Academic Editors: Brian Tomlinson and Takatoshi Kasai
Most of the published literature on Atrial fibrillation (AF) originates from the northern hemisphere and mainly involves Caucasian patients, with limited studies in certain ethnicities and races. This scoping review was conducted to collect and summarize the pertinent evidence from the published scientific literature on AF in South Asians and Middle Eastern Arabs. MEDLINE, Embase and CENTRAL databases were included in our search. After screening 8995 records, 55 studies were selected; 42 from the Middle East and 13 from South Asia. Characteristics of the included studies were tabulated, and their data were summarized for study design, setting, enrolment period, sample size, demographics, prevalence or incidence of AF, co-morbidities, risk factors, AF types and symptoms, management, outcomes, and risk determinants. Identified literature gaps included a paucity of community or population-based studies that are representative of these two ethnicities/races. In addition, studies that addressed ethnic/racial in-equality and access to treatment were lacking. Our study underscores the urgent need to study cardiovascular disorders, particularly AF, in South Asians and Middle Eastern Arabs as well as in other less represented ethnicities and races.
There has been an increasing interest in the impact of ethnicity and race on cardiovascular disorders. Patients from the same race and/or ethnicity share common genetics as well as environmental exposures. This may be responsible for the differences observed between different races/ethnicities in the incidence, presentation and outcomes of cardiovascular disorders as well as for the differences in response to therapy [1]. In addition, racial and ethnic inequalities and bias may also play an important role. Variations in access to care, economic status and education have all been linked to race/ethnicity and can affect awareness of the disease, which may lead to underdiagnosis and insufficient treatment.
Atrial fibrillation (AF) is the most common arrhythmia encountered in clinical practice, accounting for approximately one-third of hospitalizations for cardiac rhythm disturbances [2]. Most of the published literature on AF originates from North America and Europe involving mainly Caucasian patients [3]. Other ethnicities are largely under-represented particularly South Asians and Middle Eastern Arabs. Therefore, our aim was to perform a scoping review to identify and summarize the current evidence on AF in these two ethnicities with the overarching objective to identify research gaps and inform future research in this area.
A Scoping review is a systematic method of knowledge synthesis that examines and maps evidence on a specific subject matter, thus, identifying key concepts, theories, sources of evidence, and research [4]. This scoping review follows the five steps proposed by Arskey and O’Malley [5]: (1) identifying the research question, (2) identifying relevant studies, (3) selecting studies, (4) collecting data, and (5) mapping, summarizing and describing the results.
Studies that reported various aspects of AF including epidemiology, co-morbidities, risk factors, management and/or clinical outcomes in the Middle East or South Asia were included. Exclusion criteria included studies on sub-clinical AF, post-operative AF, genetics, cost-effectiveness, and smart devices. The countries in this review were categorized according to the world regions as per the World Health Organization (WHO), i.e., South-East Asia and Eastern Mediterranean regions [6]. For the purpose of this review, the terms Middle East(ern) and South Asia(n) will be used.
A comprehensive electronic literature search using MEDLINE, EMBASE, and CENTRAL was performed on September 26, 2020. Boolean terms (“OR” and “AND”), Medical Subject Headings (MeSH), Emtree and broad keywords were used. The search terms were combined and included: “atrial fibrillation”, “Middle East”, “South Asia”, “Arab”, individual country and nationality. Search limitations were not applied. Reviews and meta-analyses, international registries and the references’ lists of the retrieved articles were manually screened to identify additional relevant studies. The detailed search strategy is described in Supplementary Table 1.
All records (N = 8995) were reviewed on the titles and abstracts levels. Duplicate publications, posters, abstracts, irrelevant studies or those that did not meet the inclusion criteria were excluded. Relevant abstracts (n = 305) were screened and 84 of them were retrieved in full texts. After excluding 29 studies (Supplementary Table 2), the search strategy resulted in 55 studies [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61] (Figs. 1,2). However, 17 studies [45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61] were related to three registries [30, 33, 41] (Supplementary Table 3) and were used when provided additional data or analysis. The included studies were tabulated, and their data were extracted for the study design, setting, enrolment period, sample size, demographics, prevalence or incidence of AF, co-morbidities, risk factors, AF types and symptoms, management, outcomes, and risk determinants (Supplementary Tables 3,4,5,6,7).
 Fig. 1.
Fig. 1.Flowchart of search strategy and identification of relevant studies with summary of the inclusion and exclusion process.
