTD, such as the presence of chromosome 9 open reading frame 72 (C9orf72) or transactivation response DNA-binding protein 43 (TDP-43) mutations [29, 30, 31]. Investigating the thalamus in MNDs with and without FTD may therefore help to elucidate the mechanisms and consequences of this complex interaction [31, 32].
Advanced magnetic resonance imaging (MRI) techniques, such as volumetric analyses [33], diffusion tensor imaging (DTI) [34, 35, 36], and functional MRI (fMRI) [34], have enabled the in vivo assessment of thalamic structure and function in MNDs, providing valuable insights into the pathophysiology and progression of these diseases [37, 38, 39]. However, despite the increasing evidence regarding thalamic alterations in MNDs gained using advanced MRI techniques, there is a lack of comprehensive syntheses of the current literature on this topic. Previous reviews have focused on specific aspects of thalamic pathology or specific MND subtypes but not on the overall picture of thalamic involvement in different MNDs. We aimed to analyze the MRI results of the included studies to assess the overall significance of thalamic alterations in MND. This review provides a comprehensive overview of the current state of knowledge regarding thalamic involvement in MNDs and outlines the development of innovative diagnostic, targeted therapeutic, and translational imaging strategies.
We followed the Preferred Reporting Items for Systematic Reviews and
Meta-Analyses (PRISMA) 2020 statement to conduct and report this systematic
review [40] (Supplementary Material). We searched the PubMed and Scopus
electronic databases from their inception to June 2023. We used a combination of
Medical Subject Headings (MeSH) terms and free-text words to construct our search
strategy. The search terms were related to MRI, the thalamus, ALS, MND, and all
other forms of MND. We also checked the reference lists of relevant articles and
reviews for additional studies. We included studies that involved adult patients
(
We used a two-stage process to select and extract data from eligible studies. In the first stage, five reviewers (ZNAP, RK, FM, SMJ, and MM) screened the titles and abstracts of the retrieved records and obtained the full texts of potentially eligible records. In the second stage, the two reviewers (SM and SG) extracted data from the included studies using a standardized data extraction form. The data included general information, study characteristics, and alterations in thalamus outcomes. Any disagreements between the reviewers were resolved by discussion or consultation with a fifth reviewer (SG). We contacted the authors of the original studies for missing or unclear data. Two reviewers (SG and SM) independently evaluated the risk of bias in the included studies using the Newcastle-Ottawa Scale checklist [41].
A systematic review of 52 studies was conducted (Fig. 1 and Table 1 (Ref. [21, 31, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91])). These studies included 3009 patients with MNDs and 2181 HCs. We reviewed the findings of these studies that used MRI to investigate thalamic alterations in patients with MNDs.
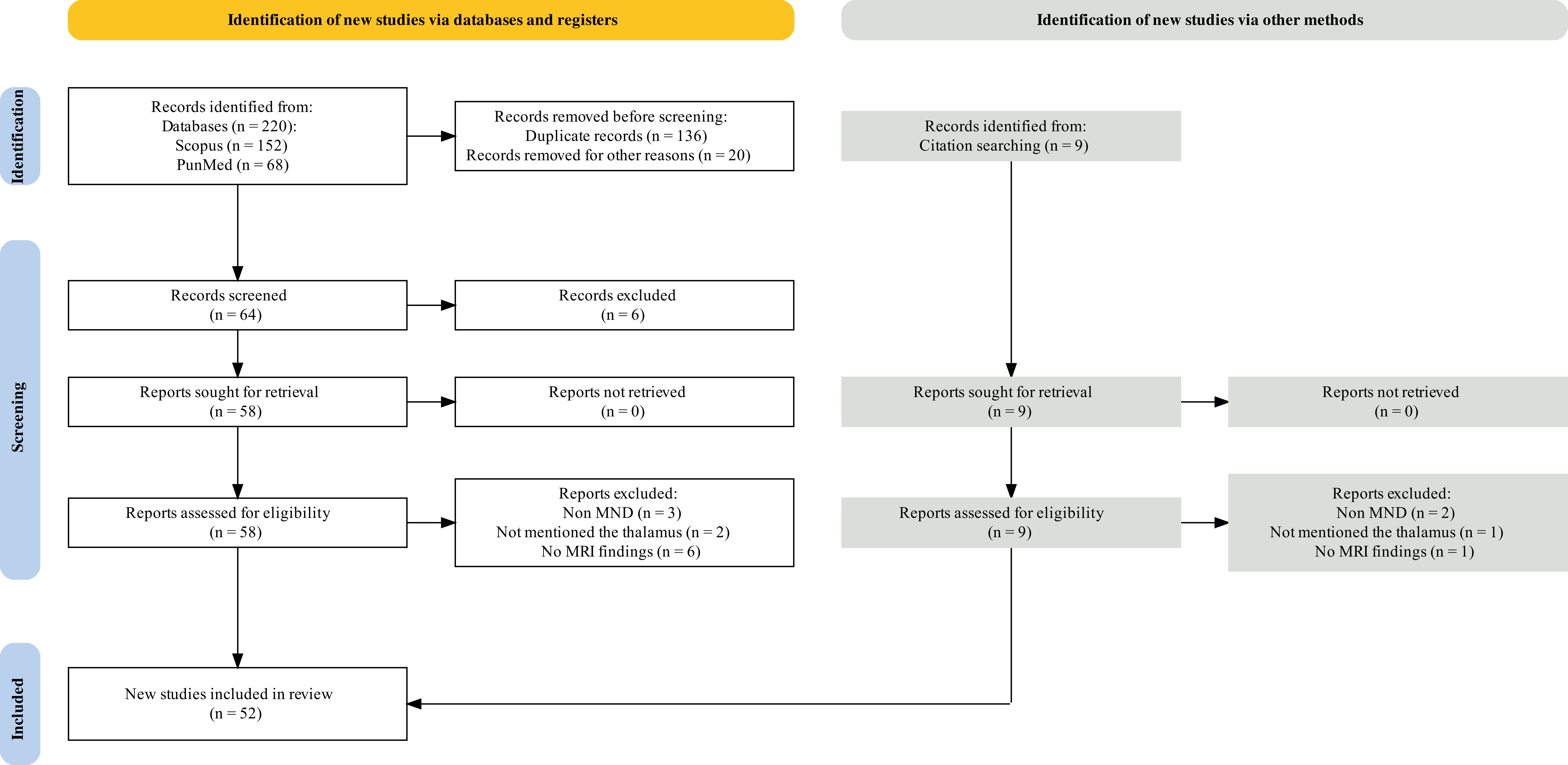 Fig. 1.
