1 Department of Cardiac Surgery, The Barts Heart Centre, St. Bartholomew’s Hospital, Barts Health NHS Trust, EC1A 7BE London, UK
2 School of Medicine, University of Zagreb, 10000 Zagreb, Croatia
3 Andrija Stampar School of Public Health, 10000 Zagreb, Croatia
4 Department of Cardiac Surgery, University Hospital Centre Rijeka, 51000 Rijeka, Croatia
5 Department of Vascular Surgery, University Hospital Centre Zagreb, 10000 Zagreb, Croatia
6 Polyclinic for Radiology and Neurology “Dijagnostika 2000”, 10000 Zagreb, Croatia
7 Department of Radiology, University Hospital Centre Rijeka, 51000 Rijeka, Croatia
8 School of Medicine, University of Rijeka, 51000 Rijeka, Croatia
9 Department of Vascular Surgery, University Hospital Centre Rijeka, 51000 Rijeka, Croatia
10 Department of Pathology, University Hospital Merkur, 10000 Zagreb, Croatia
11 Department of Diagnostic and Interventional Radiology, University Hospital Merkur, 10000 Zagreb, Croatia
Academic Editor: Peter A. McCullough
Abstract
Components of carotid atherosclerotic plaque can be analysed preoperatively by non-invasive advanced imaging modalities such as magnetic resonance imaging (MRI). The expression of matrix metalloproteinase-9 protein (MMP-9), which has a potential role in remodelling of atherosclerotic plaques, can be analysed immunohistochemically. The aim of the present prospective pilot study is to analyse histological characteristics and expression of MMP-9 in carotid plaques of patients undergoing carotid endarterectomy (CEA) and to investigate the correlation with preoperative clinical symptoms and MRI features. Preoperative clinical assessment, MRI imaging, postoperative histological and immunohistochemical analyses were performed. Fifteen patients with symptomatic (7/15; 47%) and asymptomatic carotid artery stenosis undergoing CEA were included. Among symptomatic patients, 5 (71%) had recent stroke and 2 (29%) had recent transient ischaemic attack with a median timing of 6 weeks (IQR: 1, 18) before the surgery. Both groups did not significantly differ in respect to preoperative characteristics. Prevalence of unstable plaque was higher in symptomatic than asymptomatic patients, although it was not significant (63% vs. 37%, p = 0.077). The expression of MMP-9 in CD68 cells within the plaque by semiquantitative analysis was found to be significantly higher in symptomatic as compared to asymptomatic patients (86% vs. 25% with the highest expression, p = 0.014). The average microvascular density was found to be higher and lipid core area larger among both symptomatic patients and unstable carotid plaque specimens, although this did not reach statistical significance (p = 0.064 and p = 0.132, p = 0.360 and p = 0.569, respectively). Our results demonstrate that MRI is reliable in classifying carotid lesions and differentiating unstable from stable plaques. We have also shown that the expression of MMP-9 is significantly higher among symptomatic patients undergoing CEA.
Trial Registration: This study has been registered at the ISRCTN registry (ID ISRCTN46536832), isrctn.org Identifier: https://www.isrctn.com/ISRCTN46536832.
Keywords
- Atherosclerosis
- Carotid endarterectomy
- Unstable plaque
- Magnetic resonance imaging
- Matrix metalloproteinase
Carotid artery disease is the principal cause of ischemic stroke and the benefit of carotid surgical revascularization in both symptomatic and asymptomatic patients has been demonstrated by several trials [1, 2, 3, 4]. Atherosclerotic plaque formation is associated with endothelial cells damage, which leads to lipid accumulation, immigration of monocyte-derived macrophages, release of growth factors and activation of vascular smooth muscle cells (VSMC) [5]. Atherosclerotic plaque rupture leads to local platelet activation and thrombosis that may result in embolization and the onset of clinical symptoms [6]. Accumulating evidence confirm the key role of matrix metalloproteinase (MMP) in plaque development and pathogenesis of atherosclerosis [7, 8, 9]. MMP, among which is MMP-9, are enzymes that are produced by infiltrating macrophages and VSMC and may have an important role in plaque remodelling and destabilization by promoting the degradation of extracellular matrix [8, 9, 10]. Increased serum levels of MMP-9 have been detected in the coronary circulation among patients with acute coronary syndrome, which suggested active process of plaque rupture and increased risk of cardiovascular adverse events [11, 12, 13]. Similarly, it was found that serum level of MMP-9 was significantly higher in patients with carotid artery stenosis and unstable carotid plaques undergoing carotid endarterectomy (CEA) [8, 14]. However, Baroncini et al. [15, 16] reported that MMP-9 is a part of atherogenesis process but may not be a factor associated with acute disruption events.
Several non-invasive imaging techniques have been evaluated for the characterization of the carotid plaque because carotid angiography is able to detect plaque ulceration but is unable to demonstrate plaque morphology [17]. Despite the fact that duplex ultrasound is the first-line imaging modality, magnetic resonance imaging (MRI) has the advantage to image the aortic arch with supra-aortic arteries as well as to detect the presence of specific plaque features (vulnerable plaque, intra-plaque haemorrhage, lipid-necrotic core) suggesting higher stroke risk [18, 19, 20, 21, 22]. Recent reports suggested that preoperative MRI may be useful in the detection of the vulnerable plaque and can help in identifying patients with high risk of cardiovascular events [19, 20]. Increased MMP-9 expression in plaques with increased neovascular changes along with MRI scan of carotid arteries was studied but this analysis was performed in an animal study model [23].
The aim of the present prospective pilot study is to analyse histological characteristics and expression of MMP-9 in carotid plaques of patients undergoing CEA and to investigate the correlation with preoperative clinical symptoms and MR features.
This pilot study is a prospective observational single-blind study of patients who underwent CEA at the Department of Cardiovascular Surgery in the Magdalena - Clinic for Cardiovascular Diseases and at the Department of Vascular Surgery in the University Hospital Merkur in Croatia over a 3-year study period. Institutional ethic committee approvals and Zagreb University School of Medicine ethic committee approval were obtained, and all patients gave their written informed consent. This study has been registered at the ISRCTN registry with study ID ISRCTN46536832. The study was conducted as per the Helsinki Declaration guidelines [24]. Identity of patients during data collection was anonymised and protected using a unique patient number.