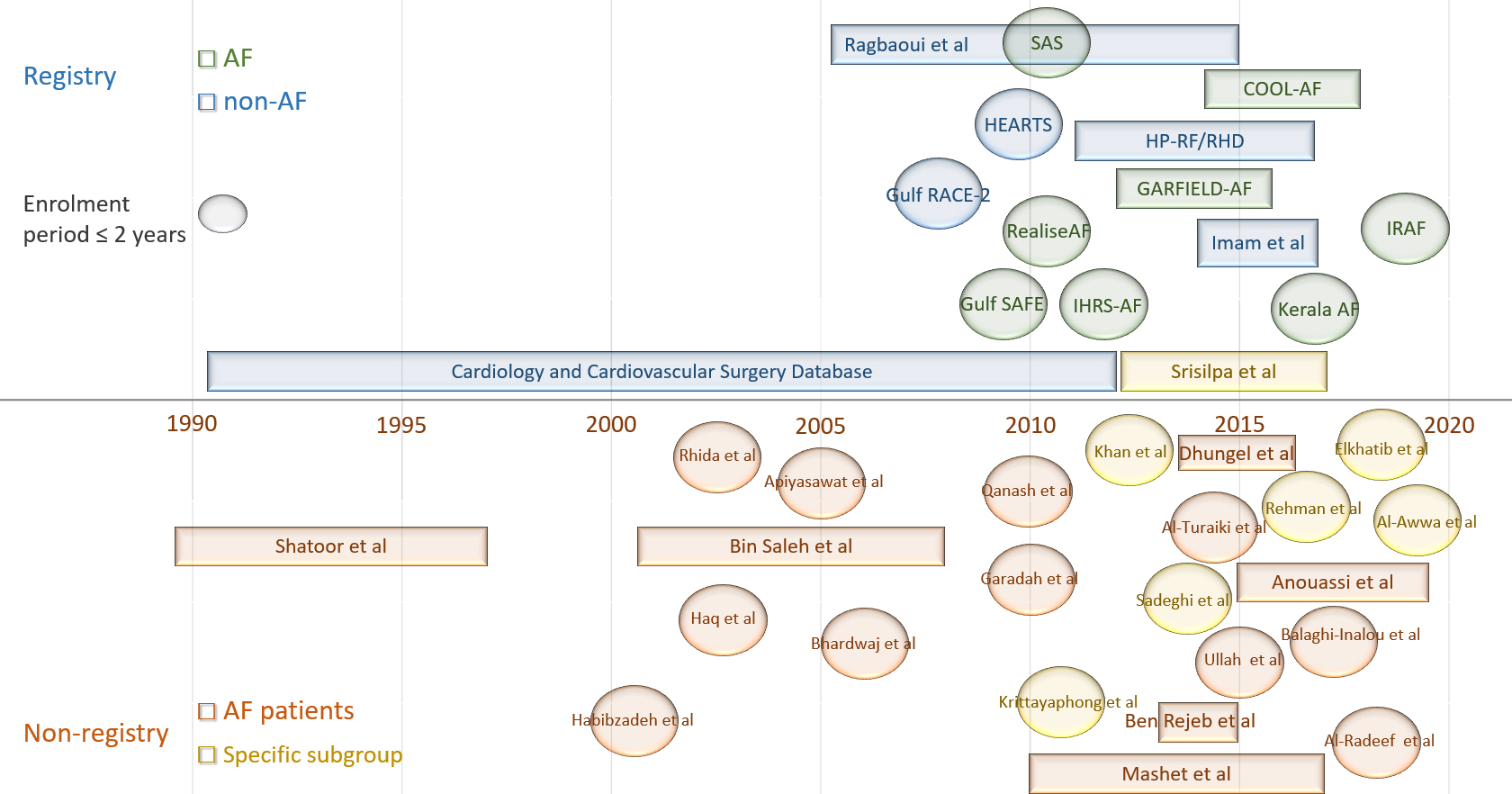 Fig. 2.
Fig. 2.The selected studies. Visual summary of the studies: AF or non-AF registry (Top) and single- or multi-center non-registry (Bottom) studies. The studies enrolled either AF patients only or specified subgroups which included proportion of patients with AF. The studies which are represented in an oval shape have an enrollment period of two years or less. Abbreviations/acronyms: AF, atrial fibrillation; COOL-AF, Cohort of Antithrombotic Use and Optimal INR Level in Patients With Non-Valvular Atrial Fibrillation in Thailand; GARFIELD-AF, Global Anticoagulant Registry in the FIELD-Atrial Fibrillation; Gulf RACE-2, second Gulf Registry of Acute Coronary Events; Gulf SAFE, Gulf Survey of Atrial Fibrillation Events; HEARTS, Hearts Function Assessment Registry Trial in Saudi Arabia, HP-RF/RHD, Himachal Pradesh- Rheumatic Fever/Rheumatic Heart Disease; IHRS-AF, Indian Heart Rhythm Society-Atrial Fibrillation; IRAF, Iranian registry of atrial fibrillation; RealiseAF, Real Life global Survey Evaluating patients with atrial fibrillation; SAS, Saudi Atrial Fibrillation Survey.
