1. Introduction
Ischemic heart disease is one of the most common cardiovascular diseases in the
world, and percutaneous coronary intervention (PCI) is an effective means to
treat it [1]. The number of PCI procedures is increasing year on year. According
to current guidelines, using dual antiplatelet therapy (DAPT) consisting of
aspirin and P2Y inhibitors after drug-eluting stent placement can reduce
the risk of postoperative thrombotic complications [2, 3, 4]. The routine duration of
DAPT in patients with chronic coronary syndrome (CCS) is 6 months. The routine
duration of DAPT in patients with acute coronary syndrome (ACS) is 12 months
[1, 2, 3, 4]. Following DAPT, single antiplatelet therapy (SAPT) is used for secondary
prevention, and aspirin is generally used as the first choice due to positive
results from previous randomized clinical trials [5].
Recently, consideration of the potential risk of aspirin-related
gastrointestinal complications has prompted research into non-aspirin treatments
following PCI [6]. Two studies demonstrated that clopidogrel showed similar
clinical outcomes in patients after PCI compared to aspirin [7, 8]. Recent
evidence suggests that SAPT with P2Y inhibitor is superior in balancing
bleeding and ischemic risk [9, 10, 11]. An extended HOST-EXAM (Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis–Extended Antiplatelet Monotherapy) study with more than 5
years of follow-up showed that clopidogrel monotherapy showed a lower rate of
compound net clinical events in patients with no clinical events 12 6
months after stent PCI compared to aspirin monotherapy [12]. A meta-analysis
which included five clinical trials found that clopidogrel showed a lower major adverse cardiovascular
events (MACE) and stroke rate after DAPT completion after PCI compared to aspirin, while there
were no significant differences between the two groups in mortality, major
bleeding, myocardial infarction, and repeated revascularization [13]. We know
that P2Y platelet receptor inhibitors are not just clopidogrel. Most
recently, an analysis of the GLOBAL LEADERS trial found that ticagrelor
monotherapy showed a lower ischemic composite endpoint compared to aspirin
monotherapy. In contrast, ticagrelor monotherapy showed a higher major bleeding
endpoint [14]. It is still controversial which antiplatelet monotherapy should be
continued after a period of DAPT in the post-PCI population. Therefore, an
up-to-date and comprehensive analysis of this issue is necessary.
The aim of this meta-analysis was to bringing together data from all available
prospective clinical studies investigating the efficacy and safety of P2Y
inhibitors versus aspirin in the post-PCI population after completion of DAPT.
2. Methods
Our current meta-analysis follows the performing and reporting specifications of
the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA)
guidelines [15]. We registered the protocol on the International Platform of
Registered Systematic Review and Meta-analysis Protocols database (Inplasy
protocol: INPLASY 2022120011) and is available on inplasy.com
(https://inplasy.com/inplasy-2022-12-0011). Our research did not require ethical
approval.
2.1 Search Strategy
Three independent researchers conducted an extensive electronic search of
relevant articles published between January 1, 2015 and November 20, 2022. The
database includes Embase, PubMed and the Cochrane database. We independently
hand-selected relevant randomized controlled trials (RCTs) and screened any
relevant studies. The literature search strategy is shown in Supplementary Table 1.
2.2 Inclusion and Exclusion
Document management was performed using EndNote X9 version (Thomson Corporation,
Stanford, CT, USA) software, and the eligibility of the identified items was
independently evaluated by two investigators. First, the title and abstract were
first screened. Eligible articles were retained for reading in full-text review.
The inclusion criteria for eligible studies included: (1) Patients receiving dual
antiplatelet therapy after PCI. (2) Treatment with P2Y inhibitor or
aspirin monotherapy. (3) Outcome indicators: MACE, all-cause death, cardiac
death, myocardial infarction, major bleeding, stent thrombosis, repeat
revascularization and any stroke. The exclusion criteria include: (1) Clinical
study of DAPT compared with SAPT. (2) Studies evaluating antithrombotic drugs
other than aspirin or P2Y inhibitors. (3) There is not enough data to
extract, such as abstracts of some meetings, literature reviews, pharmacological
introductions, etc. (4) Retrospective studies were also excluded.
