1 Department of Clinical and Molecular Medicine, Sapienza University of Rome University, 00189 Rome, Italy
2 Department of Cardiology, University of Foggia, 71122 Foggia, Italy
3 UOC Cardiologia, G da Saliceto Hospital, 29121 Piacenza, Italy
4 Dipartimento di Scienze Cardiovascolari, Respiratorie, Nefrologiche, Anestesiologiche e Geriatriche, Sapienza University of Rome, 00161 Rome, Italy
5 Division of Cardiology, University of Brescia, 25121 Brescia, Italy
6 Department of Clinical sciences and Community health, Cardiovascular Section, University of Milano, 20122 Milano, Italy
7 Centro Cardiologico Monzino, IRCCS, 20138 Milano, Italy
Academic Editor: John Lynn Jefferies
Abstract
Erectile dysfunction (ED) is a major concern in heart failure (HF) due its high
prevalence as well as its negative impact on the quality of life, this condition
being usually unrecognized and thus untreated. A number of possible causes might
contribute to the above mentioned tight association, i.e., shared risk factors,
comorbidities and several physiologic HF abnormalities such as impaired exercise
tolerance, psychogenic factors and neurohumoral, metabolic and vascular changes.
Medications have been blamed for playing also a pivotal role in the ED occurrence
and, particularly, the
Keywords
- heart failure
- erectile dysfunction
- beta-blocker
- therapy
The erectile dysfunction (ED) is defined as the consistent or recurrent inability to achieve and/or maintain an erection sufficient to permit satisfactory sexual performance [1]. The ED can be classified based on etiology as psychogenic, organic or mixed psychogenic and organic, the latter being the most common one. The organic form may be associated with neurological disorders, androgen deficiency, vascular causes such as penile arterial insufficiency or veno-occlusive dysfunction, obesity, diabetes mellitus or other systemic disease and, finally, drugs, cigarette smoking or chronic alcoholism. In addition, sexual function progressively declines with aging [2, 3]. Any alteration in the pathway briefly described below might lead to ED (Table 1).
| Cause | Mechanism | |
| Psychogenic | Depression | Decreased libido |
| Anxiety | Increased sympathetic tone | |
| Psychological stress | Impaired NO release | |
| Vasculogenic | Hypertension | Decreased arterial flow |
| Diabetes | Endothelial dysfunction | |
| Atherosclerosis | Increased endothelin 1 or noradrenalin | |
| Impaired vasomotion | Decreased prostacyclin | |
| Neurogenic | Stroke or Alzherimer’s disease | Failure to initiate nerve impulse |