Clinical history taking and physical examination including a neurological exam were performed by a surgeon. Clinical data including history of cardiovascular disease, risk factors for atherosclerosis, neurological event and timing since the most recent one, current medical treatment and computed tomography (CT) scans of brain were recorded. Stroke aetiology was determined based on a work-up including neurological examination, duplex ultrasound, MRI, CT scan of brain and echocardiography if required. Routine biochemical tests and highly sensitive C-reactive protein (hsCRP) were performed preoperatively. Before surgery, all patients underwent a duplex ultrasound plus MRI to grade internal carotid artery (ICA) stenosis and assess the supra-aortic and intracranial arterial system. The severity of carotid artery stenosis was defined using the North American Symptomatic Carotid Endarterectomy Trial Collaborators’ (NASCET) criteria [25]. Inclusion criteria were that the patient should be already scheduled for CEA based on the indication for surgery with the consent to participate in the study. CEA was indicated according to the ESVS guidelines and recommendations of multicentric randomised controlled trials [1, 4, 25, 26, 27]. Exclusion criteria were contraindications to MRI (pacemaker, metallic implant, claustrophobia), presence of suboptimal MRI visualization of the carotid plaque or surgical specimen inadequate for histological or immunohistochemical analysis.
Patients were divided into two groups based on the presence of symptoms and the timing of the most recent symptom. Symptomatic patients were any patients who suffered a carotid stenosis territory symptom within the previous 6 months including ipsilateral stroke, transient ischaemic attack (TIA) or amaurosis fugax. Patients without any history of recent neurological symptoms or with nonspecific, non-hemispheric symptoms such as dizziness or vertigo were considered as asymptomatic.
MRI was performed using a 1.5-Tesla Vantage (Toshiba Medical Systems, Tokyo,
Japan) or Avanto Scanner (Siemens, Erlangen, Germany) with a head and neck coil.
Patient was placed in a standard dorsal position and the coil was combined with a
head holder to stabilize position and prevent head rotation. A software
syngo MR B19 2017 was used. A standardized MRI protocol was used to
obtain four different contrast-weighted images (time-of-flight [TOF] and T1-, PD-
and T2-wighted) of the carotid arteries 2 cm proximal and 2 cm distal to the
carotid bifurcation in axial and coronary planes [22, 28]. Fat suppression was
used to reduce signal from subcutaneous fat tissue. MR angiography of carotid
arteries was performed with 3-dimensional TOF sequence (repetition time [TR]/time
to echo [TE]/flip angle [FA] = 30 ms/8 ms/20
Each carotid lesion was classified using the modified American Heart Association (AHA) classification of carotid plaques identified by MRI [28]. The Modified AHA classification was derived by Cai et al. [28] from experience in reviewing MR images and extensive literature review. In this classification, we used: (I–II) near-normal wall thickness with no calcification (AHA classification types I and II were merged into the type I–II due to a lack of the current resolution of MRI), (III) diffuse intimal thickening or small eccentric plaque with no calcification, (IV–V) plaque with a lipid or necrotic core surrounded by fibrous tissue with possible calcification (for a similar reason, AHA classification types IV and V were merged into the type IV–V), (VI) complex plaque with possible surface defect, haemorrhage or thrombus, (VII) calcified plaque, and (VIII) fibrotic plaque without lipid core and with possible small calcifications. According to this modified classification, types IV–V and VI were classified as unstable plaques, while the others were classified as stable plaques. MRI criteria for the diagnosis of plaque vulnerability were thin fibrous cap, large lipid-rich or necrotic core, and plaque disruption or intra-plaque haemorrhage. MR images were stored in a digital archive as DICOM files and independently analysed by two experienced investigators blinded to the clinical history and histological findings. Any discrepancies were resolved by discussion between the two investigators.
The carotid atherosclerotic plaque was excised during CEA using a standard
surgical technique with minimal and atraumatic manipulation of the specimen
previously described by Sef et al. [29]. The carotid plaque specimen was
obtained immediately after the surgical excision and then stored in fresh 4%
paraformaldehyde solution at 4
The sections were stained with standard haematoxylin-eosin (H&E) and with
Masson method for detecting collagen tissue which will be semiquantitatively
analysed according to the study by Verhoeven et al. [30]. Each specimen
was cut into 5-mm-long sections from the central part of the plaque resulting in
a total of 5 sections for histological analysis. Plaque specimens were analysed
for lipid, fibrous tissue and calcium. Lipid core area was expressed as a
percentage of the total plaque area (TPA) as described previously using the
following semiquantitative scale: 0 =
Immunohistochemical analysis of MMP-9 expression was performed in the central section of the plaque and one of the adjacent sections. Primary antibodies used in the study were: MMP-9 (Leica Mikrosysteme Vertieb GMBH, UK, monoclonal, clone 15W2, 1:50 dilution), CD68 PG-M1 (DakoCytomation, Denmark, monoclonal, clone PG-M1, RTU), smooth mucle actin (SMA) (DakoCytomation, Denmark, monoclonal, clone 1A4, RTU), CD34 (DakoCytomation, Denmark, monoclonal, clone QBend 10, RTU).
Specimen sections were deparaffinized and antigens MMP-9, CD68 PG-M1, CD34 and
SMA were unmasked in PT-module (DakoCytomation, Denmark) at 97
Data were collected prospectively. Categorical variables were tabulated as numbers with percentages. Continuous variables were shown as median values, with corresponding IQR. Analysis of the normal distribution of data (Kolmogorov-Smirnov test) was performed and, according to the results, appropriate non-parametric statistical analysis was performed. Differences among continuous variables were analysed using t-test and Mann-Whitney U test. Differences in categorical variables were analysed using Fisher’s exact test. Difference between the groups was considered significant at the level of 0.05. Statistical analysis was performed using the licensed software IBM SPSS Statistica (version 25.0. www.statsoft.com, StatSoft, Inc. 2017).