2.3 Bias & Quality Assessment
The three researchers independently evaluated, screened and examined the
literature according to a unified and standardized method, and included the
literature according to strict inclusion and exclusion criteria, and then
conducted data collection and analysis. We evaluated the quality of the selected
articles according to the quality evaluation criteria of the Newcastle-Ottawa
Scale and Cochrane Reviewer Handbook 5.1.0 [16].
2.4 Data Synthesis and Analysis
This meta analysis selected Revman 5.3 (The Nordic Cochrane Center, Copenhagen,
Denmark) and Stata 14.0 (STATA Inc., College Station, TX, USA) for data
management and analysis. The data which met homogeneity (p 0.10 and
I 50%) through a heterogeneity test were meta-analyzed with a
fixed effect model. If homogeneity (p 0.10 or I 50%)
was not met, and heterogeneity could not be excluded, a random effects model was
used to combine effects, but it should be noted that the type of data analyzed
should consider sensitivity analysis and subgroup analysis. We merged the results
from all relevant studies to estimate the pooled risk ratio (RR) and associated
95% confidence intervals (CIs) for dichotomous outcomes. Statistically
significant was defined as p 0.05.
3. Results
The search and research selection process is summarized in a flow chart (Fig. 1). Of the 5127 studies identified by electronic search, 1782 studies were
excluded due to duplications. After reading the title and abstract, we excluded
3219 studies that did not meet the inclusion criteria. The remaining 126 studies
were evaluated by reading the full text. Data from 5 trials evaluating P2Y
inhibitor versus aspirin monotherapy after coronary stenting were included.
 Fig. 1.
Fig. 1.
The flow chart of study selection process.
Table 1 (Ref. [7, 8, 11, 12, 14]) shows the main features of the included trials. In
our analyses, a total of 24,460 patients were assigned to aspirin (n = 10,661) or
P2Y inhibitor monotherapy (n = 13,799). All of the studies were on
clopidogrel monotherapy following dual antiplatelet therapy after coronary
stenting except for the trial reported by Masafumi, where patients were on
ticagrelor [14]. The observational trials reported by Doo Sun Sim and Natsuaki
[7, 8] showed 12-month follow-up outcomes, the observational trial reported by
Masafumi reported 23-month follow-up outcomes [14], while the randomized trial
reported by Park [11] showed 36-month follow-up outcomes, the randomized trial
reported by Jeehoon Kang showed 5-year follow-up outcomes [12]. In trials between
P2Y inhibitor and aspirin, no difference was observed in the proportion of
patients who failed at follow-up. Table 2 (Ref. [7, 8, 11, 12, 14]) summarizes the
baseline characteristics of the patients and surgeries included in our analyses.
There were no significant differences in baseline data between the two groups in
our analyses.
Table 1.The main features of the included trials.
|
Year of publication |
Region |
Number of patients |
P2Y |
Type of trial |
Multicenter |
follow-up |
| Overall |
Aspirin |
P2Y |
| Park [11] |
2016 |
South Korea |
3243 |
2472 |
771 |
Clopidogrel |
Observational Trial |
No |
36 months |
| Doo Sun Sim [8] |
2020 |
South Korea |
1819 |
1286 |
533 |
Clopidogrel |
Observational Trial |
Yes |
12 months |
| Natsuaki [7] |
2020 |
Japan |
2819 |
1480 |
1339 |
Clopidogrel |
Observational Trial |
Yes |
12 months |
| Jeehoon Kang [12] |
2023 |
South Korea |
5438 |
2728 |
2710 |
Clopidogrel |
Randomized Trial |
Yes |
60 months |
| Masafumi Ono [14] |
2022 |
United Kingdom |
11,121 |
5813 |
5308 |
Ticagrelor |
Randomized Trial |
Yes |
23 months |
Abbreviation: P2Y, P2Y inhibitor.