| Spinal cord or pelvic injury | Interrupted neural transmission | |
| Diabetes | Peripheral neuropathy | |
| Hormonal | Hypogonadism | Loss of libido |
| Hyperprolactinemia | Inadequate NO release | |
| Drug-induced | SSRI | Central suppression |
| Unknown mechanism | ||
| Digoxin | Smooth muscle sodium-pump inhibition | |
| Spironolactone | Androgen suppression | |
| Diuretics | Unknown mechanism | |
| Cigarette smoking | Vascular insufficiency | |
| Alcohol abuse | Alcoholic neuropathy | |
| NO, nitric oxide; SSRI, selective serotonin reuptake inhibitors. | ||
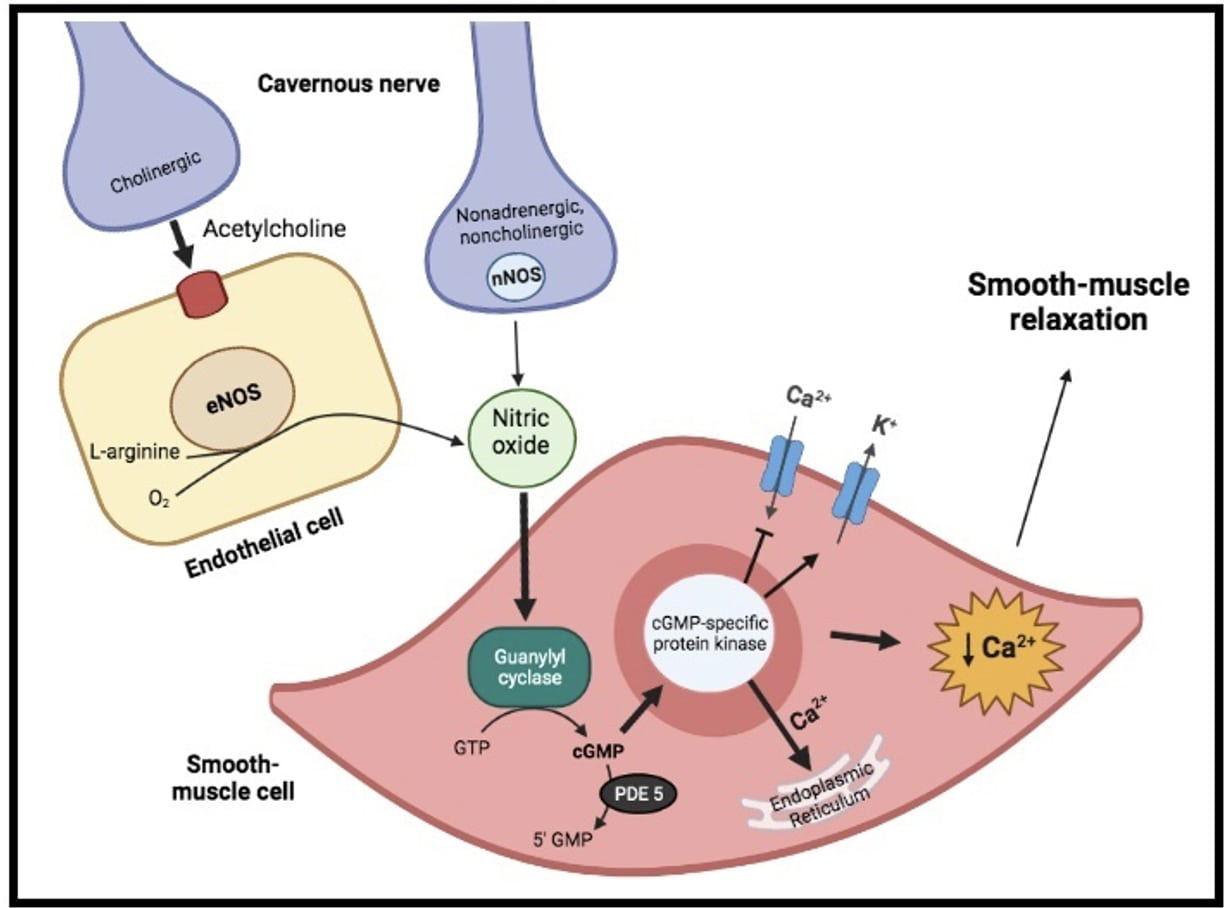
Physiologically, penile erection results from the integrative synchronized action of neuronal and vascular systems, both of them being modulated by psychological factors and hormonal status. Indeed, on sexual stimulation, the cavernous nerve terminals release neurotransmitters resulting in relaxation of the trabecular smooth muscle and vasodilation of the arteries and arterioles supplying the erectile tissue (Fig. 1). Thus, penile blood flow extremely increases and sinusoidal spaces rapidly expands. In addition, the enlargement of the sinusoids compresses the subtunical venular plexuses against the tunica albuginea decreasing the venous outflow to a minimum. A cessation of neurotransmitters release, the metabolization of second messengers by phosphodiesterase or sympathetic discharge during ejaculation (i.e., adrenergic receptors’ activation on the cavernous arteries and trabecular smooth muscles) can lead to detumescence. During this phase, contraction of the trabecular smooth muscle allows venous drainage of the lacunar spaces and relief of the erection [4]. In such a context, nitric oxide (NO), released from parasympathetic nerve terminals and vascular endothelium, is probably the principal neurotransmitter involved. Indeed, within the smooth muscle cells, NO stimulates a soluble guanylyl cyclase, which in turn increases the production of cyclic guanosine monophosphate (cGMP), the intracellular second messenger mediating smooth muscle relaxation. Thereafter, cGMP activates a specific protein kinase leading to phosphorylation of certain proteins to cause opening of potassium channels, closing of calcium channels and sequestration of intracellular calcium by the endoplasmic reticulum. The resultant fall in intracellular calcium leads to smooth muscle relaxation, that is essential for maximal penile engorgement. Eventually, during the return to the flaccid state, cGMP is metabolized by type 5 phosphodiesterase (PDE-5), resulting in detumescence [4, 5].