Fig. 1.Flow diagram outlining the study selection process using the PRISMA guideline. PRISMA, preferred reporting items for systematic reviews and meta-analyses; MRI, magnetic resonance imaging; MND, motor neuron disease.
| Technique | First author (Year) | Subjects [Patients/Controls] | MND [Types] | MRI [Field Strength/Coils] | Thalamic findings |
| T1-w | McKenna (2023) [21] | 70/100 | 10 bvFTD, 15 nfvPPA, 5 svPPA | 3T | |
| 20 ALS-FTD C9+ | |||||
| 20 ALS-FTD C9– | |||||
| Dieckmann (2022) [42] | 100/72 | ALS | 1.5T/4 | ||
| Ahmed (2021) [43] | 151/58 | 58 bvFTD, 41 ALS-FTD, 52 ALS | 3T/8 | ||
| Liu (2021) [44] | 76/94 | ALS | 3T/dStream head coil | ||
| Chipika (2020) [45] | 133/117 | 100 ALS, 33 PLS | 3T/8 | ||
| Finegan (2020) [46] | 133/117 | 100 ALS, 33 PLS | 3T/8 | ||
| Chipika (2020) [47] | 133/117 | 88 ALS C9–, 12 ALS C9+, 33 PLS | 3T/8 | ||
| Johns (2019) [48] | 17/18 | ALS | 4.7T | ||
| Finegan (2019) [49] | 133/117 | 100 ALS, 33 PLS | 3T/8 | ||
| Bede (2018) [50] | 88/50 | 10 bvFTD, 11 nfvPPA, 5 svPPA, 14 ALS-FTD C9+, 12 ALS-FTD C9–, 36 ALSnci | 3T/8 | ||
| Branco (2018) [51] | 54/38 | 12 ALSci, 14 ALSbi, 28 ALSni | 3T/16 | ||
| Bocchetta (2018) [31] | 341/99 | 141 bvFTD, 76 SD | 1.5T and 3T | ||
| 103 PNFA, 7 FTD-MND, 14 PPA-NOS | |||||
| Bertrand (2018) [52] | 80 participants | 41 ALS C9+, 39 ALS C9– | 3T | ||
| Devenney (2017) [53] | 56/23 | 20 ALS-FTD, 36 bvFTD | 3T | ||
| Masuda (2016) [54] | 51/24 | 25 ALSCD, 7 ALS-FTD, 19 ALS | 3T | ||
| Westeneng (2016) [55] | 170/90 | 165 ALS C9–, 14 ALS C9+ | 3T | ||
| Bede (2016) [56] | 70/40 | ALS | 3T/8 | ||
| de Albuquerque (2016) [57] | 32/32 | ALS | 3T/8 | ||
| Machts (2015) [58] | 67/39 | 7 ALS-FTD, 18 ALS-plus, 42 ALSnci | 3T/32 | ||
| McMillan (2015) [59] | 20/25 | ALS | 3T/8 | ||
| Menke (2014) [60] | 36/36 | ALS | 3T/12 | ||
| Lillo (2012) [61] | 35/18 | 10 ALS, 10 ALS-FTD, 10 bvFTD | 3T | ||
| Sha (2012) [62] | 104/64 | 15 bvFTD C9+, 11 FTD-MND C9+, 5 ALS CP+ | 1.5T, 3T, and 4T | ||
| 48 bvFTD C9–, 19 FTD-MND C9–, 6 ALS C9– | |||||
| Rohrer (2010) [63] | 28 (4 FTD-MND)/50 | 9 with type 1, 5 with type 2, and 10 with type 3 FTLD-TDP (MND in type 2 and 3) | 1.5T | ||
| Tartaglia (2009) [64] | 11/10 | PLS | 1.5T | ||
| Chang (2005) [65] | 20/22 | 10 ALS, 10 ALS-FTLD | 1.5T/ quadrature head coil | ||
| DTI | El Mendili (2022) [66] | 29/24 | 13 fast and 16 slow progressors | 3T/32 | |
| Trojsi (2019) [67] | 36/35 | ALS phenotype: 14 classic, 2 bulbar, 10 flail arms, 10 flail leg | 3T/8 | ||
| Zhang (2017) [68] | 38/35 | ALS phenotype: 7 bulbar, 30 limb, 1 bulbar and limb | 3T/8 | ||
| Barbagallo (2014) [69] | 26/22 | ALS | 3T/8 | ||
| Bede (2014) [70] | 27/42 | ALS | 3T/8 | ||
| Sharma (2013) [71] | 14/12 | ALS phenotype: 12 limb, 2 bulbar | 3T/8 | ||
| Sach (2004) [72] | 15/12 | 9 ALS, 6 no UMN | 1.5T | ||
| fMRI | Dey (2023) [73] | 52/52 | ALS | 3T/20 and 32 | |
| Xu (2017) [74] | 20/21 | ALS | 3T/8 | ||
| Zhou (2013) [75] | 12/12 | ALS | 3T | ||
| Mahammadi (2009) [76] | 27/22 | 10 ALS without bulbar signs, 12 ALS with bulbar signs, 5 Kennedy syndrome | 3T | ||
| T1-w and DTI | Finegan (2021) [77] | 40/100 | PLS | 3T/8 | |
| Christidi (2018) [78] | 56/25 | 28 ALS-non-PLC, 28 ALS-PLC | 3T/8 | ||
