Academic Editor: Giacomo Mugnai
Background: A 70 years-old superobese man (weighted 230 kg) was referred to our hospital for recurrent syncope due to asystole alternating to atrial fibrillation. Convectional pacing was highly challenging; therefore, it was decided to implant a leadless pacemaker in a multidisciplinary intervention with surgical management of the femoral venous access. Methods: In a fully equipped operating room with bariatric table and appropriately dimensioned fluoroscope, a vascular surgeon performed surgical isolation of the right common femoral vein. After that, we proceeded to insert sheaths via the femoral vein, and through that a steerable transcatheter delivery system for the device. Results: The implant was successful without complication. Conclusions: Leadless pacemaker implantation can be effectively and safely performed even in superobese patients. Vascular access, fluoroscopic guidance and electronic interrogation could be easily managed and do not constitute a limit.
The Micra leadless pacemaker (Medtronic, Minneapolis, MN, USA) is currently used in clinical practice for the treatment of bradyarrhythmia all around the world, with good safety and efficacy performance [1]. The device is implanted in the right ventricle through a femoral vein with the use of a large introducer sheath and positioned at right ventricular septum under fluoroscopy guidance [2, 3]. However, in superobese patients, venous access and fluoroscopy could potentially constitute a limit and lead to implant failure. Furthermore, some issues may also be related to telemetry for device interrogation. Indeed, the distance between device and the head of the programmer depends on orientation of antennas of device and programmer and in the worst case should be less than 10 cm. At present, no data is available about feasibility of Micra in superobese patients.
A 70 years-old man was referred to our hospital for recurrent syncope. His
medical history was positive for diabetes mellitus, arterial hypertension,
dyslipidaemia and “super obesity”: he was 165 cm high and weighted 230 kg. Body
mass index (BMI) was 84.4 kg/m
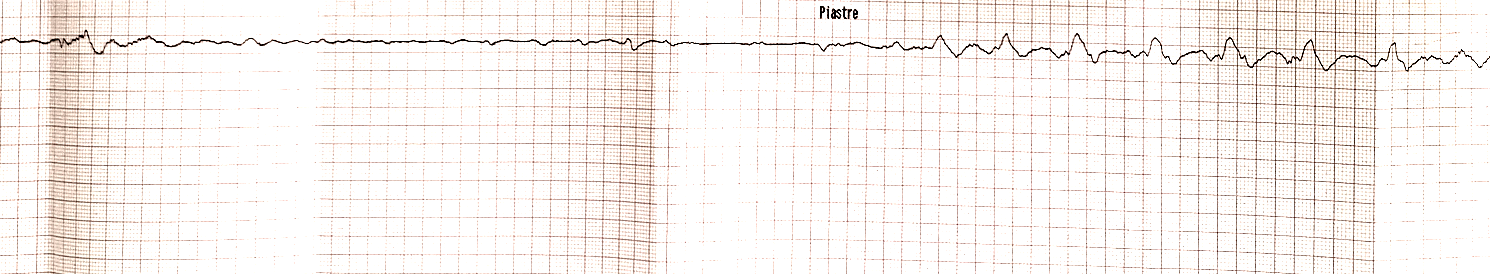 Fig. 1.
Fig. 1.Electrocardiogram strip showing asystole alternating to atrial fibrillation.
A “heart team” discussion was done to decide the better care of the patient: a conventional pacemaker inserted percutaneous through venous access as cephalic, axillary or subclavian was judged highly challenging because of BMI of the patient. Obtaining a cephalic or axillary or even subclavian venous access with conventional technique is highly difficult in this kind of patient and the conventional leads may result too short to reach the right ventricle. Moreover, the presence of diabetes, temporary pacing, renal insufficiency, the need for oral anticoagulants and positive inflammatory indices in superobese patient increased the risk of infection of the device [4]. Therefore, conventional pacing procedure was discarded. The chosen option was leadless pacemaker implantation in a multidisciplinary intervention with surgical management of the femoral venous access. With an estimated battery longevity of 13.6 years, considering age and life expectancy of the patient, the leadless pacemaker was deemed appropriate [1].