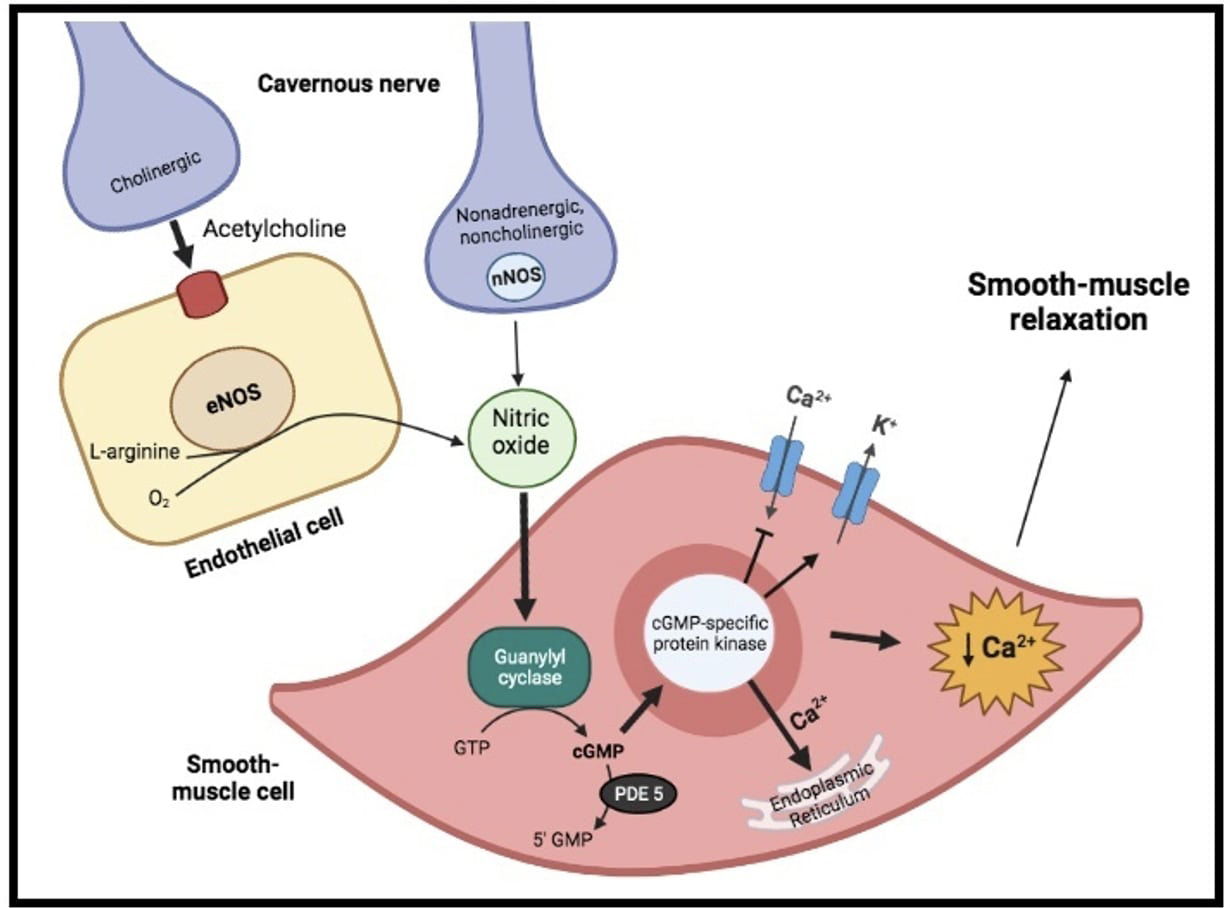 Fig. 1.
Fig. 1.Physiology of erectile function. On sexual stimulation, the
cavernous nerve terminals release neurotransmitters resulting in production of
nitric oxide (NO). Within the smooth muscle cells, it stimulates a soluble
guanylyl cyclase, which increases the production of cyclic guanosine
monophosphate (cGMP), the intracellular second messenger mediating smooth muscle
relaxation. Indeed, cGMP activate a specific protein kinase, leading to opening
of potassium (K
In this narrative review we sought to analyze the pathophysiological mechanisms
involved in the development of ED in patients with heart failure (HF) and,
particularly, we tried to answer a historical question on a possible detrimental
role of
Several studies sought to investigate the overlap between ED and HF, showing a prevalence ranging from 60% to 75%, regardless of the HF etiology [6, 7]. In general, patients suffering from HF experience a decrease in libido and in frequency of coitus, negative changes in sexual performance and a general dissatisfaction related to their sexual function. Even, it has been reported that about one quarter of them cease all sexual activity [8]. However, despite the well-known tight association between these conditions, cardiologists address rarely the presence of an ED concern in contrast with patients’ expectations who would like their physicians to be interested in this issue [9]. Accordingly, a large percentage of HF patients remains without a diagnosis and thus untreated.