| Tu (2018) [79] | 20/31 | ALS | 3T/12 | ||
| Senda (2017) [80] | 67/38 | 19 slow progressions ALS, 36 intermediates progression ALS, 12 rapid progressions ALS | 3T/32 | ||
| Bede (2013) [81] | 39/44 | 30 ALS C9–, 9 ALS C9+ | 3T/8 | ||
| Mahoney (2012) [82] | 11/- | 3 FTD-MND C9+, 8 FTD-MND C9– | 1.5T and 3T | ||
| Thivard (2007) [83] | 15/25 | ALS | 1.5T | ||
| T1-w and fMRI | Devenney (2021) [84] | 75/25 | 28 ALS, 9 ALS-plus, 11 ALS-FTD, 27 bvFTD | 3T/8 | |
| T1-w and QSM | Li (2022) [85] | 34/34 | ALS | 3T/8 | |
| T1-w, DTI, and fMRI | Menke (2018) [86] | 16/- | 13 ALS, 3 PLS | 3T/12 | |
| DTI and fMRI | Li (2009) [87] | 10/10 | 5 ALS with dysphagia, 5 ALS without dysphagia | 3T/8 | |
| PD-w and fMRI | Stoppel (2014) [88] | 40/42 | ALS | fMRI; 3T/8 PD-w; 1.5T/(quadrature head coil) | |
| ● The thalamus is involved in motor system activity | |||||
| ● Motor-related functional alterations | |||||
| ● ALS | |||||
| ASL | Wang (2022) [89] | 18/20 | ALS-FTD | 3T/8 | ● |
| T2*-w | De Reuck (2017) [90] | 50/28 | 12 ALS, FTLD | 7T | |
| T2-w | Kato (1993) [91] | 13/- | ALS | 0.5T |
Abbreviations: ACE-III, third edition of the Addenbrooke’s Cognitive
Examination; AD, axial diffusivity; ALS, amyotrophic lateral sclerosis; ALSFRS-R,
Revised ALS Functional Rating Scale; ALS-FTD, ALS-frontotemporal dementia; ALSci,
ALS with cognitive impairment; ALSbi, ALS with behavioral impairment; ALSni, ALS
with normal cognitive and behavioral performance; ALSnci, ALS with no cognitive
impairment; ALS-plus, strong criteria for cognitive and/or behavioral impairment;
bvFTD, behavioral variant FTD; C9–, without C9ORF72C9orf72 mutation;
C9+, with C9ORF72C9orf72 mutation; CBF, cerebral blood flow; cFL,
calculated functional loss rate; cFS, calculated functional state; CO, cortical
thickness; CST, corticospinal tract; DTI, diffusion tensor imaging; DGN, deep
gray nuclei; ECAS, Edinburgh Cognitive and Behavioural ALS Screen; FA, fractional anisotropy; FC,
functional connectivity; FTD-MND, frontotemporal lobe dementia with motor neuron
disease; FTLD, frontotemporal lobar degeneration; FTLD-TDP, FTLD with
transactivation response DNA-binding protein 43; fMRI, functional MRI; GM, gray matter; GMV, gray
matter volume; IDM, intensity-driven modulator; M1, primary motor cortex; MD,
mean diffusivity; MND, motor neuron disease; MRI, magnetic resonance imaging;
MTR, magnetization transfer ratio; nfvPPA, nonfluent/agrammatic variant primary
progressive aphasia; PD-w, proton density-weighted; PLC, pathological laughing
and crying; PLS, primary lateral sclerosis; PMC, premotor cortex; PPA, primary
progressive aphasia; RD, radial diffusivity; rD50, relative disease
aggressiveness; ROI, region of interest; S1, primary somatosensory cortex; QSM,
quantitative susceptibility mapping; svPPA, semantic
variant primary progressive aphasia; T, Tesla; TA, tensor-based analysis; TMT,
Trail Making Test; UMN, upper motor neuron; VBM, voxel-based morphometry; SD, semantic dementia;
PNFA, progressive nonfluent aphasia; PPA-NOS, primary progressive aphasia not otherwise specified; ALS-CD, ALS with cognitive deficiency; CC, corpus callosum; T1-w, T1-weighted; HCs, healthy controls.
