†These authors contributed equally.
Academic Editor: Alpo Vuorio
Although immunization with the 2019 coronavirus disease (COVID-19) mRNA vaccine is considered to be an effective measure to reduce the number of serious cases or deaths associated with COVID-19, rare cases of cardiac complications have been reported in the literature, encompassing acute myocardial injury, arrhythmia, vasculitis, endothelial dysfunction, thrombotic myocardial infarction and myocarditis. Interestingly, patients diagnosed with myocarditis after receiving the COVID-19 mRNA vaccine exhibit abnormal cardiac magnetic resonance (CMR) findings, suggesting CMR can be a valuable non-invasive diagnostic tool. In populations immunized with the COVID-19 mRNA vaccine, the risk in teenagers and young men is significantly higher. Myocardial injury in male patients is mainly myocarditis, while in female patients, myocarditis and pericardial effusion are predominantly found. Generally, the symptoms of myocarditis are relatively mild and complete recovery can be achieved. Moreover, the incidence rate associated with the second dose is significantly higher than with the first or third dose. This article brings together the latest evidence on CMR characteristics, influencing factors and pathogenesis of myocarditis caused by the COVID-19 mRNA vaccine. At the same time, we make recommendations for populations requiring immunization with the COVID-19 mRNA vaccine.
As of June 2022, more than 530 million patients with the 2019 coronavirus disease (COVID-19) novel coronavirus have been confirmed worldwide, and the death toll has exceeded 6 million [1]. Mahmood et al. [2] reported that treatment with Ramdesivir and convalescent plasma (CP) is the best treatment to combat COVID-19. COVID-19 mRNA vaccine has a positive effect on preventing coronavirus infection. Dagan et al. [3] reported that the study of large-scale COVID-19 mRNA vaccination in Israel showed that COVID-19 mRNA vaccine could effectively prevent COVID-19 infection and reduce COVID-19 severe patients [3, 4, 5]. However, the COVID-19 mRNA vaccine has also been associated with an increased incidence of relatively rare diseases, such as myocardial injury, myocarditis thrombosis, tubulitis, macrovasculitis and Kawasaki disease [5, 6, 7, 8, 9, 10, 11, 12, 13, 14]. It has been established that the COVID-19 mRNA vaccine produces antibodies to S protein through mRNA and membrane s glycoprotein, prevents the binding of S protein with angiotensin converting enzyme2 (ACE2), and produces cellular immunity and humoral immunity, which eventually leads to myocarditis [15]. COVID-19 mRNA vaccine-related myocardial injury has been widely reported, and cardiac magnetic resonance cardiac magnetic resonance (CMR) is an important diagnostic tool for evaluating myocardial structural and functional changes [16]. The American College of Cardiology and the Cardiovascular Magnetic Resonance Association advocates that CMR is a valuable diagnostic tool for COVID-19 patients with incomplete evidence of myocardial tissue composition, myocardial injury and cardiac function decline [16, 17]. Shiyovich et al. [18] showed that CMR has a high diagnostic performance in diagnosing and evaluating myocarditis caused by COVID-19 vaccine treatment, especially in patients with good ejection function; CMR imaging findings are consistent with “typical myocarditis”. This review sought to provide a comprehensive overview of the imaging characteristics of CMR in the diagnosis and evaluation of patients with myocarditis caused by COVID-19 mRNA vaccine treatment and analyze the influencing factors and potential pathogenesis of myocarditis. Finally, we made some suggestions for immunization with the COVID-19 mRNA vaccine.
Overwhelming evidence substantiates that the immunogenicity of the COVID-19 mRNA
vaccine can trigger many rare cardiovascular and blood disease reactions,
including myocarditis, pericardial effusion, myocardial infarction, atypical
Kawasaki disease, arterial thrombosis, cutaneous small vessel vasculitis, large
vessel vasculitis, etc. [7, 8, 9, 10, 11, 12, 14]. In a study on 27 patients with cardiac
inflammation caused by COVID-19 vaccine, most complained of chest pain,
palpitations, joint pain and dyspnea, exhibiting elevated cardiac troponin I (HS
cTnI) levels, and 77.8% of patients had ST-segment elevation or T wave inversion
in electrocardiogram (ECG) (Table 1a,1b, Ref. [5, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37]). Amir et al. [20] retrospectively collected 15 cases of
myocarditis related to the BNT162b2 mRNA COVID-19 vaccine in five major
children’s medical centers in Israel. The majority of patients were male,
exhibiting symptoms and signs of myocardial involvement (such as chest pain and
arrhythmia), 93.3% of patients had elevated troponin levels, 13.3% of patients
had pericardial effusion, 20% of patients had ventricular dysfunction, and
86.7% of patients had nonspecific ST/T changes in ECG [20]. Puchalski et
al. [21] retrospectively analyzed five adolescents with body mass index (BMI) values of 24.8 to 30
(4 obese and 1 overweight) vaccinated with Pfizer mRNA vaccine, which exhibited
retrosternal chest pain (n = 5), elevated body temperature (n = 4), diarrhea and
shoulder pain (n = 1), and dry cough (n = 1). Troponin levels were significantly
increased in all cases and decreased rapidly a few days later. Echocardiography
showed that the left ventricular ejection fraction ranged from 61 to 72% [21].