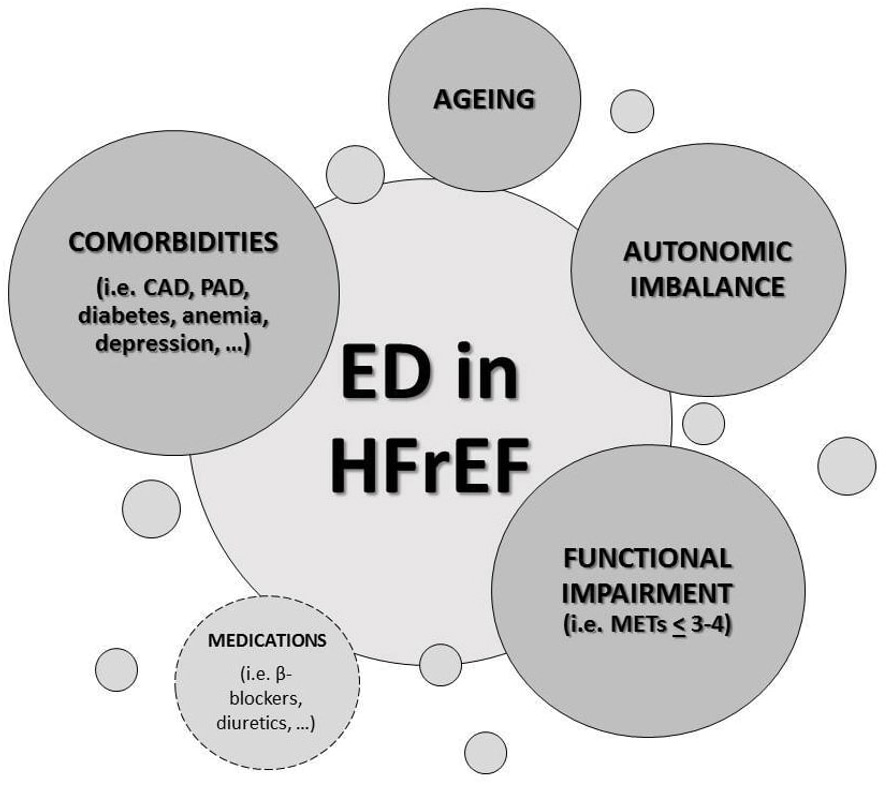
A number of possible reasons may contribute to the high ED incidence in HF
patients (Fig. 2). Firstly, depression and anxiety, usually described conditions
in HF, may play a pivotal role in sexual dysfunction as well as the concomitant
treatments with selective serotonin reuptake inhibitors (SSRIs), whose sexual
side effects are well known [6], could magnify the issue. Another important