Symbols mean:
MRI techniques include T1-weighted (T1-w), T2-weighted (T2-w), T2*-weighted (T2*-w), DTI, fMRI, arterial spin labeling (ASL), and quantitative susceptibility mapping (QSM). These techniques measure different aspects of thalamic structure and function, such as volume, shape, density, diffusion, connectivity, CBF, iron deposition, and signal intensity. We also compared the findings across different subtypes of MND, including PLS, pathological laughing and crying ALS (PLC-ALS), dysphagia-ALS, ALS with strong criteria for cognitive and/or behavioral impairment (ALS-plus), C9orf72 mutation ALS, and ALS-FTD. In our review, there were studies with different ranges of magnetic field strength (0.5, 1.5, 3, 4, 4.7, and 7 Tesla), but 82% of the studies used a 3 Tesla device. Sixty-one percent of the studies reported the channel coil. A standard 8-channel coil was used in 72% of the studies. Despite the use of 32-channel coils in four studies, none of the studies utilized 64-channel coils. It may be worth considering the use of 64-channel coils in future studies due to their superior imaging characteristics.
Table 1 summarizes the findings of various studies that used MRI techniques for the thalamus (Fig. 2). Comparisons of the thalamus in patients with MNDs with HCs revealed significant alterations. These alterations included reduced gray matter volume (GMV), reduced fractional anisotropy (FA), increased mean diffusivity (MD), increased axial diffusivity (AD), increased radial diffusivity (RD), reduced cerebral blood flow (CBF), increased iron deposition, and reduced functional connectivity (FC). Some of these alterations are associated with disease severity, disease progression rate, functional disability, cognitive performance, and clinical symptoms. We observed differences and similarities between the studies in terms of the location, extent, and direction of the thalamic alterations, and some showed bilateral thalamic involvement [45, 46, 47, 56, 57, 60, 71, 77, 79, 82, 83, 85, 86, 87]. However, other studies showed unilateral or asymmetric involvement [48, 52, 59, 62, 63, 78, 80, 81, 87]. Some studies revealed more widespread and severe alterations in certain subtypes of MND, such as PLS [46, 49, 64], PLC-ALS [78], C9orf72 mutation ALS [59, 81, 82], dysphagia ALS [87], rapidly progressing ALS [80], and ALS-FTD [31, 53, 58]. Specific regions or subregions of the thalamus were more affected than others, such as the mediodorsal nucleus [67], anterior thalamic radiations [82], superior and inferior regions [81], sensorimotor and visual networks [86], and posterior thalamus [65].
 Fig. 2.
Fig. 2.Anatomical location of the thalamus and adjacent regions.
Forty percent of T1-w studies reported reduced GMV in the thalamus of patients with MND compared with HCs [21, 42, 53, 59, 60, 77, 78, 79, 80, 82, 83, 84, 85, 86]. Thalamic atrophy was more pronounced in ALS-FTD [21, 31, 43, 53, 54, 58, 65, 82], PLC-ALS [78], PLS [31, 45, 47, 55], C9orf72 mutation carriers [31, 45, 47, 55, 81, 82], and rapidly progressing ALS [80]. Thalamic atrophy was correlated with reduced survival, disease severity, duration, and progression rate, as well as impaired cognition and functional disability [31, 51, 62, 80, 88]. Specific thalamic subregions were more affected, including the mediodorsal nucleus [45, 47], anterior thalamic radiations [80, 82, 86], superior and inferior regions [81], and motor and sensory nuclei [47, 50].
Eighty-one percent of DTI studies reported an increase in MD, RD, and AD, as well as a decrease in FA in the thalamus of patients with MND compared with HCs [47, 50, 66, 68, 69, 71, 72, 77, 78, 80, 81, 83, 86]. DTI changes were more severe in ALS-FTD [47, 50], PLS [45, 47], and C9orf72 mutation carriers [47, 81]. DTI measures were correlated with reduced survival, increased severity, duration, and disease progression, as well as impaired cognition [67, 68, 69, 74, 78, 83, 88].
Seventy-five percent of fMRI studies reported reduced FC between the thalamus and cortical regions in patients with MND compared with HCs [74, 75, 76, 84, 86, 88]. FC was more reduced in ALS-FTD [84] and correlated with increased disease severity and progression [74, 87, 88].
Our literature review shows that MRI techniques can detect various aspects of thalamic structure and function such as volume, shape, density, diffusion, connectivity, CBF, iron deposition, and signal intensity.
Atrophy of the thalamus was a common finding across several studies [21, 31, 42, 44, 46, 47, 49, 52, 54, 55, 56, 57, 58, 59, 61, 62, 63, 64, 65, 80, 88]. Thalamic atrophy might therefore have far-reaching implications. McKenna et al. [21] (2023) found variability in thalamic atrophy patterns, suggesting the differential involvement of various thalamic nuclei in MNDs. Variations were also observed based on the specific analytical methods used. This underscores the complexity of thalamic involvement in MNDs and the need for careful methodological considerations in future research. Several studies found reductions in GMV in the thalamus [42, 43, 44]. These changes may be associated with cognitive and motor functions, as suggested by the associations between thalamic GMV and cognitive function level and motor functional parameters [42, 51, 92]. Ahmed et al. [43] (2021) also reported that decreased thalamic volume can lead to poorer performance on the Trail Making Test (TMT), a neuropsychological test of visual attention and task switching [93, 94, 95]. Furthermore, advanced volumetric analyses have revealed thalamic atrophy in ALS [96]. This suggests a greater thalamic involvement at the severe end of the ALS-FTD spectrum. Among the FTD subtypes, thalamic atrophy is most pronounced in FTD with MND (FTD-MND) [31] .