Six cases of myocarditis were reported out of nearly 200000 citizens vaccinated
with the mRNA COVID-19 vaccine in Italy. All six patients were hospitalized due
to fever and elevated troponin and were treated with colchicine and ibuprofen.
One patient exhibited atrial tachycardia, and another showed right ventricular
involvement. Only a female patient was diagnosed with myocarditis and pericardial
effusion. The median high-sensitivity troponin and C-reactive protein (CRP) levels in these 6 patients
were 2373 ng/mL and 4
| First Author (Ref. #) Study Design | Country | Number of vaccinations | Number of Cases | Men | Age, ya |
Type of vaccination | COVID-19 vaccine doses prior to symptom onset | Time (days) from vaccine inoculation to symptoms | Patient characteristics during acute myocarditis (Clinical manifestation and laboratory examination) | Patient characteristics during the postacute stage |
| Mohammadi et al. [36] Retrospective observational study | Iran | No symptoms | 1 | 1 | 20 | AstraZeneca | 3 | 4 | Severe chest pain, Troponin I = 3.34 | No symptoms |
| Dedda et al. [19] Retrospective observationalstudy | Europe | No symptoms | 27 | 25/27 | 36.6 |
Pfizer/BioNTech/ Moderna/AstraZeneca | 1 (n = 27), 2 (n = 15) | n = 22/27; average 8 |
Chest pain (n = 25), palpitations (n = 10), arthralgias and myalgias (n = 9), and dyspnea (n = 7), (n = 27) cases (HS cTnT) or (HS cTnI) were elevated | Short-term follow-up from presentation was uneventful for 25/27 patients (median = 20 days; range = 2–82 days) and unavailable in two cases. |
| Bae et al. [24] Retrospective observational study | Korea | No symptoms | 1 | 0 | 38 | mRNA1273 (Moderna) | 1 | 4 | Chest pain, mild dyspnea, and sweating, CK-MB, ng/mL ( |
Not reported |
| Amir et al. [20] Retrospective observational study | Israe | 224000 | 15 | 15 | 17 |
BNT162b2 | 2 (n = 14/15), 3 (n = 1/15) | 4.4 |
Clinical manifestation Not reported, (14/15) patients had elevated troponin T levels | After 6 months, clinical symptoms were resolved in all patients, and one patient exhibited mild pericardial effusion. Individuals with preexisting CAD or myocarditis had abnormal ECG findings |
| Oka et al. [33] Retrospective observational study | Japan | No symptoms | 1 | 1 | 50 | BNT162b2 | 2 | 10 | Syncope and resting chest pain; ST-segment elevation on ECG and significantly increased Cardiac troponin I | At 2 weeks after discharge, syncope, heart failure, ECG atrioventricular block, echocardiographic LVEF was 60%, and cardiac troponin I level increased slightly. |
| Christophe et al. [31] Retrospective observational study | Switzerland | 93968 | 3 | 3 | 28.7 |
mRNA-1273/BNT162b2 | 2 (n = 3) | 2.3 |
All hospitalized (100%, n = 3) patients had mild to moderate symptoms On admission, 100% (3/3) patients had troponin elevation, and 100% (n = 3) had ECG abnormalities | Not reported |
| Das et al. [32] Retrospective observational study | The United Arab Emirates | No symptoms | 1 | 1 | 27 | Pfizer/BioNTech | 2 | 3 | severe chest discomfort, patients had troponin elevation, ECG abnormalities | Not reported |
| Ansari et al. [37] Retrospective observational study | Germany | No symptoms | 1 | 1 | 23 | mRNA1273 (Moderna) | 2 | 1 | On admission, angina pectoris, the ECG was abnormal, the symptoms were serious, and the level of high-sensitivity troponin I increased | Patient asymptomatic |
| Nunn et al. [23] Retrospective observational study | Germany | No symptoms | 4 | 3 | 29.5 |
Pfizer/BioNTech | 2 (n = 3/4) | 7.5 |
On admission, 75% (3/4) of patients had mild symptoms, and 125% (1/4) had moderate to severe symptoms. 4 patients had troponin elevation | Not reported |
| Puchalski et al. [21] Retrospective observational study | Poland | No symptoms | 5 | 5 | 16.6 |
Pfizer/BioNTech | 1 (n = 2/5), 2 (n = 3/5) | 6.4 |