Table 2.Baseline clinical characteristics of patients.
|
Park 2016 [11] |
Doo Sun Sim 2020 [8] |
Natsuaki 2020 [7] |
Jeehoon Kang 2023 [12] |
Masafumi Ono 2022 [14] |
| Aspirin |
P2Y |
Aspirin |
P2Y |
Aspirin |
P2Y |
Aspirin |
P2Y |
Aspirin |
P2Y |
| (n = 2472) |
(n = 771) |
(n = 1286) |
(n = 533) |
(n = 1480) |
(n = 1339) |
(n = 2728) |
(n = 2710) |
(n = 5813) |
(n = 5308) |
| Patient Characteristics |
|
|
|
|
|
|
|
|
|
|
| Mean age, y |
62 |
64 |
61.1 |
60.9 |
69.7 |
68.1 |
63.3 |
63.3 |
64.1 |
63.7 |
| Male (%) |
73.3 |
73.9 |
78.2 |
78.5 |
73.0 |
79.0 |
75.4 |
74.3 |
77.7 |
77.9 |
| Diabetes (%) |
33.7 |
42.2 |
21.4 |
20.7 |
39.0 |
39.0 |
33.9 |
33.6 |
24.1 |
24.3 |
| Hypertension (%) |
53.2 |
64.5 |
46.0 |
45.7 |
83.0 |
74.0 |
61.3 |
61.4 |
72.8 |
73.4 |
| Dyslipidemia (%) |
28.5 |
33.5 |
13.3 |
13.2 |
80.0 |
74.0 |
70.6 |
69.5 |
70.4 |
69.6 |
| Current smoking (%) |
17.3 |
22.6 |
62.9 |
63.0 |
21.0 |
27.0 |
21.9 |
19.7 |
26.8 |
26.5 |
| Chronic kidney disease (%) |
8.1 |
10.2 |
NA |
NA |
30.0 |
35.0 |
11.9 |
12.9 |
12.2 |
12.2 |
| Prior cerebrovascular accident (%) |
3.2 |
6.1 |
3.2 |
3.1 |
9.2 |
5.3 |
4.8 |
4.2 |
2.2 |
2.4 |
| Prior myocardial infarction (%) |
19.0 |
18.4 |
2.9 |
2.8 |
17.0 |
14.0 |
15.8 |
16.7 |
22.9 |
21.8 |
| Clinical presentation (%) |
|
|
|
|
|
|
|
|
|
|
| Stable angina |
58.9 |
58.0 |
NA |
NA |
67.0 |
62.0 |
28.7 |
27.6 |
55.5 |
51.7 |
| UA/NSTEMI |
26.5 |
31.3 |
NA |
NA |
NA |
NA |
53.7 |
55.2 |
31.6 |
34.7 |
| STEMI |
14.7 |
10.8 |
NA |
NA |
NA |
NA |
17.7 |
17.2 |
12.9 |
13.6 |
| LVEF, % |
62 |
62 |
53.3 |
53.5 |
NA |
NA |
NA |
NA |
NA |
NA |
| Procedural Characteristics |
|
|
|
|
|
|
|
|
|
|
| Angiographic disease extent |
|
|
|
|
|
|
|
|
|
|
| 1-vessel disease (%) |