The selected articles were categorized based on the region, i.e., Middle East (n = 42) and South Asia (n = 13) (Tables 1,2, Ref [12, 15, 20, 22, 36, 38, 39] and Supplementary Table 3). Studies from the Middle East included 15 single-center [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21] and four multi-center [22, 23, 24, 25] studies, five non-AF registries [26, 27, 28, 29, 30] with seven related publications [45, 46, 47, 48, 49, 50, 51], and three AF registries [31, 32, 33] with eight related publications [52, 53, 54, 55, 56, 57, 58, 59]. Studies from South Asia included three single-center [34, 35, 36] and two multi-center [37, 38] studies, one non-AF [39] and five AF [40, 41, 42, 43, 44] registries. Two additional publications [60, 61] came out from the COOL-AF registry [41]. By disregarding the 17 registry-related articles that were mentioned above [45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61], the studies were performed in the following countries: India (n = 6), Kingdom of Saudi Arabia (KSA) (n = 7), Pakistan (n = 4), Thailand (n = 4), Iran (n = 4), Qatar (n = 2), combined six adjacent Middle Eastern countries (n = 2), and one study in each of the following: Bahrain, Egypt, Iraq, Jordan, Kuwait, Morocco, Nepal, Tunisia, and United Arab Emirates (UAE). With the exception of two papers [14, 32], the studies enrolled patients from the hospital settings, between 1989 and 2019. In the single- and multi-center studies, sample size, age range and female distribution of AF patients in the Middle Eastern and South Asian regions were 3514 and 9899 patients, 58.8–68.4 and 51.2–64.6 years, and 36.0–65.0% and 48.8–58.1%, respectively. The respective data from AF registries were 3181 and 9974 patients, 56.0–61.7 and 54.7–67.4 years, and 40.5–48.5% and 40.1–51.5%, respectively. Comparable age ranges and female proportions were seen in the studies (n = 7) and registries analyses (n = 5) that investigated AF in specified patient populations such as acute coronary syndrome (ACS) [15, 18, 27], acute [26] and chronic heart failure [29], hypertension [38], and haemodialysis (HD) [22]. However, in two [20, 36] out of four studies [12, 20, 28, 36] that enrolled patients with stroke, patients were older (71 years), while patients with rheumatic heart disease (RHD) were younger (40.2 years) and with higher female proportion (72.3%) [39].
| Characteristic | ME region | SA region | Total | |
| Study design | ||||
| Cohort | 4 | 1 | 5 | |
| Case–control | 1 | - | 1 | |
| Cross-sectional | 9 | 2 | 11 | |
| Retrospective | 5 | 2 | 7 | |
| Registries* | ||||
| AF registry | 11 | 7 | 18 | |
| Non-AF registry | 12 | 1 | 13 | |
| Study Setting | ||||
| Community/Primary care | 1 | - | 1 | |
| Hospital patients/no population denominator | 26 | 9 | 35 | |
| Hospital patients/population denominator | 14 | 4 | 18 | |
| Hospital patients and community | 1 | - | 1 | |
| Year of final data collection | ||||
| 2005 and before | 5 | 1 | 6 | |
| 2006–2015 | 28 | 5 | 33 | |
| 2016 onwards | 9 | 7 | 16 | |
| Publication date | ||||
| Before 2012 | 9 | - | 9 | |
| 2012 onwards | 33 | 13 | 46 | |
| *Numbers of studies included the additional publications (n = 17) related to the
included registries. Abbreviations: AF, atrial fibrillation; ME, Middle Eastern; SA, South Asian. | ||||
| Single- and multi-center studies* | Non- AF registries | AF registries | ||||
| ME region | SA region | ME region | SA region [39] | ME region | SA region | |
| Number of studies | 17 | 5 | 5 | 1 | 3 | 5 |
| Enrolment period | 1989–2019 | 2005–2017 | 1991–2017 | Ongoing since 2011 | 2009–2018 | 2010–2017 |
| Enrolment duration | 8 weeks–7.5 years | 1–5 years | 9 months–22 years | - | 8 months–1 year | 2 months–4 years |
| Sample size | 36–7450 | 119–13207 | 2593–41453 | 1918 | 400–2043 | 301–3421 |
| Number of AF patients | 3514 | 9899 | 5097 | 459 | 3181 | 9974 |
| Incidence/Prevalence | 2.8–5.8% | 3.4%** | 2.7–17.8% | 23.9% | - | - |