In this prospective pilot study, 15 patients with symptomatic (7 patients, 47%) and asymptomatic carotid artery stenosis undergoing CEA were included. Among symptomatic patients, 5 (71%) had recent stroke and 2 (29%) had recent TIA with a median timing of the most recent event of 6 weeks (IQR: 1–18) before the surgery (Table 1). Demographic and clinical characteristics including risk factors, NASCET degree of stenosis, medications and preoperative laboratory results are summarized in Table 1. Symptomatic and asymptomatic patients did not significantly differ in respect to preoperative characteristics. Symptomatic patients had significantly higher hsCRP and D-dimer levels (p = 0.016 and p = 0.036, respectively). Mean NASCET degree of stenosis was higher in the symptomatic as compared to the asymptomatic group (95% [80–95] and 80% [76.3–83.8], p = 0.021, respectively). There was no significant difference in the length of hospitalization and perioperative complications between the two groups, and only one patient in the symptomatic group had an episode of perioperative TIA which has recovered after the surgery.
| Characteristic | Symptomatic (N = 7) | Asymptomatic (N = 8) | p |
| Age, years | 70 (60, 77; 48–77) | 69 (64.3, 72.8; 64–78) | 0.867 |
| Gender, male | 7 (100) | 4 (50) | 0.077 |
| Degree of Stenosis, % | 95 (80, 95; 80–99) | 80 (76.3, 83.8; 70–85) | 0.021 |
| Timing of neurologic symptoms, weeks | 6 (1, 18; 1–20) | 0 | |
| Diabetes mellitus | 4 (57) | 3 (38) | 0.619 |
| Arterial hypertension | 6 (86) | 6 (88) | 0.733 |
| Ischemic heart disease | 3 (43) | 3 (38) | 0.622 |
| Dyslipidaemia | 4 (57) | 6 (75) | 0.608 |
| COPD | 0 (0) | 1 (13) | 0.533 |
| Chronic kidney disease | 2 (29) | 1 (13) | 0.569 |
| Smoking, active | 3 (43) | 1 (13) | 0.386 |
| Smoking, previous | 0 (0) | 2 (25) | |
| Aspirin | 7 (100) | 7 (88) | 0.533 |
| Statins | 4 (57) | 6 (75) | 0.608 |
| Haemoglobin, g/L | 140 (117, 156; 114–162) | 136 (128.3, 142.8; 127–150) | 0.955 |
| Leukocyte count, |
8.7 (7.3, 10.9; 6.2–11) | 7.95 (7, 9.6; 5.3–11.5) | 0.336 |
| C-reactive protein | 13.3 (5.2, 27; 1.5–36.4) | 0.8 (0.4, 1.5; 0.4–1.8) | 0.016 |
| Serum glucose, mmol/L | 5.3 (4.6, 6.8; 4.4–6.8) | 5.45 (5.3, 8.5; 4.9–10.4) | 0.336 |
| PT | 0.805 (0.74, 0.91; 0.72–1) | 0.75 (0.56, 0.92; 0.56–0.92) | 0.548 |
| Fibrinogen, g/L | 5.65 (3.0, 7.5; 2.6–7.6) | 2.9 | 0.800 |
| D-dimer, mg/L FEU | 0.87 (0.61, 1.19; 0.55–1.49) | 0.4 (0.1, 0.46; 0.1–0.46) | 0.036 |
| Total Cholesterol, mmol/L | 4.25 (2.98, 4.7; 2.6–4.8) | 3.65 (2.55, 4.45; 2.2–4.7) | 0.486 |
| LDL Cholesterol, mmol/L | 2.55 (1.15, 3.13; 0.7–3.3) | 2 (1.1, 3.1; 1.1–3.1) | 0.857 |
| HDL Cholesterol, mmol/L | 0.92 (0.74, 1.07; 0.7–1.09) | 0.91 (0.9–1.2) | 0.629 |
| Triglycerides, mmol/L | 1.82 (1.2, 2.1; 1.04–2.2) | 0.8 (0.54, 1.29; 0.45–1.45) | 0.057 |
| Perioperative complications | 1 (14)* | 0 (0) | 0.467 |
| Length of hospitalization, days | 6 (3, 6; 2–8) | 3 (3, 6; 2–6) | 0.281 |
| Data are median (quartiles; minimum-maximum) or count (percent). *Transient ischaemic attack. aPTT, activated partial thromboplastin time; ICA, internal carotid artery; FEU, fibrinogen equivalent units; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PT, prothrombin time. | |||
Carotid plaque features detected on preoperative MRI scan are summarized in Tables 2,3. Prevalence of unstable plaque was higher in symptomatic than asymptomatic patients, although this was not statistically significant (63% vs. 37%, p = 0.077) (Table 2, Fig. 1). The most common type of carotid plaque as per modified AHA classification was type IV–V (10 patients, 65%) (Table 3).