44.9 |
39 |
53.6 |
53.9 |
NA |
NA |
49.9 |
50.6 |
69.5 |
69.1 |
| 2-vessel disease (%) |
33.6 |
38.0 |
30.3 |
30.1 |
NA |
NA |
31.3 |
31.4 |
21.8 |
22.7 |
| 3-vessel disease (%) |
21.5 |
23.0 |
13.6 |
13.4 |
NA |
NA |
18.7 |
18.1 |
8.7 |
8.3 |
| Target vessel location |
|
|
|
|
|
|
|
|
|
|
| LM |
NA |
NA |
1.3 |
1.2 |
1.2 |
2.9 |
4.9 |
5.2 |
2.3 |
2.6 |
| LAD |
NA |
NA |
47.4 |
47.6 |
57.0 |
55.0 |
NA |
NA |
52.2 |
50.4 |
| LCX |
NA |
NA |
18.7 |
18.4 |
24.0 |
18.0 |
NA |
NA |
31.4 |
31.6 |
| RCA |
NA |
NA |
32.6 |
32.8 |
26.0 |
29.0 |
NA |
NA |
36.4 |
37.6 |
| Treated lesions per patient |
1.0 |
1.0 |
NA |
NA |
1.21 |
1.12 |
1.30 |
1.32 |
1.4 |
1.4 |
| No. of stents per patient |
1.0 |
1.0 |
1.15 |
1.16 |
1.37 |
1.26 |
1.5 |
1.5 |
NA |
NA |
| Maximal stent diameter, mm |
3.5 |
3.5 |
3.18 |
3.18 |
NA |
NA |
3.08 |
3.08 |
NA |
NA |
| Stent total length, mm |
28 |
32 |
29.0 |
29.1 |
33.0 |
30.3 |
35.3 |
36.3 |
NA |
NA |
Data are median (25th–75th percentiles) or number of patients (%). NA means that the study didn’t present that data.
Abbreviation: y, years; UA, unstable angina; NSTEMI, non ST segment elevation myocardial
infarction; STEMI, acute ST segment elevation myocardial infarction; RCA, right
coronary artery; LM, left main coronary artery; LAD, left anterior descending
coronary artery; LCX, left circumflex coronary artery; LVEF, left ventricular
ejection fraction.
The safety and efficacy outcomes are summarized in Fig. 2. Patients who received
a P2Y inhibitor showed a risk of MACE than patients who received aspirin
(odd ratio (OR) 0.70 [95% CI 0.60–0.80], I = 0%, p 0.00001)
monotherapy following dual antiplatelet therapy 12 months after stent
implantation. Specifically, the benefit of MACE in patients receiving P2Y