C9orf72 mutation carriers show more extensive thalamic atrophy than C9orf72-negative carriers across diseases [59, 62]. The early radiological signs of C9orf72 mutation are linked to specific degeneration in the thalamus and focal hippocampus, which can be detected before any changes in the cortical gray matter (GM) occur [97]. This validates the early involvement of subcortical GM in C9orf72-related neurodegeneration [97]. Unexpectedly, hypermethylation of C9orf72 was associated with faster thalamic atrophy over time [59]. Vertex-based shape analyses localized the atrophy to superior, inferior, anterior, ventral, lateral, and medial thalamic subregions [49, 56]. Changes in diffusivity, magnetization transfer ratio (MTR), susceptibility, and homogeneity were also noted [48, 57], reflecting microstructural damage [98, 99] that aligns with other neurodegenerative diseases such as multiple sclerosis (MS) [100, 101, 102]. Genotype-specific patterns of thalamic atrophy were also identified [45]. This suggests that genetic factors may influence the nature and extent of thalamic alterations in patients with MNDs. Furthermore, Devenney et al. [53] (2017) found that thalamic atrophy is associated with psychotic symptoms in patients with ALS-FTD carrying the C9orf72 mutation. Another interesting finding is that hypermethylation may protect the thalamus from this mutation [59], suggesting a possible avenue for therapeutic intervention [103].
Carriers of C9orf72 mutation show more thalamic atrophy than non-carriers, especially in the motor and sensory nuclei [21, 45, 47, 50, 52, 53, 55, 62]. Patients with PLS showed more thalamic atrophy than patients with ALS, especially in the pulvinar and lateral geniculate nuclei, which are involved in visual processing, reflecting the predominant upper motor neuron (UMN) involvement in PLS [45, 46, 47]. These findings are consistent with those of previous studies [50, 104]. Furthermore, changes in cognition, structure, and microstructure were identified in young individuals with the C9orf72 mutation. When focusing on individuals under the age of 40 years, those with the C9orf72 mutation showed right thalamic atrophy compared with those without the mutation [52].
ALS patients with cognitive impairment also show more thalamic atrophy than those without cognitive impairment [43, 51, 58]. Thalamic atrophy in MNDs was associated with various clinical outcomes, such as motor function, cognitive performance, behavioral symptoms, survival time, and disease progression [29, 105]. Thalamic volume or density correlated negatively with clinical severity scores, indicating that greater thalamic loss is associated with worse functional status [85, 106, 107, 108]. Thalamic volume or density also correlated positively with cognitive features, such as those measured using the third edition of the Addenbrooke’s Cognitive Examination (ACE-III) or TMT [43], suggesting that a smaller or less dense thalamus is related to poorer cognitive abilities. Thalamic volume or density also predicted survival time and disease progression rate in some studies, showing that lower thalamic values were associated with shorter survival or faster deterioration [62].
Thalamic atrophy in MNDs is not uniform across the whole thalamus, but affects specific nuclei or regions depending on the disease subtype and phenotype. For example, motor and sensory thalamic nuclei are commonly affected in ALS and PLS, reflecting impairment of the corticospinal and corticobulbar tracts [45, 47, 50]. Mediodorsal peritoneal-reuniens nuclei were frequently involved in ALS with or without FTD and C9orf72 mutation, suggesting a role in cognitive and behavioral functions [45, 47, 67]. Pulvinar and lateral geniculate nuclei are preferentially affected in PLS, which is possibly related to visual processing deficits [45, 47]. The anterior, ventral, and lateral thalamic nuclei are more atrophic in patients with ALS-FTD than in those without FTD, indicating a link between executive dysfunction and language impairment [47, 58]. According to the literature, despite the high occurrence of non-motor symptoms in rare MNDs such as post-polio syndrome (PPS), research has revealed that thalamic atrophy may not be a consistent characteristic of all rare forms of MND. This indicates that the underlying mechanisms and clinical manifestations of these disorders may be more intricate than previously assumed [109, 110].
The involvement of the thalamus in different subtypes of FTD was also reported [31, 61]. Thalamic atrophy was most pronounced in bvFTD, followed by ALS-FTD and ALS [61], again emphasizing the varied nature of thalamic involvement in different MNDs, suggesting that comorbidity with FTD exacerbates thalamic degeneration [21, 111]. Thalamic atrophy correlated with executive dysfunction and poorer cognitive performance, highlighting the role of thalamo-cortical circuits in cognition [43, 58, 67]. Associations with extrapyramidal motor deficits indicate the involvement of thalamo-basal ganglia connections [47]. Correlations with disease stage and progression suggest the potential use of the thalamus as a biomarker [44, 59]. These findings support previous research suggesting a connection between FTD and focal thalamic degeneration [21]. The specific thalamic traits associated with each phenotype align with well-established patterns of cortical vulnerability. Furthermore, it is likely that the primary symptoms of FTD phenotypes stem from dysfunction of the thalamocortical circuitry rather than solely from focal cortical changes. Given the compelling evidence of a significant burden of thalamic disease in the early stages of most FTD subtypes, it is important to consider the practical relevance, diagnostic role, prognostic significance, and monitoring potential of thalamic metrics in future FTD research [21, 29, 31].