| MR features | p | ||
| Stable | Unstable | ||
| Asymptomatic | 4 (100) | 4 (37) | 0.077 |
| Symptomatic | 0 | 7 (63) | |
| Data are count (percent). | |||
| AHA | n (%) |
| Type I–II: near normal thickness, no calcification | 0 (0) |
| Type III: diffuse intimal thickening or small eccentric plaque with no calcification | 1 (7) |
| Type IV–V: plaque with lipid or necrotic core surrounded by fibrous tissue with possible calcification | 10 (65) |
| Type VI: complex plaque with possible disruption, haemorrhage or thrombus | 1 (7) |
| Type VII: calcified plaque | 2 (14) |
| Type VIII: fibrotic plaque without lipid core and with possible small calcifications | 1 (7) |
| Data are count (percent). | |
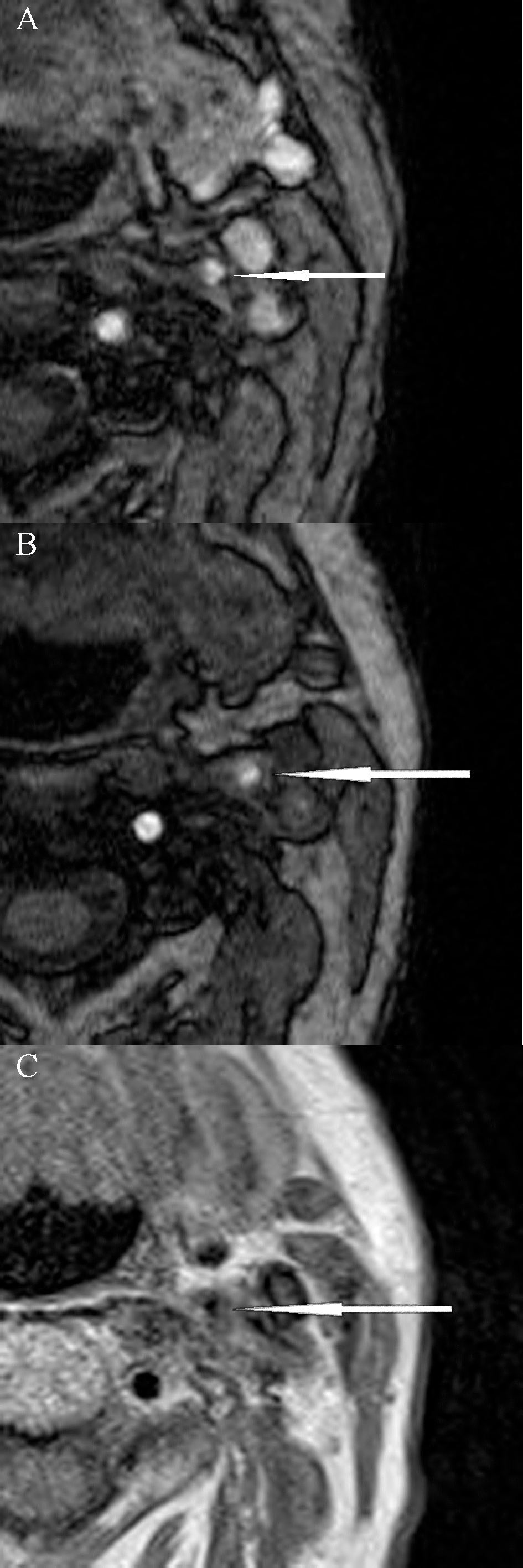
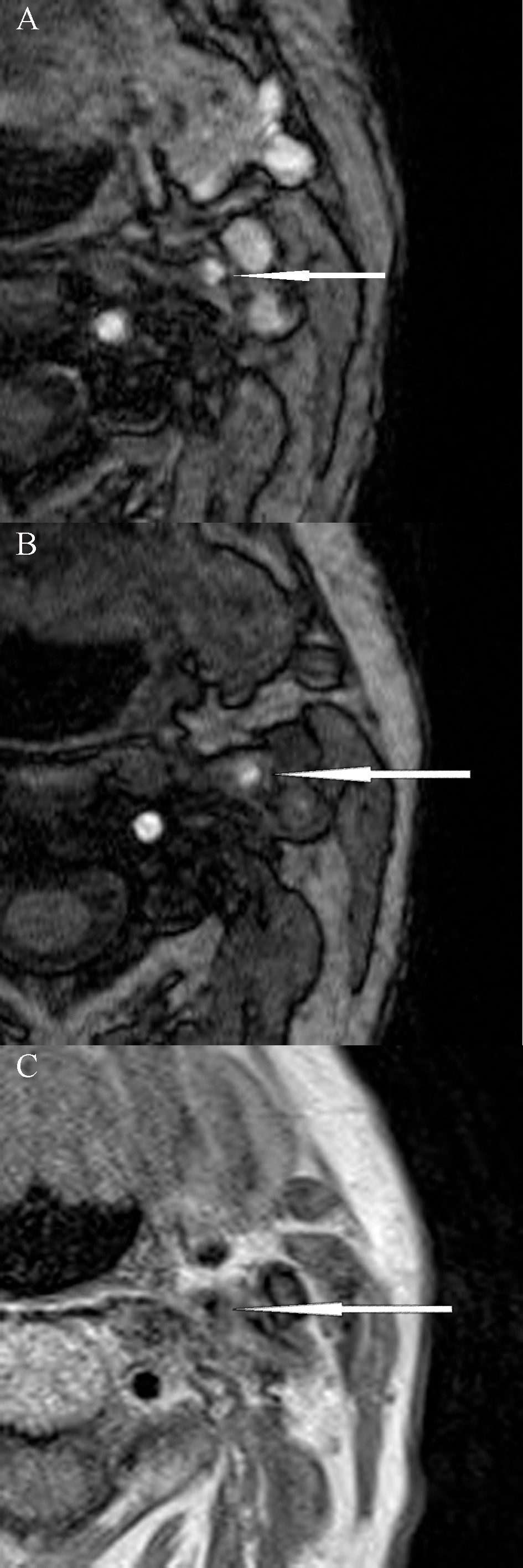 Fig. 1.
Fig. 1.Magnetic resonance imaging of unstable carotid plaque (modified AHA classification type VI) in a patient with symptomatic left internal carotid artery stenosis. (A) and (B) 3D-time of flight (TOF) sequences; (C) Proton density (PD) sequence.