The subsequent day, in a fully equipped operating room with bariatric table and appropriately dimensioned fluoroscope, a vascular surgeon performed surgical isolation of the right common femoral vein (Fig. 1). After that, two electrophysiologists proceeded to insert sheaths of increasing size until 23-Fr-internal/27-Fr-external via the femoral vein, and through that a steerable transcatheter delivery system for the device (Medtronic, Minneapolis, MN, USA). Fluoroscopic intraoperative images were of sufficient quality to guide the procedure (Fig. 2) and the Micra leadless pacemaker was inserted in the right ventricle and positioned in the interventricular septum. At that moment, it was observed that the temporary pacemaker was located at right ventricular outflow tract but it was pacing efficiently with acceptable electrical parameters. Therefore, it was decided to lease temporary pacing and during deployment and fixation of Micra to myocardial wall, particular attention was dedicated to not engage the temporary pacemaker in the fixation mechanism. After Micra deployment electrical parameters of sensing, pacing threshold and impedance were optimal. Vascular haemostasis and suture were performed by the vascular surgeon. No complications occurred. Postoperative imaging with X-rays performed in the ward was of poor quality and unable to show the implanted device (Fig. 3). Echocardiogram and device interrogation with dedicated programmer confirmed the good position and performance of Micra. The device was programmed in VVI mode with a lower rate of 60 bpm. After a few days, electrical control of the device confirmed optimal electrical parameters and the patient was discharged at home. One month after intervention device control confirmed optimal electrical parameters showing 35% of ventricular pacing.
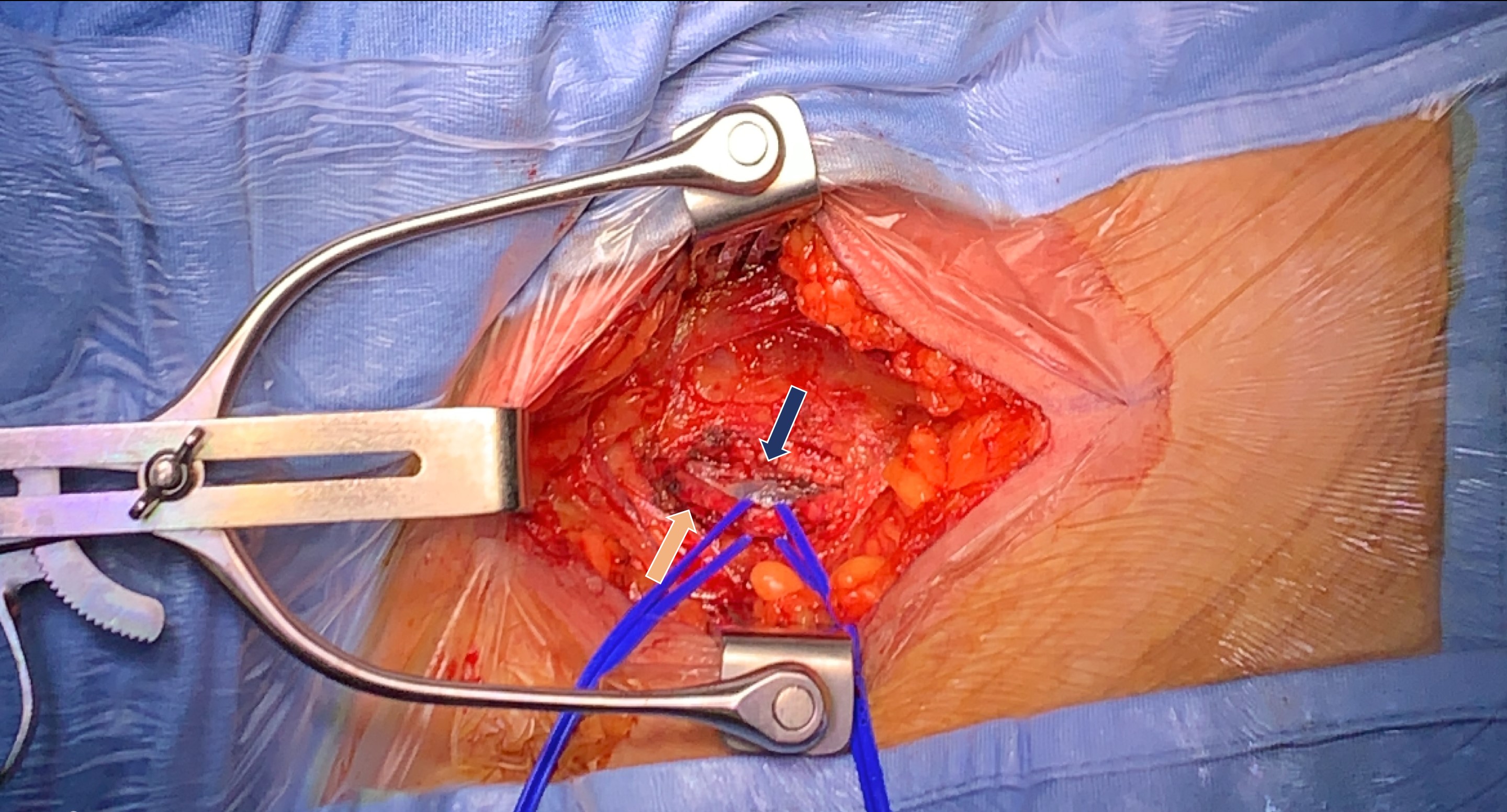 Fig. 2.
Fig. 2.Surgical isolation of femoral vein (blue arrow) and artery (orange arrow).
 Fig. 3.
Fig. 3.Intraoperative fluoroscopy (left) and postoperative chest X-rays (right). Note the position of temporary pacemaker in right ventricular outflow tract (white arrow). Visualization of leadless pacemaker (and transcatheter delivery system) was of good quality with intraoperative fluoroscopy (left panel, black arrow) and of insufficient quality with chest X-rays (right panel).