contributing cause for ED in this setting is the exercise impairment degree,
sexual function being related with New York Heart Association (NYHA) functional
class, the 6-minute walk test and the peak oxygen uptake (pVO
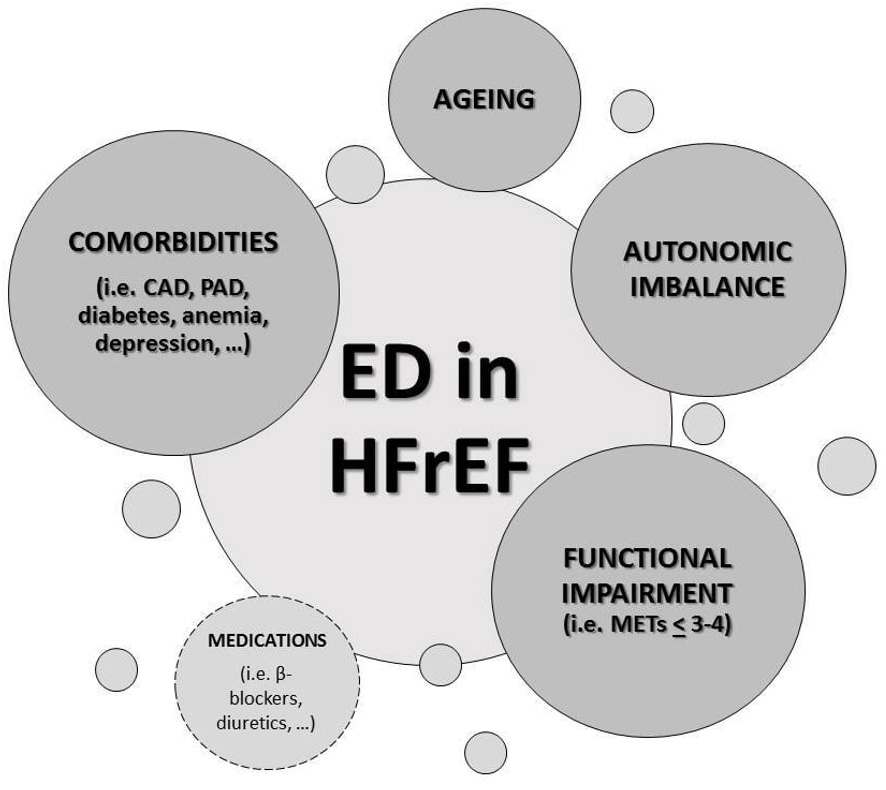 Fig. 2.
Fig. 2.Causes of erectile dysfunction (ED) in heart failure with
reduced ejection fraction (HFrEF). ED, erectile dysfunction; HFrEF, heart