| Age (year) | 58.8–68.4 | 51.2–64.6 | 52.0–67.0 | 40.2 | 56.0–61.7 | 54.7–67.4 |
| Female sex (%) | 36.0–65.0% | 48.8–58.1% | 30.4–48.9% | 72.3% | 40.5–48.5% | 40.1–51.5% |
| * Data from studies that investigated AF in specified patient populations (e.g.,
stroke) are not included in this table unless stated [12, 15, 20, 22, 36, 38]. ** In hypertensive patients [38]. Abbreviations: AF, atrial fibrillation; ME, Middle Eastern; SA, South Asian. | ||||||
The incidence and prevalence of AF was reported in association with other diseases. The overall incidence of AF during hospitalization was 1.8% in patients admitted with acute myocardial infarction (AMI) in Qatar [48], with the incidence in Arabs being higher than that in South Asians (3.1% vs. 0.8%). From the earlier data of the same Qatari registry (1991 to 2002) [45], the overall annual incidence among Qataris was higher than that of other nationalities (12% vs. 8.0%). However, the rate (8.0–9.3%) remained consistent over years for the overall patient population, i.e., those hospitalized with acute cardiac illness [30, 46, 47, 50]. The incidence of AF in the Gulf RACE-2 registry was 2.7% among patients hospitalized with ACS [27]. Two studies from Pakistan [15, 18] reported similar frequency (9.1%) in patients hospitalized with AMI. The prevalence of AF among general hospital admissions was 3.4% in Bahrain [13], 4.2% in Kuwait [19], and 5.8% in Pakistan [24]. Whereas, a lower prevalence (2.8%) was reported in the only study that was conducted in a primary health care center in Iran [14]. The prevalence of AF was 7.8% among patients on maintenance HD in Jordan [22], and 10.6% in patients with chronic heart failure in Morocco [29]. In Iran, the prevalence of AF among patients with stroke was 11.1% [20], while in Qatar, it was 6.4%. In the latter Qatari study, 2.6% of stroke patients were newly diagnosed with AF [28]. The highest AF prevalence reported was 17.8% in patients hospitalized with heart failure [26], and 23.9% in patients with RHD [39] (Table 2 and Supplementary Table 3).
The most common co-morbidities associated with AF were hypertension, diabetes
mellitus, heart failure and stroke which were comparable between the Middle
Eastern and the South Asian registries (hypertension 52.0–63.0% and
31.4–68.5%, diabetes mellitus 30.0–48.0% and 16.1–36.2%, heart failure
27.0–31.7% and 15.5–27.2%, stroke 9.0–13.0% and 9.1–17.8%, respectively).
Analyses from the Gulf SAFE registry [33] showed similar frequencies of the first
three aforementioned co-morbidities in patients with stroke [57], and similar
frequencies of hypertension and heart failure in patients with diabetes mellitus
[54]. In non-AF registries (stroke and heart failure), AF patients with stroke
[28] and acute heart failure [26] had higher frequencies of both hypertension
(72.3% and 70.8%) and diabetes (67.7%, including pre-diabetics, and 55.9%),
than that for AF patients with chronic heart failure (55.0% and 65.9%) [29] and
ACS (39.0% and 44.2%) [27], respectively. Valvular heart diseases were more
prominent in South Asia (40.7–50.3% versus 8.0–24.0%) than in the Middle
East. In the non-registry studies, there were noticeable variations in the
reported frequencies of co-morbidities accompanied with variations in the terms
of definitions used for the associated conditions. The mean HAS-BLED score ranged
between 1.1 and 1.9 across the included studies, while mean
CH
| Morbidity/Risk | Single- and multi-center studies* | AF registries | ||
| ME region | SA region | ME region | SA region | |
| Heart failure | 14.0–45.6% | 12.3–56.5% | 27.0–31.7% | 15.5–27.2% |
| Hypertension | 23.7–79.5% | 10.2–30.5% | 52.0–63.0% | 31.4–68.5% |
| Diabetes mellitus | 12.0–68.3% | 5.0–15.0% | 30.0–48.0% | 16.1–36.2% |
| Prior stroke | 11.7–23.0% | 0.6% [37] | 9.0–13.0% | 9.1–17.8% |
| CAD/IHD | 3.5–55.8% | 13.6% [35] | 28.0–28.5% | 5.4–34.8% |
| Valvular heart disease | 23.6–58.7% | 5.1–14.9% [34, 35] | 8.0–24.0% | 40.7–50.3% [42, 44] |
| Chronic kidney disease | 18.9–36.8% | 1.3–1.8% [35, 37] | - | 4.5–10.3% |