Differences between preoperative symptoms and histological and
immunohistochemistry features of carotid plaques are demonstrated in Table 4. The
expression of MMP-9 in CD68 cells within the carotid plaque was found to be
significantly higher in symptomatic as compared to asymptomatic patients (86%
vs. 25% with the highest expression, p = 0.014). In the symptomatic
group, 6 out of 7 plaque specimens had the expression of MMP-9 in
| Symptoms | p | |||
| Asymptomatic (N = 8) | Symptomatic (N = 7) | |||
| n (%) | n (%) | |||
| MMP-9 CD34 | No expression | 3 (37.5) | 1 (14) | 0.273 |
| 1–25% | 0 (0) | 3 (43) | ||
| 26–50% | 0 (0) | 0 (0) | ||
| 51–75% | 2 (25) | 2 (29) | ||
| 3 (37.5) | 1 (14) | |||
| MMP-9 CD68 | No expression | 0 (0) | 1 (14) | 0.014 |
| Rare or 1 cluster |
4 (50) | 0 (0) | ||
| 2 (25) | 0 (0) | |||
| 2 (25) | 6 (86) | |||
| MMP-9 SMA | No expression | 0 (0) | 1 (13) | 0.143 |
| Rare or 1 cluster |
6 (75) | 2 (29) | ||
| 0 (0) | 2 (29) | |||
| 2 (25) | 2 (29) | |||
| MVD CD34 | n, median (interquartile ranges) | 6 (2.5, 10.0; 2, 15) | 17 (6, 23; 1, 34) | 0.064 |
| lipid core/TPA | 0 (0) | 0 (0) | 0.132 | |
| 10–40% | 6 (75) | 2 (29) | ||
| 2 (25) | 5 (71) | |||
| SMA cells | Low density and no circumference | 6 (75) | 2 (29) | 0.132 |
| High density and 100% circumference | 2 (25) | 5 (71) | ||