100% (5/5) of patients chest pain, 100% (5/5) of patients Increased troponin levels | Three months later (1 patient with a follow-up appointment postponed for one month due to moderate infectious symptoms), 1 patient reported a single episode of sharp chest pain lasting for a few seconds |
| Frustaci et al. [25] Retrospective observational study | Italy | 100000 | 3 | 2 | 56.3 |
BNT162b2 | 2 (n = 3/3) | Not reported | 100% (n = 3/3) patients chest pain , beyond elevation of troponin I (3.5 |
Not reported |
| Shaw et al. [34] Retrospective observational study | USA | No symptoms | 4 | 2 | 22.0 |
Pfizer/Moderna | 1 (n = 2/4), 2 (2/4) | 8.8 |
100% (n = 4/4) had chest pain and elevated troponin I (n = 4/4) | Not reported |
| Manfredi et al. [22] Retrospective observational study | Italy | 231989 | 6 | 4 | 17.5 |
Pfizer/BioNTech and Moderna | 2 (n = 6/6) | Not reported | 100% (n = 6/6) fever ,The median high-sensitive Troponin-I (Hs-TnI) was 2373 (Q1, Q3: 576, 8123) ng/mL | 3.0 |
| Gomes et al. [35] Retrospective observational study | Portugal | No symptoms | 1 | 1 | 32 | SARS-CoV-2 mRNA | 2 | 2 | Syncope and chest pain, Myocardial biomarkers (high-sensitivity cardiac troponin T 834 ng/L and NT proBNP 433 mg/mL) increased | After discharge, epicardial involvement during late gadolinium enhancement was significantly improved, and T1 and T2 normalized |
| Meyer-Szary et al. [28] Retrospective observational study | Poland | No symptoms | 3 | 3 | 19.3 |
Spikevax Moderna Comiranty | 2 (n = 3/3) | 1.7 |
Elevated troponin I, 100% (n = 3/3) Severe stinging chest pain | Not reported |
| Kravchenko et al. [27] Retrospective observational study | Germany | No symptoms | 20 | 12 | 28 |
Pfizer/BioNTech or Moderna | 1 (n = 5/20), 2 (n = 15/20) | 1.1 |
85% of patients (17/20) had chest pain, 55% of patients (11/20) had dyspnea, and 10% of patients (2/20) had a fever, troponin T levels (3938 |
Not reported |
| Patel et al. [30] Retrospective observational study | USA | No symptoms | 5 | 5 | 24.6 |
Pfizer/Moderna | 1 (n = 1/5), 2 (n = 4/5) | 1.8 |
Chest pain and elevated troponin I in 100% (n = 5/5), dyspnea in 60% (n = 3/5) | Not reported |
| Chelala et al. [29] Retrospective observational study | USA | No symptoms | 5 | 5 | 17.2 |
Pfizer-BioNTech/Moderna | 2 (n = 5/5) | 3.6 |
Chest pain and elevated troponin I in 100% (n = 5/5) of patients and abnormal ECG in 40% (n = 2/5) | Not reported |
| Choi et al. [26] Retrospective observational study | Korea | No symptoms | 1 | 1 | 22 | BNT162b2 mRNA | 1 | 22 | Chest pain, ventricular fibrillation, laboratory examination not reported | Not reported |
| First Author (Ref. #) Study Design | Histopathological evidence | LGE | Myocardial parametric mapping | LV/RV structure and function, pericardial disease |
| Mohammadi et al. [36] Retrospective observational study | Not reported | subepicardial/mid-wall enhancement in the basal inferior and anterior apical segments of LV | subepicardial/mid-wall enhancement in the basal inferior and anterior apical segments of LV STIR T2WI (increased signal intensity in the inferior basal segment; myocardial inflammation | Echocardiography and cardiac troponin were normal, without any symptoms |
| Dedda et al. [19] Retrospective observational study | Not reported | 85% (n = 23) patients had LGE and T2 enhancement | CMR revealed typical mid-subepicardial nonischemic late gadolinium enhancement (LGE) in 23 cases and matched positively with CMR T2 criteria of myocarditis. | Not reported |
| Bae et al. [24] Retrospective observational study | A small number of eosinophils, T lymphocytes and macrophages | The patient had LGE and elevated T2 | LGE in the left anterior inferior septum and the middle of the left anterior inferior septum. T2 left ventricular basal wall to middle wall high signal | Normal left and right ventricular functions, no regional wall motion abnormalities, normal diastolic function, normal ejection fraction of 67%, right ventricular systolic pressure of 29 mmHg |