| Smoking | 22.7–42.0% | - | 23.0% [55] | 16.0–18.6% [42, 43] |
| HAS-BLED score (mean) | 1.2–1.9 | - | 1.1 [33] | 1.5–1.6 |
| CH |
2.6–4.1 | 1.8 [37] | 2.3 [33] | 2.9–3.0 |
| CHADS |
2.0–2.3 | - | 1.6 [33] | 1.8 [41] |
| CHADS |
5.0% | - | 28.5% [33] | 11.6–27.2% |
| CHADS |
24.0–26.0% | - | 27.5% [33] | 19.9–36.2% |
| CHADS |
69.0–71.0% | - | 44.0% [33] | 36.7–68.5% |
| * Data from studies that investigated AF in specified patient populations (e.g.,
stroke) are not included in this table unless stated [12, 15, 20, 22, 36, 38]. Abbreviations: AF, atrial fibrillation; CAD, coronary heart disease; IHD, ischemic heart disease; ME, Middle Eastern; SA, South Asian. | ||||
There were few studies that reported the types and symptoms of AF. The frequency of new onset AF was reported in two Middle Eastern studies as 27.0% [24] and 59.0% [13], which was higher than that reported in the AF registries [32, 33, 41, 42, 44]. The proportion of patients with permanent AF was higher in AF registry studies as compared with that in non-AF registry studies. The proportion of patients with persistent AF was higher in the latter studies. The most common symptoms at presentation, i.e., palpitation and dyspnea, were reported in more than half of the patients across the studies (Table 4, Ref [12, 13, 15, 20, 22, 24, 32, 34, 36, 38, 42], and Supplementary Table 5). A single-centre study from Nepal [35] reported AF types and symptoms in patients with valvular and non-valvular AF. Paroxysmal AF was more common in non-valvular AF (55.2% versus 7.8%), while permanent AF was more common in valvular AF (51.0% versus 10.4%). Symptoms did not differ between the two groups (Supplementary Table 5).
| AF type/symptom | Single- and multi-center studies* | AF registries | ||
| Type | ME region | SA region | ME region | SA region |
| New onset | 27.0–59.0% [13, 24] | - | 13.0–37.0% | 2.3–14.3% |
| Paroxysmal | 27.0–48.0% | - | 17.0–24.5% | 16.4–39.4% |
| Persistent | 26.0–58.4% | 41.6% [34] | 10.0–17.5% | 10.4–33.0% |
| Permanent | 28.0–32.0% | 58.4% [34] | 33.0–45.0% | 8.5–47.2% |
| Symptoms | ||||
| Palpitation | 24.5–48.0% |
- | 21.3% [32] | 55.0% [42] |
| Dyspnea | 14.0–59.3% |
- | 20.5% [32] | 66.0% [42] |
| Chest pain | 12.0–38.0% |
- | - | 21.0% [42] |
| Dizziness/syncope | 6.0–18.0% |
- | 20.5% [32] | 23.1% [42] |
| Fatigue | 5.0% |
- | 13.3% [32] | 48.7% [42] |
| * Data from studies that investigated AF in specified patient populations (e.g.,
stroke) are not included in this table unless stated [12, 15, 20, 22, 36, 38]. Abbreviations: AF, atrial fibrillation; ME, Middle Eastern; SA, South Asian. | ||||
Rate control was the strategy reported in more than half of the patients. Rhythm control was approached in up to one-third of the patients using amiodarone in up to 40% of them. Beta-blockers were used in more than 50% of the patients, digoxin in 30% and calcium channel blockers in approximately 20% of them. Only two AF registries [31, 32] reported the use of ablation. In the Saudi registry (SAS), 3.8% of patients had been ablated [32]. The Iranian registry (IRAF) reported ablation use in 34.0% and 29.8% of patients with familial and non-familial AF, respectively [31]. The use of vitamin K antagonists (VKAs) across the studies ranged from about 40% to 70%, and more than 90% in two studies [34, 41]. Of the 14 studies [17, 20, 24, 26, 31, 32, 34, 35, 40, 41, 43, 44, 46, 59] that reported anticoagulation agents use, seven studies [20, 31, 35, 40, 41, 43, 44] recruited patients in the non-vitamin K oral anticoagulants (NOACs) era, i.e., beyond 2010. Of the seven studies, two did not report NOACs use [20, 35], four South Asian AF registries [40, 41, 43, 44] reported use between 1.9% to 9.1%, and the IRAF registry reported higher average NOACs than VKAs use (45.7% versus 23.0%) [31]. Across the AF registries, aspirin and clopidogrel use ranged from 19.5% to 54.4% and 11.0% to 19.5%, respectively (Table 5, Ref [32, 55, 59] and Supplementary Table 6).