In ALS, thalamic changes occur bilaterally [56, 57], whereas leftward asymmetry has been reported in ALS-FTD and PLS [49, 65]. Lateralized thalamic differences were also found between ALS cognitive subgroups [51]. These findings indicate that lateralization may reflect cognitive dysfunction. Finally, age-related changes in thalamic volume were observed in HCs but were more pronounced in patients with PLS [64]. This implies that normal aging may be accelerated or exacerbated in patients with MNDs, leading to greater thalamic atrophy.
Only one study used the ASL technique to assess CBF in the thalamus of MND patients [89]. This study reported a decrease in CBF in the thalamus of patients with ALS-FTD compared with HCs, suggesting reduced metabolic activity, perfusion in the thalamus, and hypoperfusion of the thalamus in patients with ALS-FTD [21, 112, 113]. Two studies used T2*-w [90] and T2-w [91] to detect iron deposition and signal intensity changes, respectively. T2*-w showed a significant increase in iron deposition in the thalamus of patients with frontotemporal lobar degeneration (FTLD), but not in patients with ALS, suggesting a possible role of iron accumulation in neurodegeneration. T2-w reported high-intensity signals in the thalamus of one in 13 patients with ALS, suggesting a possible abnormality in water content or myelination.
Previous research has demonstrated that utilizing multimodal neuroimaging measures, particularly thalamic metrics, as input features in machine-learning models can aid in categorizing individual subjects into diagnostic groups. Studies have revealed that thalamic structural measures, in conjunction with white matter (WM) integrity metrics, play a crucial role in distinguishing between patients with ALS, HCs, and phenotypic variants such as PLS [114, 115, 116]. The consistent significance of thalamic characteristics in feature importance analyses across studies underscores the value of assessing thalamic integrity for diagnostic purposes. This suggests that quantifying thalamic involvement could facilitate the development of computational models capable of accurately distinguishing ALS from other conditions, such as PLS or HCs, at the individual level. This underscores the practical clinical significance of identifying thalamic signatures to support the early and precise diagnosis and prognosis of MNDs using machine learning [114, 115, 116]. Although large-scale validation is necessary, these findings confirm the potential of personalized diagnostic algorithms.
The thalamus, particularly the left mediodorsal nucleus, plays a crucial role in cognitive processes including memory and executive function. As a “higher order thalamic relay”, the left mediodorsal nucleus facilitates interactions across various association cortices through cortical-thalamocortical connections. This function makes it a key area of interest when studying memory and cognition-related impairments in MNDs such as ALS. The mediodorsal nucleus is associated with several DTI measurements and cognitive scores, suggesting that changes in this area may serve as a biomarker for cognitive impairment. Notably, a negative relationship was found between isolated memory subscores and MD and RD measurements in the left mediodorsal nucleus of the thalamus, underscoring its significance in memory functions [11, 67].
Additionally, the mediodorsal-paratenial-reuniens nuclei of the thalamus, which are involved in memory and executive functions, also exhibit changes in neurodegenerative diseases. A study by Chipika et al. [47] (2020) reported reduced volumes of these nuclei in patients with ALS without the C9orf72 gene mutation and in patients with PLS. This reduction points to potential disruption of “relay” circuits between different cortical regions, which could result in cognitive impairment. These findings highlight the importance of the thalamus and, more specifically, its various nuclei, in understanding the interplay between neurodegenerative processes and cognitive functions, including memory [117].
DTI studies reported an increase in MD and RD and a decrease in FA in the thalamus of MND patients compared with HCs or non-impaired MND patients [67, 68, 69, 70, 71, 72]. Trojsi et al. [67] (2019) found that alterations in thalamic diffusion metrics, such as AD and MD, were associated with memory impairment and decreased visuospatial abilities in ALS patients. Specifically, they noted increased AD and MD in the mediodorsal nucleus of the left thalamus, with poorer performance on cognitive tests. This is consistent with the known role of the mediodorsal thalamus in memory and its connections to the prefrontal cortex [1, 67, 99, 118, 119]. Zhang et al. [68] (2017) also implicated thalamocortical pathways linking the thalamus to motor areas, such as the primary motor cortex, in the pathogenesis of ALS. Additional evidence for thalamic changes in ALS comes from Barbagallo et al. [69] (2014), who found that increased MD in the thalamus of patients versus HCs correlated with longer disease duration but lower Revised ALS Functional Rating Scale (ALSFRS-R) scores. Sharma et al. [71] (2013) reported similar diffusion alterations, with higher MD, AD, and RD and lower FA in the thalamus of patients with ALS. Sach et al. [72] (2004) also found reduced FA in the right thalamus in ALS. Together, these diffusion MRI studies consistently demonstrate that the thalamus undergoes microstructural changes such as axonal loss and demyelination throughout the course of ALS. Sex may also play a role, as Bede et al. [70] (2014) linked higher thalamic FA to male sex in ALS.