| Amir et al. [20] Retrospective observational study | Not reported | 26% (n = 4) of patients had elevated T2, and 93% (n = 14) of patients had LGE. | 26% (n = 4) of patients had elevated T2, 93% (n = 14) of patients had LGE. The subepicardial layer in the mid-myocardial region of the left ventricle was involved in 100% (n = 15) of patients | Not reported |
| Oka et al. [33] Retrospective observational study | A small number of eosinophils, T lymphocytes and macrophages | Elevated T2 and positive LGE | Patient had LGE and elevated T2 | The patient was discharged 22 days after admission; the echocardiography showed recovery of the LVEF to 60% |
| Christophe et al. [31] Retrospective observational study | Not reported | 100% (n = 3) of patients had positive LGE | 3 patients had LGE | Not reported |
| Das et al. [32] Retrospective observational study | Not reported | The patient had LGE and elevated T2 | Patient had both elevated T2 and positive LGE in the LV’s basal and mid-anterolateral, posterolateral, and inferoseptal segments. | Patient baseline values for LV GLS (−14.55), RV GLS (−15.8), and RVCS were all considerably lower (−6.88). |
| Ansari et al. [37] Retrospective observational study | Not reported | The patient had LGE and elevated T1 | Native T1 maps revealed a diffuse increase in relaxation times in all myocardial segments [1,344 |
A follow-up CMR performed after 3 months revealed a markedly improved LVEF (57%) |
| Nunn et al. [23] Retrospective observational study | Acellular myocarditis | 50% (n = 2) of patients had had hyperenhancement on their T2 and T1 sequences 100% (n = 4) of patients had LGE | 4 patients had LGE, and 2 patients had elevated T2 | LVEDVI was greater than 70 (81 |
| Puchalski et al. [21] Retrospective observational study | Not reported | Subepicardial, subepicardial and intraventricular LGE in segments and elevated T2. | 5 patients had LGE and elevated T2. | Echocardiography %EF of 5 patients: 64, 72, 61, 62, 68 |
| Frustaci et al. [25] Retrospective observational study | Strong infiltration of eosinophils | CMR showed three patients increase in both T2 and T1 myocardial, and LGE was present in the subepicardial myocardium. | 3 patients had LGE and elevated T1 and T2. | Two males showed severe compromise of myocardial contractility (left ventricular ejection fraction |
| Shaw et al. [34] Retrospective observational study | Not reported | 100% (n = 4) of patients had elevated T2. 100% (n = 4) of patients had LGE | 4 patients had LGE and elevated T2. 4 patients T1 (1111 MS, 1117 ms-1137 MS, 1122 ms-1128 MS, 1172 MS are greater than the normal range 950 ms-1050 MS) | 1 patient had mildly decreased systolic function (LVEF 1/4 54%), and 3 patients had a normal systolic function |
| Manfredi et al. [22] Retrospective observational study | Not reported | 100% (n = 6) of patients had LGE | Myocarditis was present in males (65% (n = 4/6)) and characterized by myocardial edema (T2w hyperenhancement) and LGE in females was predominantly myopericarditis (30% (n = 2/6)). | LVEDV (68.8 |
| Gomes et al. [35] Retrospective observational study | Not reported | Patient had LGE, elevated T1 and T2 | The delayed enhancement of epicardial gadolinium in the middle anterior wall, lateral wall and inferior wall and the increase of natural T1 and T2 | The left ventricular ejection fraction remained unchanged (58%), the segmental contractility was normal, but the overall longitudinal strain decreased slightly (–17%) |