failure with reduced ejection fraction; CAD, coronary artery disease; PAD,
peripheral artery disease; METs, metabolic equivalents of task (1 MET = 3.5
mL/kg/min O
Historically,
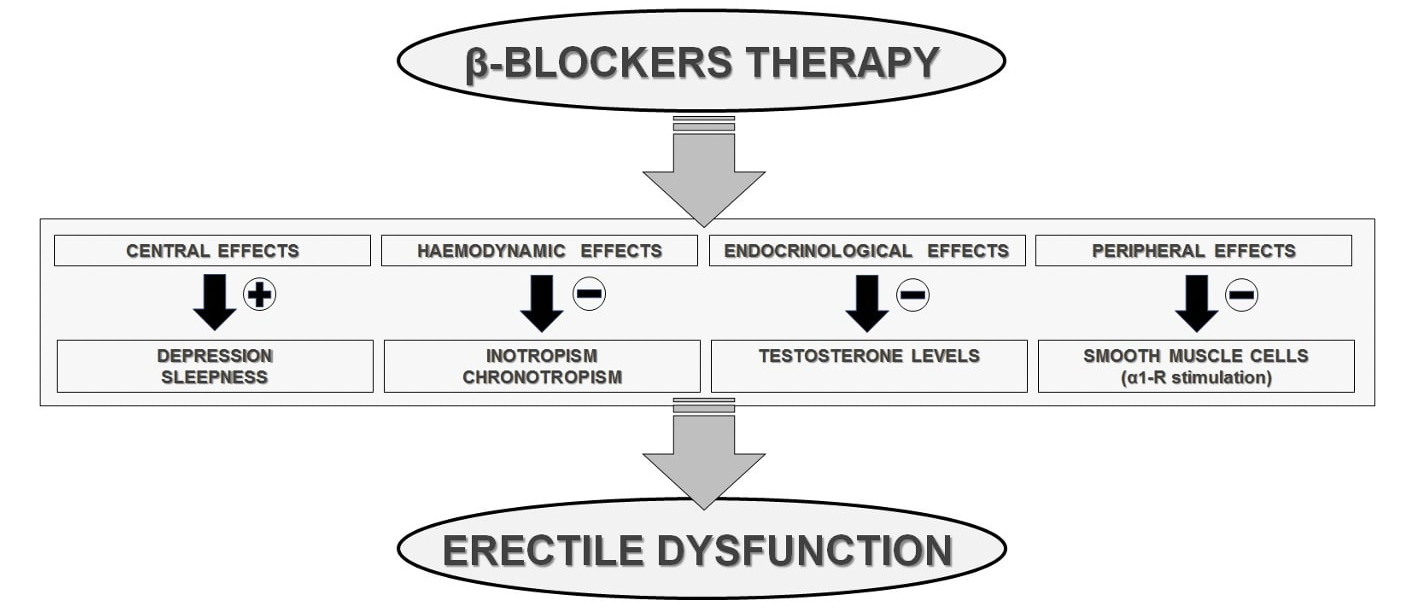
From a pathophysiological viewpoint, due to their block of the receptor sites
for the endogenous catecholamines, the
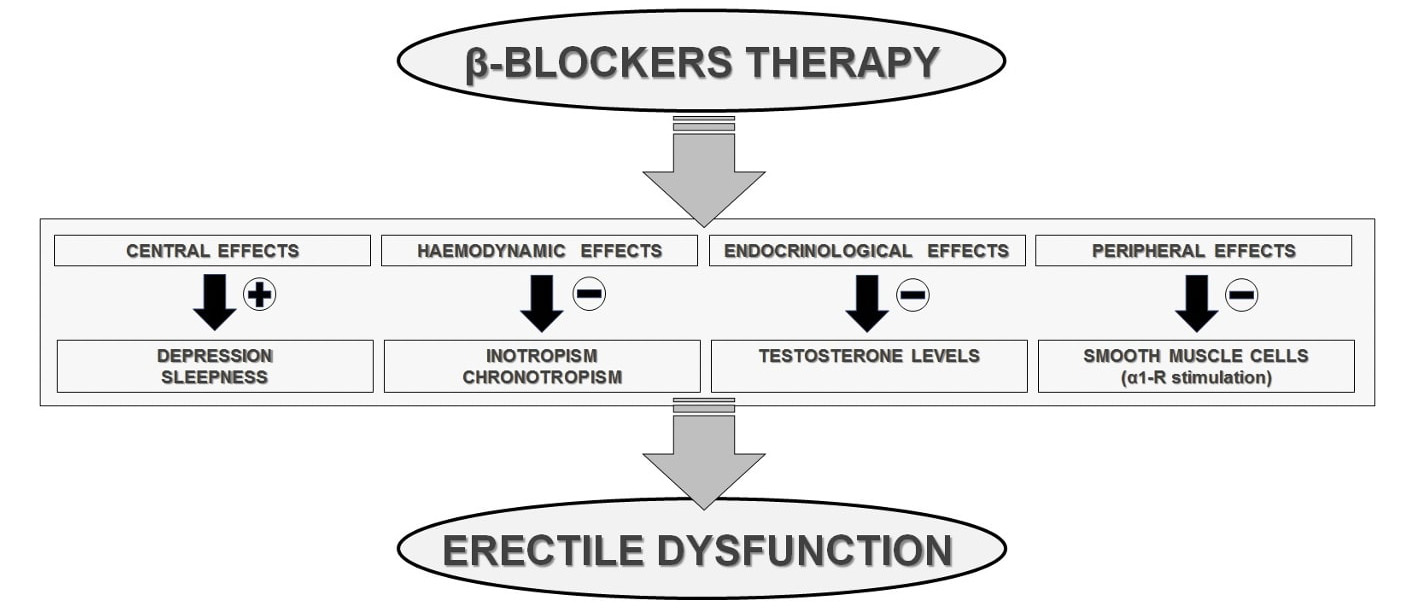 Fig. 3.
Fig. 3.