This case highlights safety and efficacy of leadless pacemaker implantation in a superobese patient.
In such a case, three aspects deserve consideration.
First, in superobese patients, fluoroscopy use is limited by the poor quality of the images. In our case, fluoroscopic guidance was useless for the positioning of temporary pacemaker in a cardiac intensive care unit setting and chest X-rays was scant for the visualization of the implanted permanent device. However, the Micra device was correctly visualized during the implantation and in such a setting intraoperative fluoroscopy was adequate for the correct positioning of the device.
Second, the vascular access is crucial for the success of the procedure and, if inadequate, could compromise the implantation of a device. Our case demonstrates that, even in a 230 kg patient, the femoral vein could be used for the insertion of the 23-Fr-internal/27-Fr-external introducer sheath that is required for the transcatheter delivery system. We observed that surgical isolation of the vein resulted in no complication. It is important to plan surgical isolation in a multidisciplinary team setting in such extreme cases.
Third, usually the device interrogation requires a distance
Finally, based on these considerations when we face difficult implant like that in superobese, the option of leadless pacemaker must be considered. This option also reduces infectious risk in such high-risk patients.
Leadless pacemaker implantation can be effectively and safely performed even in superobese patients. Vascular access, fluoroscopic guidance and electronic interrogation could be easily managed and do not constitute a limit.
MB, MM—contributed to the study design; MB, MM, ED, FV, CB, VG—data analysis; MB, MM drafting of the manuscript.
Patient gave informed consent for inclusion before he participated in the study.
We would like to express our gratitude to all those who helped us during the writing of this manuscript.
This research received no external funding.
The authors declare no conflict of interest. Matteo Bertini is serving as one of the Editorial Board members/Guest editors of this journal. Cristina Balla is serving as one of the Editorial Board members. We declare that Matteo Bertini and Cristina Balla had no involvement in the peer review of this article and have no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Giacomo Mugnai.