| Meyer-Szary et al. [28] Retrospective observational study | Not reported | Patients had LGE, elevated T1 and T2 | Increased T2 and T1 relaxation times in parametric mapping and a matching late gadolinium enhancement (LGE) area suggestive of irreversible damage | The LVEF (%) of the three patients were 65%, 58% and 63%, respectively |
| Kravchenko et al. [27] Retrospective observational study | Not reported | The T2 signal intensity of LLC-positive patients increased, and the incidence of LGE was higher. | Compared with the control group (1.6 |
Cardiac MRI parameters (LVEF, p = 0.34), (LVEDV, p = 0.34), left ventricular diastolic (LVEDVI, p = 0.05), (IVSD, p = 0.37) (ECV, p = 0.23) were compared, and there was no difference between groups |
| Patel et al. [30] Retrospective observational study | Not reported | Patients had LGE, elevated T1 and T2 | Late gadolinium enhancement on 100% (5/5) T1 weighted images and 60% (3/5) T2 weighted hyperintensity of myocardial edema | 100% (5/5) CMR LVEF is normal |
| Chelala et al. [29] Retrospective observational study | Not reported | Patients had LGE and EGE, elevated T2 | 4 patients had Ege and LGE. Ege and LGE mainly affect the epicardium of the inferior wall, inferior lateral wall, etc. | 20% (1/5) LVEF decreased,80% (4/5) LVEF is normal |
| Choi et al. [26] Retrospective observational study | Mainly neutrophils | Not reported (Death) | Not reported (Death) | Not reported (Death) |
COVID-19 disease and COVID-19 mRNA vaccine immunization have been associated
with myocarditis; however, significant heterogeneity surrounds the degree of
myocardial lesions and histopathology. The following cases can support this view.
COVID-19 induced myocarditis is also considered to be a relatively rare disease.
Macrophage and T cell infiltrations have been documented in the dead patients
with myocarditis and autopsy and the analysis of samples stained with eosin
methylene blue (EMB). Myocarditis involving macrophage and T cell infiltration
were also observed in the non-infectious death (control group) and COVID-19
cases. However, the infiltration degree in each condition is different, and in
both cases, these findings do not represent clinically relevant myocarditis. In
addition, in SARS-CoV-2 patients, myocardial tissue cells exhibit significant
macrophage inflammatory infiltration, which is related to the viral lymphoid
effect [39]. Kawakami performed an autopsy of the myocardium in 16 patients who
died of SARS-CoV-2 infection. In one case, myocarditis with macrophage and T cell
infiltration was found [39]. Bae et al. [24] reported that a 38-year-old
female was diagnosed with myocarditis 4 days after receiving the mrna-1273
vaccine (Moderna). After ventricular septal tissue sections were harvested,
hematoxylin-eosin staining and immunohistochemical staining for leukocyte common
antigen (LCA) were performed. Lymphocytic infiltration was found in muscle fibers
and stroma [24]. A 50-year-old man was diagnosed with myocarditis after
vaccination, presenting with chest pain with tachycardia and ST-segment elevation
observed on the ECG. Laboratory examination showed that cardiac troponin I was
significantly increased, the left ventricular ejection fraction (LVEF) on cardiac
ultrasound was 35%, and gadolinium enhancement was observed in late left
ventricular imaging. The right ventricular septal endocardial myocardium was
biopsied, and hematoxylin-eosin staining and immunostaining were performed. A
small number of eosinophils, T lymphocytes and macrophages were found in some
tissues, indicating that myocardial cells sustained inflammation and damage [40].
Nunn et al. [23] reported that a 31-year-old female patient with
myocarditis was inoculated with the BioNTech/Pfizer vaccine 17 days ago. The
hs-TnT and N-terminal pro b-type
natriuretic peptide (NT-proBNP) were increased.
The patient underwent a myocardial biopsy and eosin methylene blue staining.