Since 1980s, several studies tried to assess a possible negative effect of
different
| Reference | Type of study | Study sample | End points | Results | |
| Peart WS et al. [22] | Propranolol | Randomized, single blind, placebo controlled | 7513 men with mild to moderate essential hypertension | Death from hypertension or stroke and non-fatal stroke | Association between propranolol treatment and impotence |
| Croog SH et al. [23] | Propranolol | Randomized, double blind | 626 men with mild to moderate essential hypertension | Effects on quality of life | Higher side effects and sexual dysfunction than captopril |
| Fogari R et al. [24] | Atenolol | Randomized, double blind | 90 men with a newly diagnosed essential hypertension | Effects on sexual activity | Chronic worsening of sexual activity |
| Fogari R et al. [25] | Carvedilol | Randomized, double blind, placebo controlled | 160 men with a newly diagnosed essential hypertension | Effects on sexual activity | Chronic worsening of sexual activity |
| Brixius K et al. [26] | Metoprolol/Nebivolol | Randomized, double blind | 48 men with stage 1 essential hypertension | Effects on erectile function | Metoprolol decreased the erectile function/ Nebivolol improved it |
| Cordero A et al. [27] | Any |
Cross-sectional, observational | 1007 men with essential hypertension | Prevalence of ED | ED is highly prevalent in hypertensive patients treated with |
| Note that all available studies deal with hypertensive patients. | |||||
| Name | Selectivity | Lipophilicity | Ancillary effects |
| Pindolol | Nonselective | Intermediate | Intrinsic sympathomimetic activity |
| Propranolol | Nonselective | High | Membrane stabilizing effect |
| Sotalol | Nonselective | Low | Type III antiarrhythmic action |
| Timolol | Nonselective | Intermediate | |
| Nadolol | Nonselective | Low | |
| Carvedilol* | Nonselective | Intermediate | |
| Labetalol | Nonselective | High | |
| Penbutolol | Nonselective | High | Intrinsic sympathomimetic activity |
| Atenolol | Low | ||
| Bisoprolol* | Intermediate | ||
| Metoprolol* | Intermediate | ||
| Nebivolol* | Low | ||
| Esmolol | Low | ||
| Celiprolol | Low | Intrinsic sympathomimetic activity | |
| Acebutolol | Low | Intrinsic sympathomimetic activity | |
| * | |||
Most of the medications commonly used in HF therapy have been associated with
sexual dysfunction but, again, data specifically obtained in such a population
are lacking. Together with
Since that management of the cardiac disease may reduce symptoms, improve exercise capacity and decrease depression, it is highly reasonable that targeting the HF therapy according to the current guidelines might be beneficial also with respect the sexual function. Notwithstanding, it would be highly desirable to investigate the presence of ED through specific questions since it can influence patients’ adherence to treatment or lead to misguided efforts to retain satisfactory sexual activity as well as adversely affect the quality of life [13]. In this setting, cardiologists should improve their engagement in managing patients with sexual function disorders [9]. They should inform their patients about the physiologic requirements of sexual activity as well as about the advantages of targeting HF therapy with respect to sexual function. Anyway, all drugs with potential adverse sexual side effects should be discontinued or replaced when clinically feasible. Furthermore, providing an adequate sexual counseling plays a pivotal role for the management of patients with HF and ED. Similarly, optimizing the treatment of cardiovascular risk factors, practicing regular exercise, losing weight, moderating alcohol consumption and quitting smoking are all essential steps. Indeed, lifestyle changes are essential to improve erectile function, to reduce the global cardiovascular risk burden and, most likely, to reduce most of the adverse drug effects [38, 39].
If the abovementioned measures are not still enough, a more specific approach to
ED is recommended. In such a context, the PDE5 inhibitors, which block PDE-5
mediated degradation of cGMP thereby delaying detumescence, are the most commonly
used drugs for treatment of ED. They have a modest hypotensive action and a mild
nitrate-like action since PDE-5 is also present in vascular smooth muscle cells.
Properly for their mechanisms, initially HF was considered as a relative
contraindication for their use [40] but, subsequently, Katz and colleagues
demonstrated the safety of this class of drugs in patients with mild to moderate
HF [41]. In addition, several other potential hemodynamic benefits in HF patients
were reported, such as a decrease in heart rate response during exercise, an
improvement in exercise capacity and an increase in cardiac index [42, 43]. Thus,
current guidelines consider the PDE-5 inhibitors generally safe in patients with
compensated HF, except for those receiving nitrates [13] where it is possible an
increased risk of symptomatic hypotension. Instead, there are no data supporting
the efficacy of nutritional supplements, herbal therapy or vitamins in the
treatment of ED. Yohimbine, an
ED is a clinical condition highly prevalent among HF patients which adversely
affect their quality of life. The tight association between these two conditions
is most likely due to shared risk factors and common pathogenetic traits. HF
itself may worsen sexual function for countless reasons, ranging from impaired
exercise tolerance and psychogenic factors to neurohumoral, metabolic and
vascular changes. Additionally, some cardiovascular medications might contribute
to ED. Although evidence support a negative effect for diuretics and
PA, SN and MC designed the research study. DM, SC and RB performed the research. SC and GG wrote the manuscript with the support of DM and MP. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest. Damiano Magrì and Giovanna Gallo are serving as the Guest editors of this journal. We declare that Damiano Magrì and Giovanna Gallo had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to John Lynn Jefferies.