| Medication/strategy | AF registries | |||
| ME region | SA region | |||
| Baseline medications [55] | In-hospital [32, 59] | Baseline medication | ||
| Medication | ||||
| Beta-blocker | 58.3% | 66.0% | 21.0–38.5% | |
| Calcium channel blocker | 16.3% | 20.8% | 15.0–24.9% | |
| Digoxin | 36.1% | 30.3% | 22.2–31.9% | |
| Amiodarone | 9.2% | 9.0–42.3% | 7.4–37.2% | |
| Other antiarrhythmics | 3.1% | 4.0–57.7% | 0.8–33.0% | |
| Antithrombotic agent | ||||
| Vitamin K antagonists | 51.9% | 38.5% | 40.0–90.0% | |
| NOACs | - | - | 1.9–9.1% | |
| Aspirin | 54.4% | 35.5% | 19.5–23.0% | |
| Clopidogrel | 11.0% | 1.5% | 13.1–19.5% | |
| Strategy | ||||
| Rate control | - | 65.0–66.2% | 46.1–87.8% | |
| Rhythm control | - | 12.0–22.0% | 12.2–35.2% | |
| Ablation | - | 3.8% | - | |
| Abbreviations: AF, atrial fibrillation; ME, Middle Eastern; NOACs, non-vitamin K oral anticoagulants; SA, South Asian. | ||||
The main adverse outcomes reported by the studies included mortality
(in-hospital, one-year), cerebrovascular events, and bleeding. In-hospital
mortality was reported in 0.79% of patients in Bahrain [13] and was doubled in
Thailand (1.6%) [37]. The rate was 3.6% in Qatar among patients admitted with
general cardiac conditions [30], with a rate of 4.8% in Middle Eastern and 1.2%
in South Asian patients as defined in the study [47]. The rates were higher in
patients with acute heart failure (6.7%) [26], chronic kidney disease (CKD)
(11.7%) [51], ACS (14.7%) [27], and acute ischemic stroke (9.0% [20] and
26.8% [36]). One-year mortality rate ranged from 6.5% to 18.1% in four South
Asian studies [37, 40, 43, 44]. Gulf SAFE registry reported a rate of 15.0% [58]
with a significantly lower mortality rate in patients who were compliant with the
AF Better Care (ABC) pathway (7.3% versus 13.1%, p = 0.033) [55]. In
the same registry, diabetic patients had significantly higher one-year mortality
rate than non-diabetics (14.4% versus 9.6%, p = 0.003) [53].
Similarly, diabetic patients who were compliant with the ABC pathway had lower
mortality rate as well (5.8% versus 15.9, p = 0.0014) [54]. Higher
one-year mortality rates (
In hypertensive Thai patients, older age, male gender, and presence of CKD
increased the prevalence of AF. On a multivariate analysis, factors of strongest
association with increased prevalence were age of 65 years or above (adjusted odd
ratio (adj OR) 2.86, 95% confidence interval (CI), 1.89–4.33; p
In the Qatari registry, ACS was associated with increased risk of in-hospital
mortality (adj OR, 4.36, 95% CI, 1.77–10.74; p = 0.001) [30, 47] and
not the age alone as a correlate with mortality. In a multiple logistic
regression analysis, beta-blocker administration upon hospital admission was a
good correlate with reduced in-hospital mortality (adj OR, 0.36, 95% CI,
0.15–0.87; p = 0.02) [30]. Gender [46] or ethnicity [47] were not
associated with poor outcomes. A multivariate analysis found the following as
correlates with in-hospital mortality, cardiogenic shock (adj OR, 285, 95% CI,
84.0–970; p = 0.001), diabetes mellitus (adj OR, 1.52, 95% CI,
1.03–2.26; p = 0.04), and chronic renal impairment (adj OR, 1.18–3.68;
p = 0.001) [46]. In Thailand, correlates with mortality after AF
hospitalization included CKD (hazard ratio (HR), 2.01, 95% CI, 1.68–2.41;
p
In the Gulf SAFE registry, in-hospital or emergency room (ER) mortality was
strongly associated with congestive heart failure (adj OR, 2.64, 95% CI,
1.79–3.89; p
The objective of this scoping review was to assess the extent of the available literature of AF in South Asians and Middle Eastern Arabs, then identify and summarize various related aspects such as clinical features and management. A preliminary search of MEDLINE and the Cochrane Database of Systematic Reviews was conducted and no current or underway systematic reviews or scoping reviews on the topic were identified. The reason we chose a scoping review over a systematic review for this study was the broad research question. While a rigorous and detailed in-depth analysis of data is not normally required in scoping reviews, a systematic inquiry was planned and conducted with the synthesis of available evidence into several themes to comply with the study objectives. The study identified significant research gaps in the reporting of this important arrhythmia in the two prespecified ethnicities/races.