Finally, the patient was diagnosed with non-giant cell myocarditis [23]. Three
patients with myocarditis caused by BNT162b2 vaccination were reported by the
University of La Pienza in Rome, Italy. Compared with the control group (6.1
The 2018 Lake Louise criteria (LLC) upgraded version requires that the diagnosis of Acute myocarditis (AM) must meet at least one sequence sensitive to edema (T2 weighted imaging or T2 mapping) and at least one T1 sequence (T1 mapping, ECV, myocardial delayed enhancement imaging) and be positive at the same time. T1 weighted images can reflect the difference in longitudinal relaxation of myocardial tissue and reduce the influence of transverse relaxation of other tissues. In cases of acute myocarditis, gadolinium injection can enhance the early development of the myocardium. In contrast, T2 weighted images can highlight the difference in transverse relaxation. When myocardial edema occurs, the tissue shows a strong signal, and T2 time is prolonged [42, 43, 44, 45, 46, 47]. Giulia et al. [48] compared the diagnostic performance of the new and old LLC in different clinical manifestations: myocardial infarction, cardiomyopathy, and arrhythmia. Using T2-weighted short-tau inversion recovery (T2w-STIR), T2 mapping, T1 weighted images, and late gadolinium enhancement (LGE), the positive rate of the new standard LLC was 58.3%, while the positive rate of the old standard LLC was 37.9% when patients had clinical manifestations of myocardial infarct, cardiomyopathy and arrhythmia. The new LLC standard significantly improves the accuracy of CMR in diagnosing acute myocarditis, especially in patients with myocarditis whose clinical manifestations are not obvious [48]. Kravchenko et al. [27] found that the CMR results of 20 patients with myocarditis suspected to be caused by COVID-19 mRNA vaccine immunization exhibited similar manifestations to viral myocarditis, and LLC met the diagnostic criteria. Shiyovich et al. [18] reported that the CMR imaging results of myocarditis patients inoculated with Pfizer BNT162b2 vaccine were inconsistent with those of the latest early diagnosis standard of LLC, but selective bias could not be ruled out. In addition, in patients clinically diagnosed with myocarditis after vaccination, the CMR imaging results were relatively mild and consistent with the performance of “classic myocarditis”. Pan et al. [49] reported that natural T1 has a significant advantage over LLC in evaluating the sensitivity of myocarditis. Emanuele et al. [19] reported the diagnosis and manifestations of nonischemic epicardial LGE and myocarditis in 23 cases of CMR. The CMR features of acute myocarditis include a high signal (nonischemic epicardium) on the short axis of T2 weighted STIR, and the results are consistent with LGE. At the same time, acute myocarditis can be confirmed on surface images T1 and T2 [19]. It has been established that cardiac magnetic resonance tissue feature tracking is more effective than traditional LLC in diagnosing myocarditis, especially in patients with good ejection function. Therefore, CMR plays a positive role in diagnosing and evaluating myocarditis caused by immunization with the 2019 coronavirus disease vaccine [18, 50].
The CMR manifestations of myocarditis caused by COVID-19 mRNA vaccine treatment
are mainly abnormalities in the inferior epicardial wall, the inferior lateral
segment of the myocardium, and the inferior wall of the myocardium [51, 52]. The
following cases can support this view. Dedda et al. [19] reported 27
cases of myocarditis caused by the 2019 coronavirus vaccine, exhibiting chest
pain (n = 25), palpitations (n = 10), myalgia (n = 9) and dyspnea (n = 7), of
which 77.8% (n = 21/27) exhibited increased cardiac troponin T, 85.1% (n =
23/27) displayed non-ischemic late gadolinium enhancement under epicardium
matched with CMR T2 images, and 25.9% (n = 7/27) showed pericarditis.
Meyer-Szary et al. [28] reported three patients with myocarditis
immunized with the COVID-19 RNA vaccine. CMR showed that myocardial damage mainly
occurred in the lower and lower lateral segments of the myocardium, and T2
weighted stir epicardial edema signal [28]. Consistent with findings reported by
Chelala et al. [29]. In 5 patients with acute myocarditis after
immunization with the COVID-19 mRNA vaccine, CMR showed that all patients had LGE
with simple epicardial enhancement (n = 4), involvement of the inferior wall or
anterolateral wall (n = 5), epicardial enhancement (n = 1), and increased
myocardial T2 signal intensity (n = 5) [29]. Patel et al. [30] reported
the acute myocardium of 5 young men after receiving the COVID-19 mRNA vaccine.
CMR showed that myocardial edema and LGE were mainly distributed in the bottom
and middle lateral of the left ventricle, and the prognosis was good [30]. Amir
et al. [20] retrospectively analyzed 15 patients with myocarditis
induced by the BNT162b2 vaccine that were predominantly young (average age of 17
Most of the symptoms of myocarditis after COVID-19 mRNA vaccination were mild and the prognosis was good, but some patients had severe concurrent symptoms and sequelae. For example, Choi et al. [26] reported that a 22-year-old man in Korea developed chest pain 5 days after the first dose of BNT162b2 mRNA vaccine and died 7 hours later, autopsy showed that the cause of death was myocarditis [26]. Amir et al. [20] reported that a patient diagnosed with myocarditis after COVID-19 mRNA vaccine had pericardial effusion 6 months later. Oka et al. [33] reported that a Korean myocarditis patient developed syncope, heart failure and atrioventricular block 2 weeks after discharge. To sum up, myocarditis after COVID-19 mRNA vaccination needs to be paid great attention. However, it is difficult to diagnose by laboratory tests and other diagnostic methods in the early stage of the disease. CMR is sensitive and effective to discriminate early myocarditis [50].