| CD68 cells | Low density | 2 (25) | 1 (14) | 1.000 |
| High density | 6 (75) | 6 (86) | ||
| Data are count (percent) unless stated otherwise. | ||||
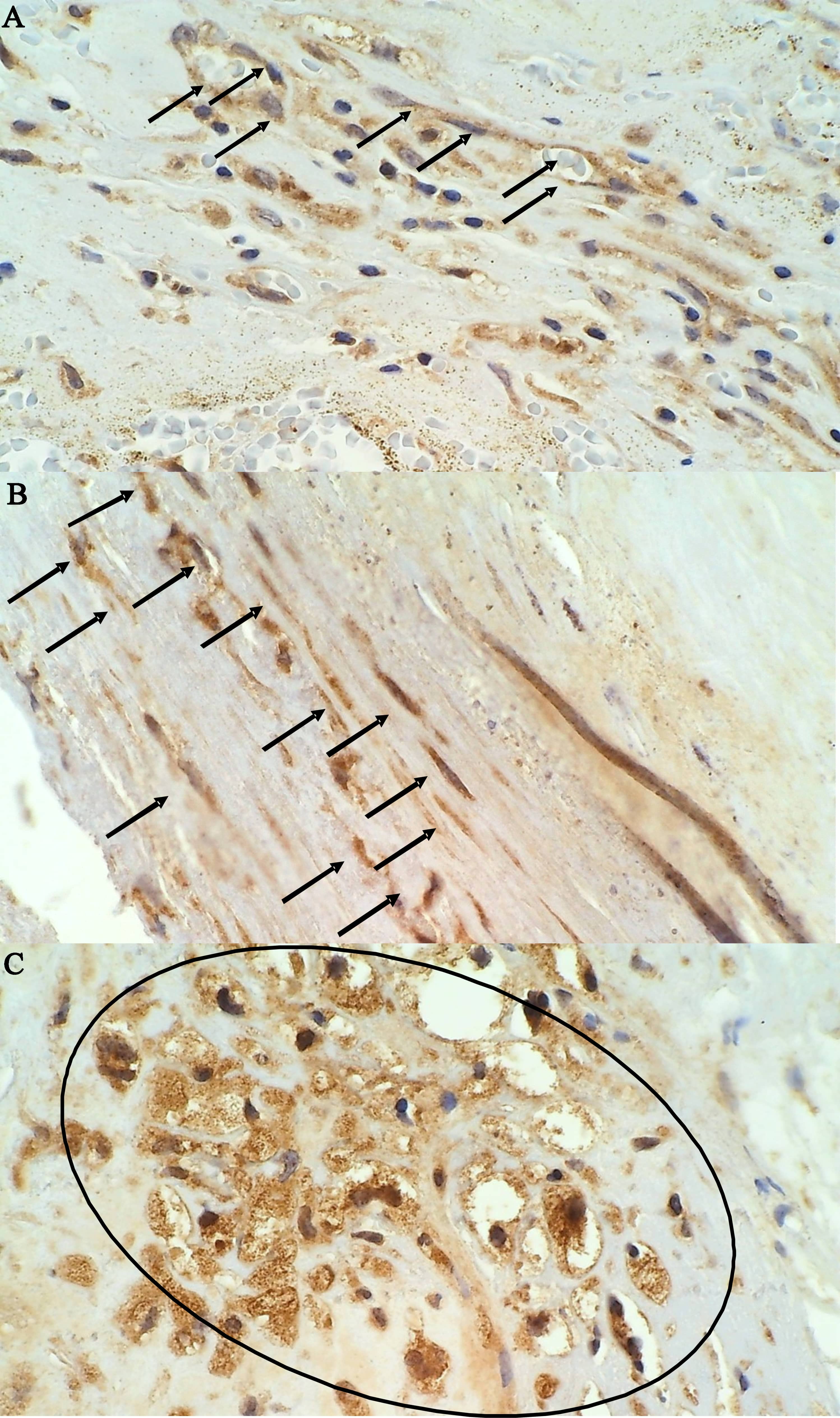
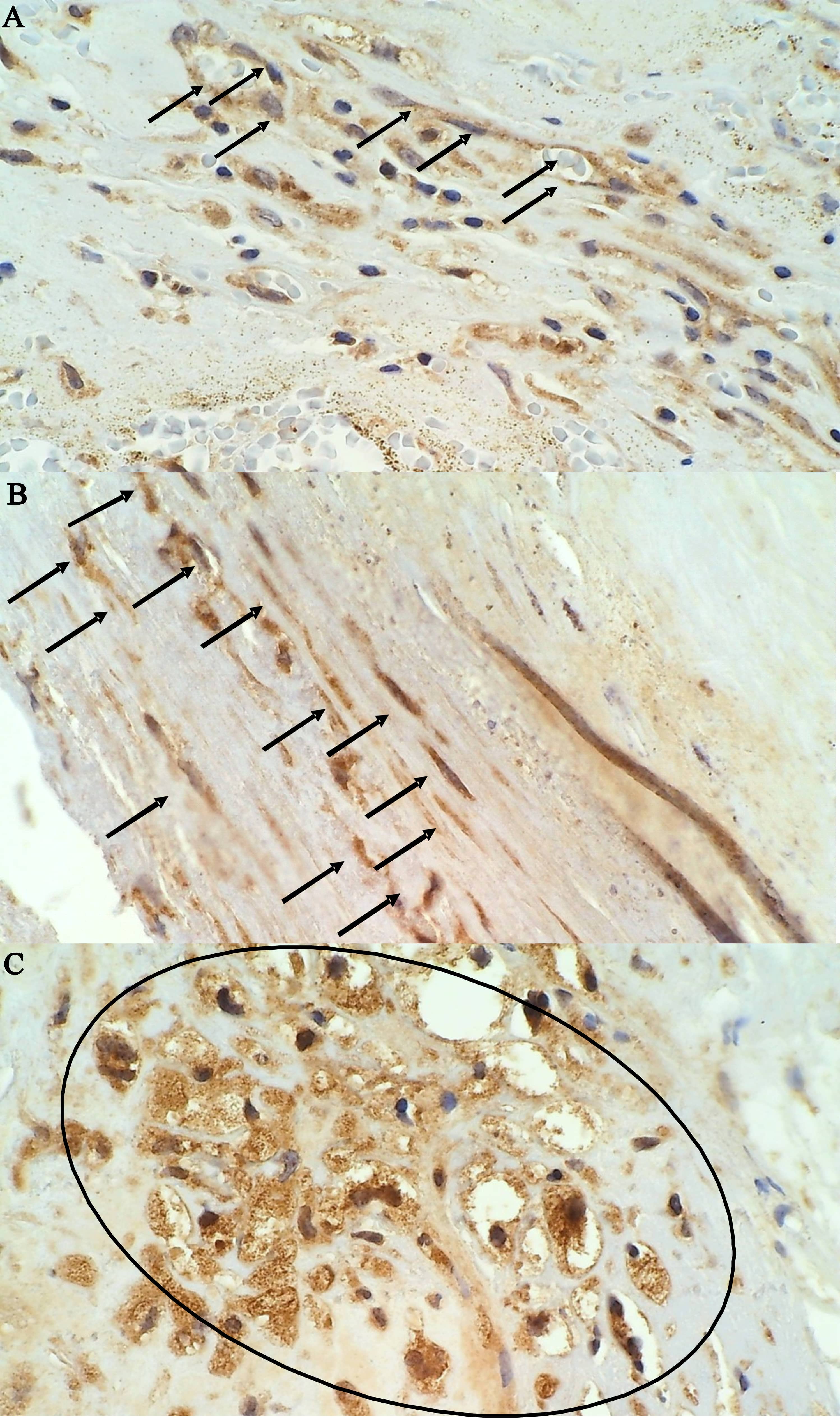 Fig. 2.
Fig. 2.Immunohistochemical staining of MMP-9 positive cells in an
unstable plaque on 400
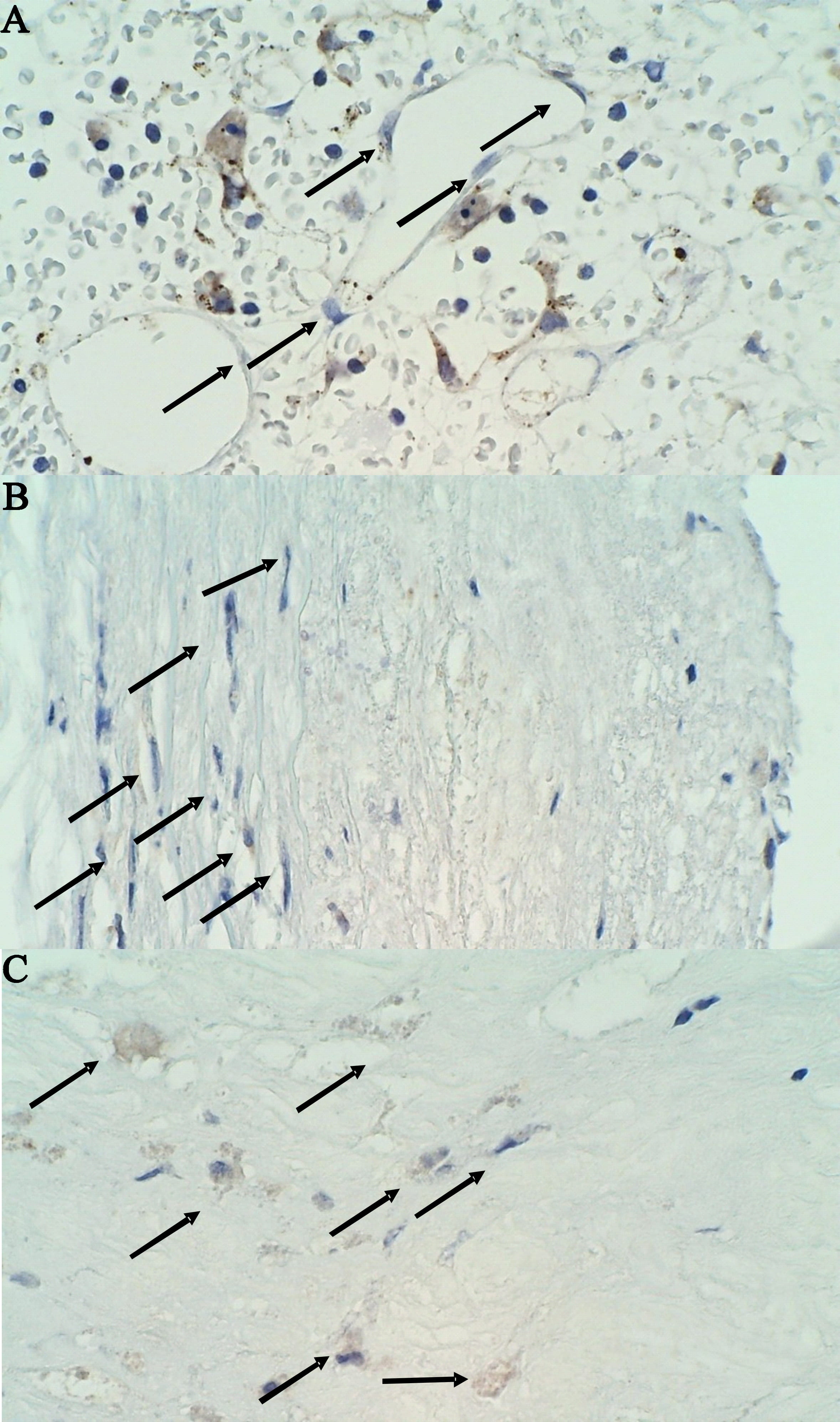
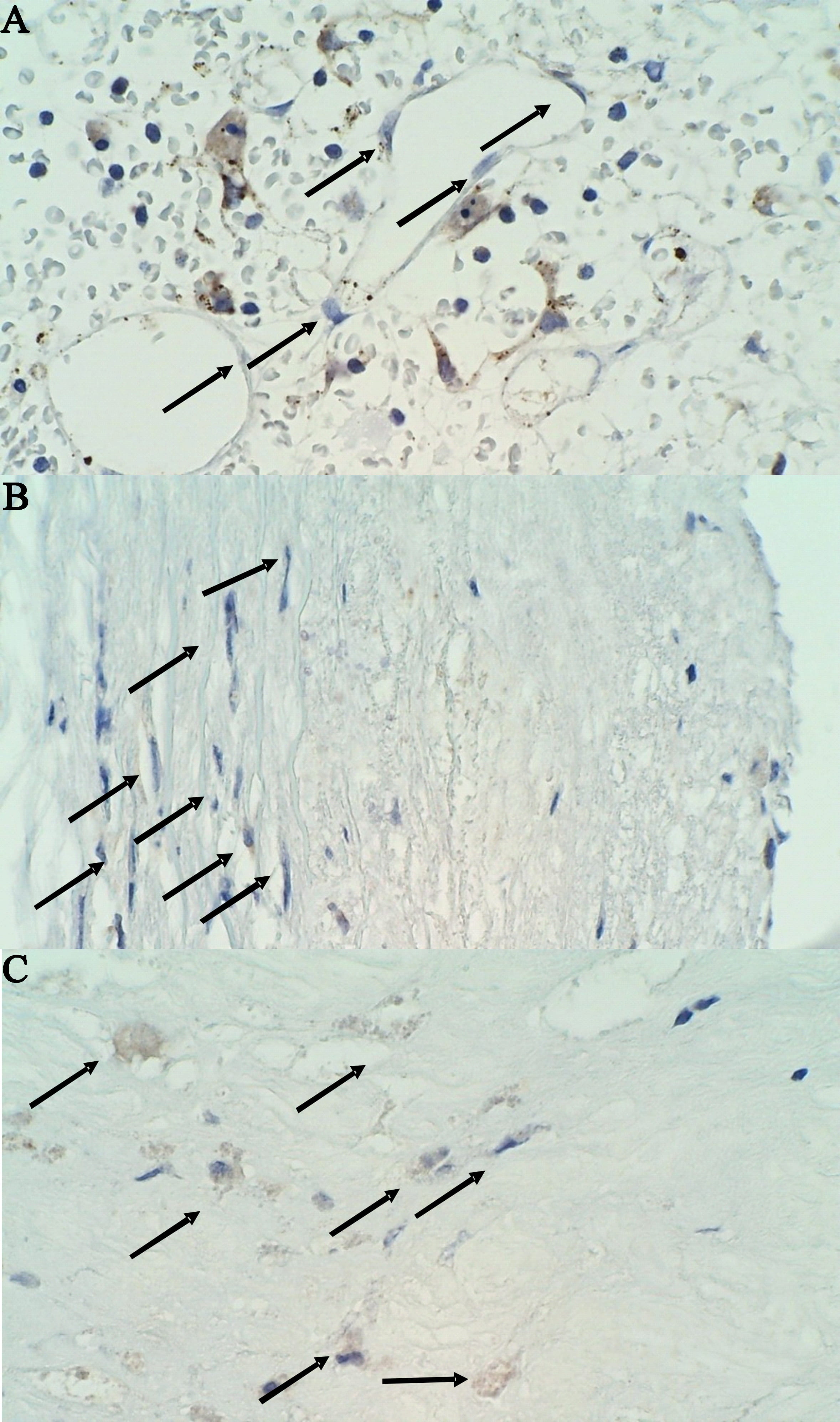 Fig. 3.
Fig. 3.Immunohistochemical staining of MMP-9 negative cells in a stable
plaque on 400
Differences between MRI features of carotid plaques and histological and immunohistochemistry features of carotid plaques are demonstrated in Table 5. There were no significant differences between several histological features and expression of MMP-9 between the patients with unstable or stable carotid plaque detected by preoperative MRI, although both the average MVD by immunohistochemical staining of CD34 and lipid core area were somewhat higher among patients with unstable than stable plaque. However, this was not significant (p = 0.360 and p = 0.569, respectively) (Table 5).
| MR features | p | |||
| Stable (N = 4) | Unstable (N = 11) | |||
| n (%) | n (%) | |||
| MMP-9 CD34 | No expression | 1 (25) | 3 (27) | 0.859 |
| 1–25% | 0 (0) | 3 (27) | ||
| 26–50% | 0 (0) | 0 (0) | ||
| 51–75% | 1 (25) | 3 (27) | ||
| 2 (50) | 2 (18) | |||
| MMP-9 CD68 | No expression | 0 (0) | 1 (9) | 1.000 |