Previous reports have suggested a lower incidence and prevalence of AF in the
individuals of non-European ancestry. Data from the United Kingdom-based West
Birmingham Atrial Fibrillation Project [62, 63] suggested a low prevalence of AF
(0.6%), in Indo-Asians aged
In our study the age range of AF patients was 58.8–68.4 years in the Middle
Eastern region, and 51.2–64.6 years in the South Asian region. Compared with our
findings, most of AF patients (
Middle Eastern Arabs and South Asians were included as part of five AF international registries [67, 68, 69, 70, 71] (Table 6). The age of patients (i.e., range 57.9–70.0 years) and female distribution (i.e., range 41.0–57.6%) were in the ranges reported in the registries of this review. The South Asian registries reported wider variabilities in the rate of heart failure, hypertension and diabetes, but not stroke, compared with those in the Middle Eastern ones. The rates of the aforementioned co-morbidities were similar to those in the international registries except for stroke in the RE-LY AF registry, which reported a higher rate in the Middle East. RHD was the highest in India (31.5%) [67]. Similarly, there was a wide range in the rates of AF types in the South Asian registries of this study. The rates of paroxysmal and permanent AF were higher in the international registries. It is important to mention that the regions and distribution of countries in the international registries were different from the WHO regions which were used in our study. The RE-LY AF registry [67] concluded with large regional variations in age, co-morbidities, risk factors, and management. Finally, substantial regional differences in stroke prevention have been found in the GLORIA-AF registry during its phase II, with an oral anticoagulation use rate of 87.4% in Africa/Middle East [63], which was higher than the rate reported in this review (i.e., range 38.5–51.9%).
| GARFIELD-AF [70] | GLORIA-AF phase I [69] | GLORIA-AF phase II [68] | RealiseAF [71] | RE-LY AF [67] | |
| Sample size | 39898 | 1063 | 15092 | 10523 | 15400 |
| Relevant regions (Countries involved) | Asia (Singapore, China, Japan, South Korea, Thailand and India) | ME (Egypt, Lebanon, Turkey, UAE) | Africa and ME (KSA, Lebanon, South Africa, UAE) | Asia (Azerbaijan, India, Taiwan, Turkey) | India |
| Others (Australia, Egypt, South Africa, UAE) | - | - | ME and Africa (Algeria, Egypt, Lebanon, Morocco, Tunisia) | ME (Egypt, Iran, KSA, Turkey, UAE) | |
| Characteristics per region | |||||
| Results presentation | Asia/Others | ME | Africa-ME | Asia/Africa-ME | India/ME |
| Region size (%) | 11117 (27.8%)/1227 (3.0%) | 59 (5.6%) | 597 (4.0%) | 1703 (16.1%)/1680 (15.9%) | 2536 (16.4%)/887 (5.7%) |
| Enrolment period | Mar 2010 to Sept 2015 | May 2011 to Jan 2013 | Nov 2011 to Dec 2014 | Oct 2009 to May 2010 | Sept 2008 to April 2011 |
| Age (year) | 67.4/68.5 | 65.0 | 70.0 | 66.4/61.1 | 57.9/58.6 |
| Female sex (%) | 41.0/42.2% | 57.6% | 47.6% | 46.0/54.2% | 50.3/43.6% |
| Heart failure | 19.8/17.8% | 25.4% | 30.3% | - | 17.7/28.4% |
| Hypertension | 68.4/76.6% | 79.7% | 80.4% | 67.5/54.7% | 41.6/56.1% |
| Diabetes mellitus | 21.7/23% | 37.5% | 42.2% | 24.9/22.7% | 20.2/36.2% |
| Prior stroke | 10.8/16.5% | 10.2% | 18.1% | - | 7.4/22.1% |
| CAD/IHD | CAD: 17.5/26.2% | CAD: 27.4% | CAD: 32.5% | CAD: 32.8/19.1% | MI: 15.5/17.2% |
| ACS: 7.2/16.2% | MI: 13.6% | MI: 16.6% | |||
| VHD | - | - | - | 38.6/31.5% | - |
| RHD | - | - | - | - | 31.1/15.3% |
| Smoking | 12.8/10.4% | - | - | 10.0/13.7% | - |
| HAS-BLED | 1.4/1.6 | - | - | - | - |
| CH |
2.9/3.3 | - | - | - | - |
| HAS-BLED |
- | 16.9% | 10.2% | - | - |
| CHADS |
- | 61.0% | - | - | - |
| CH |
- | 89.8% | 89.8% | - | - |
| New onset | - | - | - | 7.8/10.7% | - |