Statistical analysis showed that the influencing factors of myocarditis caused
by the COVID-19 mRNA vaccine include: gender, age, vaccine dose times and others
[53]. In this respect, 27 cases of myocarditis caused by the COVID-19 mRNA
vaccine reported by Emanuele et al. [19] were predominantly young (36.6
Vaccine inoculation has long been established to lead to
myocarditis and cardiomyopathy. Morgan et al. [56] reported 21 patients
with myocarditis after smallpox vaccination. Histopathological examination found
monocytes were the main type of immune cell. The pathogenesis of myocarditis has
been associated with the cross-reaction mechanism of susceptible individuals to
stimulate the vaccine to cause an autoimmune response, this mechanism is
considered to be multifactorial, and the pathogenesis is not clear, although it
is widely believed that immune-mediated mechanisms play an important role [57].
Although myocarditis caused by COVID-19 has been widely reported, the underlying
mechanism remains unclear. Current evidence suggests that the interaction between
COVID-19 spike protein and autoantibodies is involved in the pathogenesis of
myocarditis. It has been shown that the antibodies of COVID-19 spike protein and
human peptide proteins such as
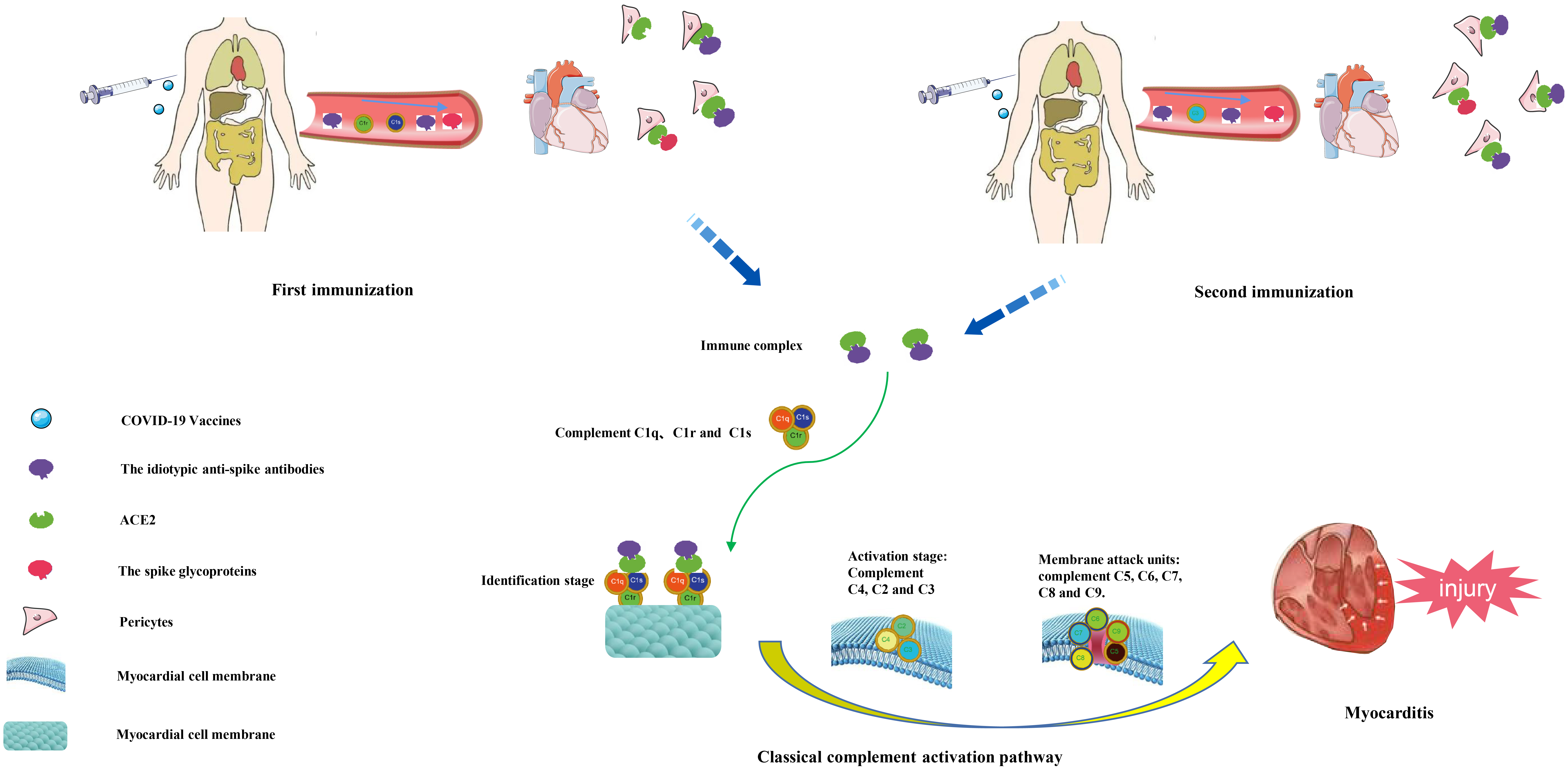 Fig. 1.