| Rare or 1 cluster |
1 (25) | 3 (27) | ||
| 1 (25) | 1 (9) | |||
| 2 (50) | 6 (55) | |||
| MMP-9 SMA | No expression | 0 (0) | 1 (9) | 0.671 |
| Rare or 1 cluster |
2 (50) | 6 (55) | ||
| 0 (0) | 2 (18) | |||
| 2 (50) | 2 (18) | |||
| MVD CD34 | n, median (interquartile ranges) | 7 (2, 13.75; 2, 15) | 10 (5, 19; 1, 34) | 0.360 |
| lipid core/TPA | 0 (0) | 0% | 0.569 | |
| 10–40% | 3 (75) | 5 (46) | ||
| 1 (25) | 6 (54) | |||
| SMA cells | Low density and no circumference | 2 (50) | 6 (54) | 1.000 |
| High density and 100% circumference | 2 (50) | 5 (46) | ||
| CD68 cells | Low density | 1 (25) | 2 (18) | 1.000 |
| High density | 3 (75) | 9 (82) | ||
| Data are count (percent) unless stated otherwise. | ||||
The atherosclerotic plaque is a dynamic lesion that undergoes continuous remodelling of the extracellular matrix on which its structural stability depends. Previous studies demonstrate that acute changes within the carotid plaque, such as IPH, fibrous cap rupture or ulceration can lead to the onset of ischemic cardiovascular events [6]. Recent reports suggest that MMP-9 may have a key role in all stages of the atherosclerotic process during which are being secreted by inflammatory cells and VSMC, although there is still lack of evidence whether MMP-9 can affect the incidence of adverse events [7, 8, 34].