| Paroxysmal | - | 67.8% | 47.9% | 26.1/17.2% | 20.5/20.8% |
| Persistent | - | 22.0% | 31.8% | 17.9/21.6% | 32.9/7.5% |
| Permanent | - | 10.2% | 20.3% | 48.2/50.5% | 46.6/71.7% |
| Amiodarone | - | - | - | 10.0/3.5% | |
| Vitamin K antagonists | - | 45.0% | 31.8% | - | 14.2/33.1% |
| NOACs | - | - | 55.6% | - | - |
| Aspirin | - | 37.5% | 9.9% | - | 24.1/38.1% |
| Rate control | - | - | - | 60.4/55.4% | - |
| Rhythm control | - | - | - | 23.2/25.7% | - |
| Abbreviations: ACS, acute coronary syndrome; CAD, coronary artery disease; IHD, ischemic heart disease; KSA, kingdom of Saudi Arabia; ME, Middle East; MI, myocardial infarction; NOACs, non-vitamin K oral anticoagulants; RHD, rheumatic heart disease; UAE, United Arab Emirates; VHD, valvular heart disease. | |||||
Our review identified several gaps in AF literature related to Middle Eastern Arabs and South Asians. Importantly, most of the available evidence was based on studies in the hospital setting with a paucity of informative community-based or population-based studies representative of these two ethnicities/races. This is particularly important in correctly identifying the AF disease prevalence and burden. Socio-economic difficulties are an important barrier for the conduct of these types of studies but can be overcome by joining internationally funded studies with samples of these and other ethnicities representative of the population. Furthermore, studies that address ethnic/racial in-equality and access to treatment, particularly oral anticoagulation, and the impact on outcomes, are lacking and should be baldly addressed in populations with mixed ethnicities and races including Middle Eastern Arabs and South Asians. In addition, how ethnicity and race affect AF presentation, symptomatology and interpretation needs to be examined. The Flow-AF registry is currently ongoing to recruit 1446 patients with a new non-valvular AF diagnosis across four Middle Eastern countries (Egypt, Lebanon, KSA, UAE). The registry will examine patients’ characteristics, patterns of treatment, clinical outcomes, and the economic aspects of treatment [72].
Although our study was conducted using rigorous and systematic search strategy, it is possible that some articles were not identified by our search. The unidentified papers could contribute to a potential bias in the presented results with unknown impact. Moreover, the gap in the time between the literature search and completing results synthesis may have contributed to missing recently published relevant papers. However, we believe that this limitation may be balanced by the meticulousness of our methodology and the included literature.
Although in this scoping study we identified and summarized studies addressing clinical features and management of AF in Middle Eastern Arabs and South Asians, the main body of evidence comes from hospital-based studies. Thus, considered a limited and incomplete evidence. There is, additionally, an evidence gap of knowledge of AF burden that should be addressed in representative community or population-based studies. Our study highlights the need to further examine the effects of ethnicity and race in heart diseases particularly in AF.
Authors AS & RK—Conceptualization, literature search, study selection, data extraction, preparing figures/tables and manuscript writing. Authors AS and SA made substantial contributions to the conception or design of the work; analysis and interpretation of data for the work. Author VS critically revised the paper for important intellectual content and final editing of the version to be published. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
Open Access funding provided by the Qatar National Library.
The authors declare no conflict of interest.