These changes indicate reduced structural integrity and increased water diffusion in the thalamus owing to neurodegeneration, axonal damage, or demyelination. As previously mentioned, consistent with the T1-w section, the scope and configuration of these modifications differed based on the type of MND, occurrence or non-occurrence of FTD or cognitive decline, and the genetic condition.
Recent resting-state and task-based fMRI studies have provided evidence for thalamus dysfunction in ALS and other MNDs. fMRI studies have reported altered FC between the thalamus and other brain regions in patients with MND compared with HCs or non-impaired patients with MND [74, 75, 76]. Dey et al. [73] (2023) found that increased FC between the premotor cortex and regions including the left thalamus was associated with greater disease progression in patients with ALS. This aligns with the role of the thalamus, particularly the ventral lateral nucleus, in motor control circuits connected to the motor cortex, suggesting that the thalamus plays a role in ALS pathophysiology [118, 120]. Xu et al. [74] (2017) specifically reported reduced nodal efficiency in the right thalamus of ALS patients, suggesting that its functional connections to the motor cortex are disrupted. Further support comes from Zhou et al. [75] (2013), who found decreased FC between the left thalamus and the right primary motor cortex in patients with ALS compared with HCs. These FC disturbances point to a failure of communication between the thalamus and the motor cortex in ALS. The thalamus relays sensory and motor signals to the cortex; therefore, impaired connectivity could contribute to motor neuron degeneration [74]. In addition to changes in the resting state, Mohammadi et al. [76] (2009) reported reduced task-based activation in the thalamus of patients with bulbar-onset ALS, indicating motor network dysfunction. Thalamic hypoactivation could be related to deficits in the coordination of volitional movements that characterize ALS. Ultimately, these changes indicate disrupted communication and coordination of neural activity in the thalamus and its target areas. The direction and pattern of these changes varied depending on the MND subtype, presence or absence of FTD or cognitive impairment, and task or resting-state conditions.
Multi-technique MRI studies have consistently demonstrated alterations in thalamic biomarkers. The key findings of the reviewed studies suggest that patients with MND exhibit reduced GMV in the thalamus compared with HCs [77, 78, 79, 83, 85]. More extensive thalamic atrophy appears to be related to the faster progression of ALS and greater functional impairment according to the ALSFRS-R score [80]. Furthermore, decreased FA and increased apparent AD, RD, and MD have been reported in the thalamus of MND patients compared with HCs [77, 86]. These findings reflect microstructural changes in the thalamus, which may be related to degeneration of WM tracts and neuronal loss.
fMRI studies have also shown differences in thalamic activation in ALS patients during motor tasks, as well as changes in FC [87, 88]. For example, reduced thalamic activation during swallowing tasks has been associated with dysphagia symptoms [87]. Together, these structural and functional changes demonstrate thalamic network dysfunction in ALS, disrupting communication with the motor cortical regions.
Li’s study in 2022 highlighted an increase in iron deposition and QSM values in the bilateral thalamus in people with ALS. Additionally, he found a decrease in GMV in the bilateral thalamus of patients with ALS, suggesting atrophy in these areas. Notably, an increase in iron deposition in the thalamus is associated with a decrease in the ALSFRS-R score, which measures ALS disease severity [85]. This is consistent with postmortem evidence of increased iron levels in the motor networks of ALS patients, which may be related to neuroinflammation and oxidative stress, contributing to disease progression [121]. Finegan et al. [77] (2021) reported a decrease in thalamic volume and diffusivity measures such as FA, AD, RD, and MD in patients with PLS compared with HC, identifying a possible marker of disease severity. Changes in these parameters were associated with an increase in PLS severity, indicating that MRI parameters could potentially be used as markers of disease progression.
Devenney et al. [84] (2021) identified a decrease in GM in the anterior and medial thalamus in patients with sensory perceptual abnormalities and patients with psychosis. They further suggested that cognitive, social, and neurobiological factors could contribute to psychosis vulnerability in patients with ALS-FTD, with thalamo-cortico-cerebellar network alterations playing a significant role. Christidi et al. [78] (2018) and Tu et al. [79] (2018) both reported a decrease in GMV in the thalamus in patients with ALS. Tu et al. [79] further suggested that thalamic diffusion correlates with cortical dysfunction and disease severity, making thalamic parcellation a potential marker of ALS.
Christidi et al. [78] (2018) reported that PLC anomalies in ALS are caused by both GM and WM abnormalities, emphasizing the importance of circuits rather than isolated sites in the genesis of this illness. PLC was studied using ALS as a natural experimental model.
Menke et al. [86] (2018) found that ALS is associated with decreased thalamic GM and shape and that changes in DTI parameters in the GM, such as increased MD, AD, and RD in the thalamus, may be indicative of ALS. Furthermore, they suggested that decreased FC between a network comprising both the thalami and an area in the visual cortex is associated with a decrease in the ALSFRS-R score. These FC changes may reflect the disruption of large-scale brain networks due to the degeneration of WM tracts connecting the thalamus and other areas such as the motor and frontotemporal cortices. These findings are consistent with those of Thivard et al. [83] (2007), who found that decreased FA in the thalamus correlates with the ALSFRS-R score and also reported decreased GMV in the bilateral thalamus in ALS patients. These results emphasize that the thalamus, WM tracts, and FC play a role in the pathophysiology of ALS. They provide knowledge on the alterations in structure and function linked to the condition and enhance our understanding of its fundamental mechanisms.