The present pilot trial has demonstrated that the prevalence of unstable plaque was higher in symptomatic than asymptomatic patients, although this was not statistically significant (63% vs. 37%, p = 0.077). Our main findings were that the expression of MMP-9 in CD68 cells within the carotid plaque was found to be significantly higher in symptomatic as compared to asymptomatic patients (86% vs. 25% with the highest expression, p = 0.014). Importantly, the average MVD by immunohistochemical staining of CD34 was found to be higher and lipid core area larger among both symptomatic patients and unstable carotid plaque specimens, although this did not reach statistical significance (p = 0.064 and p = 0.132, p = 0.360 and p = 0.569, respectively). Our study demonstrates the ability of MRI to identify patients with high-risk carotid plaque and to differentiate better which patients could benefit from early surgical revascularization. These findings are in line with the results of other studies and help in understanding that MRI can be the most accurate advanced imaging modality in distinguishing vulnerable from stable carotid plaques preoperatively, although further large prospective comparative studies are still required [7, 19, 20, 28, 35, 36]. Importantly, the most recent ESVS guidelines and recommendations of multicentric randomised controlled trials highlight clinical/imaging features in asymptomatic patients associated with an increased risk of stroke despite the best medical treatment that indicate necessity for carotid revascularisation [1, 4, 25, 26, 27]. A recent report comparing carotid MRI, ultrasound and histological findings of carotid plaque specimens has demonstrated that the findings of carotid MRI and ultrasound are closely associated with specimen morphology, but ultrasound alone is insufficient to detect the type of lesion accurately [36]. In addition, we have found that the most common type of carotid plaque as per modified AHA classification by MRI was type IV–V (10 patients, 65%), while type VI was the most common (89 out of 252 plaque specimens) in the report by Cai et al. [28]. Similarly, Million et al. [19] have reported the ability of MRI to identify high-risk carotid plaque and to differentiate symptomatic from asymptomatic patients, although their study was performed using a 3-Tesla system.
Furthermore, our results showed that MMP-9 may play a key role in remodelling and destabilization of the carotid plaque. As we hypothesized, the expression of MMP-9 in CD68 cells was significantly higher among symptomatic patients. Similarly, it was also higher in SMA cells in symptomatic patients, but this did not reach statistical difference (p = 0.143). Interestingly, symptomatic patients had significantly higher preoperative hsCRP which is in accordance with the inflammatory response and infiltration related to the destabilization of the plaque and potential increased release of MMP-9. Similarly, recent reports showed higher expression levels of MMP-9 obtained from symptomatic when compared to asymptomatic patients [8, 37, 38]. These findings highlighted MMP-9 as one of the most important enzymes related to the plaque instability. On the other hand, Baroncini et al. [15, 16] reported higher MMP-9 concentration in normal tissues and in asymptomatic patients suggesting that MMP-9 is a part of the atherosclerotic process although not associated to acute plaque disruption. Still, this finding remains part of debate as Heo et al. [38] have reported that MMP-9 is significantly associated with plaque rupture. However, recent evidence suggests that an increase in proteolytic activity may lead to an overall increase in matrix degradation which could explain our results which demonstrate that the average MVD could be higher and lipid core area larger among both symptomatic patients and unstable carotid plaque specimens.
There are several limitations to our study. Our results showed a higher prevalence of unstable plaque in symptomatic patients, and a higher average MVD and larger lipid core area among both symptomatic patients and unstable carotid plaque specimens. However, the differences did not reach statistical significance probably because of the lack of power of the study and smaller sample size which are limitations of the present pilot study. Cappendijk et al. [39] also failed to demonstrate a significant difference between symptomatic and asymptomatic patients. However, in times of constraints on resources, both additional MRI analysis and detailed histologic work-up of plaques is costly and time-consuming, thus explaining the necessity of conducting a pilot trial. Although we obtained the best possible resolution for carotid MRI, another limitation of the study could be related to the use of only non-contrast MRI and motion artefacts. Further improvement in coil design, use of 3-Tesla system and modification of imaging parameters could be required, while the use of contrast-enhanced MRI would be less justified in terms of routine preoperative diagnostic work-up. Another limitation is that we have analysed our results semiquantitatively by immunohistochemistry, and the results do not allow accurate quantification. Nevertheless, our estimates were performed independently by two investigators. In addition, it is also possible that we have excluded some regions of plaques where inflammation was more advanced.
In this pilot study, we demonstrated that carotid MRI can be reliable in classifying carotid lesions and differentiating unstable from stable plaques. Our initial results showed that the expression of MMP-9 is significantly higher among symptomatic patients undergoing CEA which suggest that MMP-9 may affect remodelling and destabilization of the carotid plaque among symptomatic patients. This study could help in defining discriminative ability of preoperative MRI, and in improving identification of patients with unstable plaque that have an indication for CEA. However, further study will be anticipated with more participants being recruited over a longer study period. Further prospective and randomized studies are needed to provide better understanding of the mechanisms by which MMP-9 can affect atherosclerotic plaque structure and remodelling.
DS conceived and designed the research study, and prepared the original draft; DS, MM, MO, BJ, SK, AS, VV provided help on investigation, methodology, data collection, validation, and analyzed the data; MM performed statistical analysis; DS, MM, MO, TM, BJ, SK, MK, AS, VV reviewed and edited the draft. All authors read and approved the final manuscript.
All patients gave written informed consent, and relevant Institutional Review Boards approved this study.
We would like to express our gratitude to Juergen Falkensammer who helped with designing the research study. Support from Inel – Medical Technologies company by providing antibodies useful for this research project is greatly appreciated. Thanks to all the peer reviewers for their opinions and suggestions.
Dr. Davorin Sef has received Leica MMP9-439 antibodies from Inel – Medical Technologies company.
The authors declare no conflict of interest.