Fig. 1.Pathogenesis of myocarditis caused by human injection of COVID-19 vaccine.
After injection of COVID-19 vaccine into human body, one idiotypic anti-spike antibody will be produced in the body. The idiotypic anti-spike antibody can come to the heart together with the spike glycoprotein through blood circulation. The idiotypic anti-spike antibody can simulate the expression of spike glycoprotein and combine with cardiac ACE2 receptor to form an immune complex. Immune complexes can activate the complement system through the classical pathway of complement. First, they bind to C1q, C1r and C1s and recognize the cardiomyocyte membrane, and then activate C4, C2 and C3 to activate the cardiomyocyte membrane. Finally, C5, C6, C7, C8, and C9 attack the cell membrane, eventually destroying the cardiomyocyte membrane and causing myocardial damage.
Herbs are widely used to treat and prevent various infectious diseases. Abiri et al. [65] discussed the mechanism of action of active compounds extracted from plants in the treatment of COVID-19, while the studies related to herbal medications are small and usually not randomized controlled trials (RCTs). However, the COVID-19 mRNA vaccine plays an active role in preventing coronavirus infection. This article focuses on the COVID-19 mRNA vaccine-induced myocarditis and puts forward the following suggestions. Myocarditis caused by the COVID-19 mRNA vaccine tends to occur in adolescents and men. In cases with chest pain, fever, and other symptoms after vaccination, emphasis should be placed on ruling out myocarditis [19, 46]. In addition, myocarditis combined with pericardial effusion should be suspected in female patients with the above symptoms after vaccination [22]. Given that pericarditis tends to occur in older men, more emphasis should be placed on increasing awareness and prevention when obtaining written informed consent [6, 7, 66]. It has been established that myocarditis mostly occurs after the second dose of vaccine injection. Accordingly, patient education on myocarditis should be prioritized when injecting the second dose of vaccine, especially in people with myocardial disease history. In certain cases, extending the interval between the first and first doses should be considered to reduce the risk of adverse events [19, 20]. CMR has significant value for the early diagnosis of myocarditis caused by the COVID-19 mRNA vaccine. CMR and cardiac examinations should be conducted as soon as possible to diagnose cases presenting with chest discomfort and other symptoms after vaccination. In general, most patients with myocarditis caused by the COVID-19 mRNA vaccine have mild symptoms and should be actively treated at the onset, resulting in a good prognosis.
Although the immunogenicity of the COVID-19 mRNA vaccine has brought a series of diseases such as myocarditis to humans, the incidence is relatively low. Indeed, immunization with the vaccine has significantly reduced the incidence rate and severe mortality of COVID-19 worldwide. As a non-invasive diagnostic tool for diagnosing myocarditis caused by the COVID-19 mRNA vaccine, CMR can effectively evaluate the myocardial function and structure changes, complement the limitations of laboratory and pathological examination during the clinical diagnosis process, and understand the health status of patients through long-term follow-up examination of CMR. By studying the pathogenesis and influencing factors of myocarditis caused by the COVID-19 mRNA vaccine, we can optimize and improve the vaccination program to efficiently reduce adverse events.
CFB, BHC, JRX—extraction and drafting of the manuscript; CFB, BHC, JRX, LMW, LSS, YZ, CS—analysis of data, manuscript revision; CFB, BHC, JRX, LMW, LSS—design and revision, statistical analysis.
Not applicable.
Not applicable.
This research was supported by the National Natural Science Foundation of China (grant no. 82171884).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