Senda et al. [80] (2017) reported similar findings, associating ALS with decreased GMV in the thalamus and FA in the cortical and subcortical regions. They suggested that the rate of ALS progression is correlated with GM atrophy and the severity and extent of decreased FA. Furthermore, they found that thalamic atrophy varies with the rate of progression and is faster in rapid progression than in slow progression.
Stoppel et al. [88] (2014) and Bede et al. [81] (2013) reported motor system involvement in the thalamus, with ALS associated with decreased MTR and thalamic atrophy. Bede also found that ALS patients with the C9orf72 gene mutation showed decreased thalamic volume and FA, but increased thalamic AD, MD, and RD compared with ALS patients without the C9orf72 mutation.
Mahoney et al. [82] (2012) and Li et al. [87] (2009) found decreased thalamic GM bilaterally in ALS patients with the C9orf72 gene mutation. Furthermore, Li found a bilateral decrease in FA in patients with ALS without dysphagia compared with HCs, and a further decrease in FA in the left thalamus in patients with ALS with dysphagia.
Interestingly, thalamic alterations may differ depending on genotype, as carriers of the C9orf72 mutation show unique volume loss and diffusion changes compared with patients without the C9orf72 mutation [81, 82]. Regional thalamic subnetworks also appear to follow distinct trajectories during the disease stages [79]. This suggests the potential of thalamic MRI metrics as imaging biomarkers for ALS genetics and progression.
These findings imply that multimodal MRI techniques can be more useful for detecting and monitoring thalamic alterations in MNDs as well as for identifying subgroups of patients with different clinical phenotypes and prognoses. Thalamic alterations in MNDs may result from various mechanisms such as axonal damage, neuronal demise, neuroinflammation, oxidative stress, excitotoxicity, or network dysfunction. Thalamic changes in MNDs can have several consequences, such as impaired motor, sensory, cognitive, emotional, and sleep functions. Thalamic alterations in MNDs may have diagnostic, prognostic, and therapeutic implications. For example, thalamic changes may help to distinguish MND subtypes or progression patterns, monitor disease progression, predict functional decline or survival time, and serve as potential therapeutic targets or biomarkers for treatment response in patients with MND.
Advanced MRI methods allow for sensitive detection and measurement of dynamic thalamic alterations in MNDs from clinical to final stages. Understanding the complex sequence of structural, functional, and metabolic thalamic modifications in disease etiology may lead to the identification of new therapeutic targets. Longitudinal multimodal and multiparametric imaging investigations with larger cohorts will provide more information on the link between thalamic biomarkers and clinical milestones. Validated thalamic imaging measures have the potential to improve differential diagnosis, track progression, predict outcomes, and evaluate therapies for patients in the future. To date, these findings encourage further research into the targeted manipulation of thalamic activity and connectivity as a treatment option for MNDs. The use of thalamic neuroplasticity helps restore motor and cognitive function. In conclusion, discovering the mysteries of the thalamus will usher in a new era of more accurate medication and improved quality of life for patients with MNDs.
Finally, Table 2 highlights the importance of the thalamus in MNDs. Thalamic damage occurs early, progresses over the course of the disease, and involves multiple pathological pathways. Multimodal MRI techniques have the potential to reveal distinct thalamic signatures that correlate with different disease subtypes and outcomes. Further research is needed to validate thalamic biomarkers and elucidate the mechanisms of thalamic degeneration in MNDs. However, the thalamus is a promising target for future prognostic tools and therapeutic interventions in these currently incurable conditions. Targeting thalamic dysfunction and network disruptions may present novel avenues for managing motor, cognitive, and behavioral symptoms, and altering disease progression in MNDs.
| Multiple MRI techniques have detected: | |
| T1-w and voxel-based morphometry analyses | |
| DTI biomarkers | |
| fMRI biomarkers | |
| Changes in other biomarkers | |
Abbreviations: AD, axial diffusivity; ALS, amyotrophic lateral sclerosis; ALS-FTD, ALS with frontotemporal dementia; CBF, cerebral blood flow; DTI, diffusion tensor imaging; FA, fractional anisotropy; fMRI, functional magnetic resonance imaging; MD, mean diffusivity; MRI, magnetic resonance imaging; MND, motor neuron disease; PLS, primary lateral sclerosis; RD, radial diffusivity; T1-w, T1-weighted.
Magnetic resonance neuroimaging studies provide robust evidence for significant thalamic involvement in the spectrum of MNDs. Structural, functional, and multimodal neuroimaging techniques have consistently demonstrated thalamic pathology in ALS, FTD, PLS, and genetic variants. Key findings showed that thalamic atrophy, microstructural degradation, disruption of network connectivity, perfusion deficits, and neurochemical alterations are interrelated biomarkers of disease progression in MNDs. Thalamic changes correlate with the clinical measures of motor impairment, cognitive decline, and reduced survival. Alterations are most extensive with rapid progression, executive dysfunction, and C9orf72 mutations. Differential targeting of the motor, sensory, associative, and limbic nuclei contributes to the heterogeneous phenotypes.
This article contains all of the data produced or analyzed during this investigation. Any further inquiries should be forwarded to the corresponding author.
SG and SM: Conceptualization, methodology, investigation, writing - original draft, visualization, writing - review & editing. SG, SM, MM, ZNAP, RK, FM, and SMJ: Formal analysis, resources, data curation, and writing the original draft. SG: Supervision, project administration. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