inhibitors was primarily due to a significant reduction in repeated
revascularization (OR 0.80 [95% CI 0.70–0.93], I = 0%, p =
0.002) and any stroke (OR 0.59 [95% CI 0.44–0.79], I = 0%, p =
0.0004). We observed no differences between patients who received aspirin and
those who received a P2Y inhibitor in terms of stent thrombosis,
myocardial infarction, cardiac death and all-cause death. The risk of major
bleeding (OR 0.86 [95% CI 0.53–1.39], I = 57%, p = 0.54) was
similar in patients who received aspirin and those who received a P2Y
inhibitor.
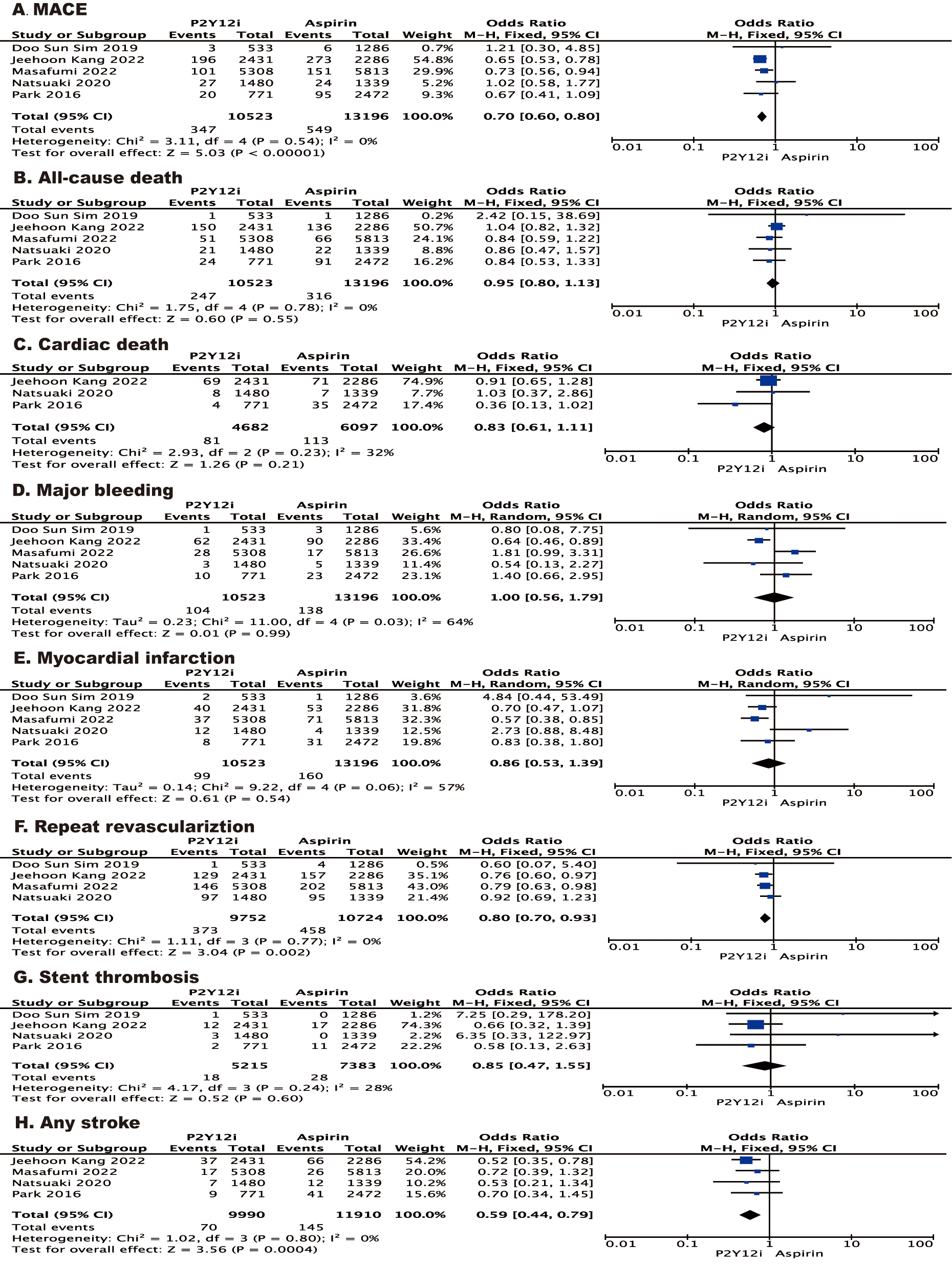 Fig. 2.
Fig. 2.
Forest plot of the effect of P2Y inhibitor vs aspirin on
the risk of outcomes for post-PCI patients after a period of DAPT. Forest plot
reporting the odds ratios of P2Y inhibitor vs aspirin: (A) MACE; (B)
all-cause death; (C) cardiac death; (D) major bleeding; (E) myocardial
infarction; (F) repeat revascularization; (G) stent thrombosis; (H) any stroke. PCI, percutaneous coronary
intervention; MACE, major adverse cardiovascular events; DAPT, dual antiplatelet therapy.
A stratified analysis of MACE according to the characteristics of patients
(i.e., age 65 years, with diabetes mellitus, male or with multivessel disease)
showed results consistent with the primary analysis (Fig. 3). In another
stratified analysis according to type of P2Y inhibitor, the results for
MACE and death from any cause were consistent with the primary analysis, while
the risk of myocardial infarction was significantly lower (OR 0.57 [95% CI
0.38–0.85], p = 0.005) and the risk of major bleeding was increased in
patients who received ticagrelor monotherapy (OR 1.81 [95% CI 0.99–3.31],
p = 0.05).
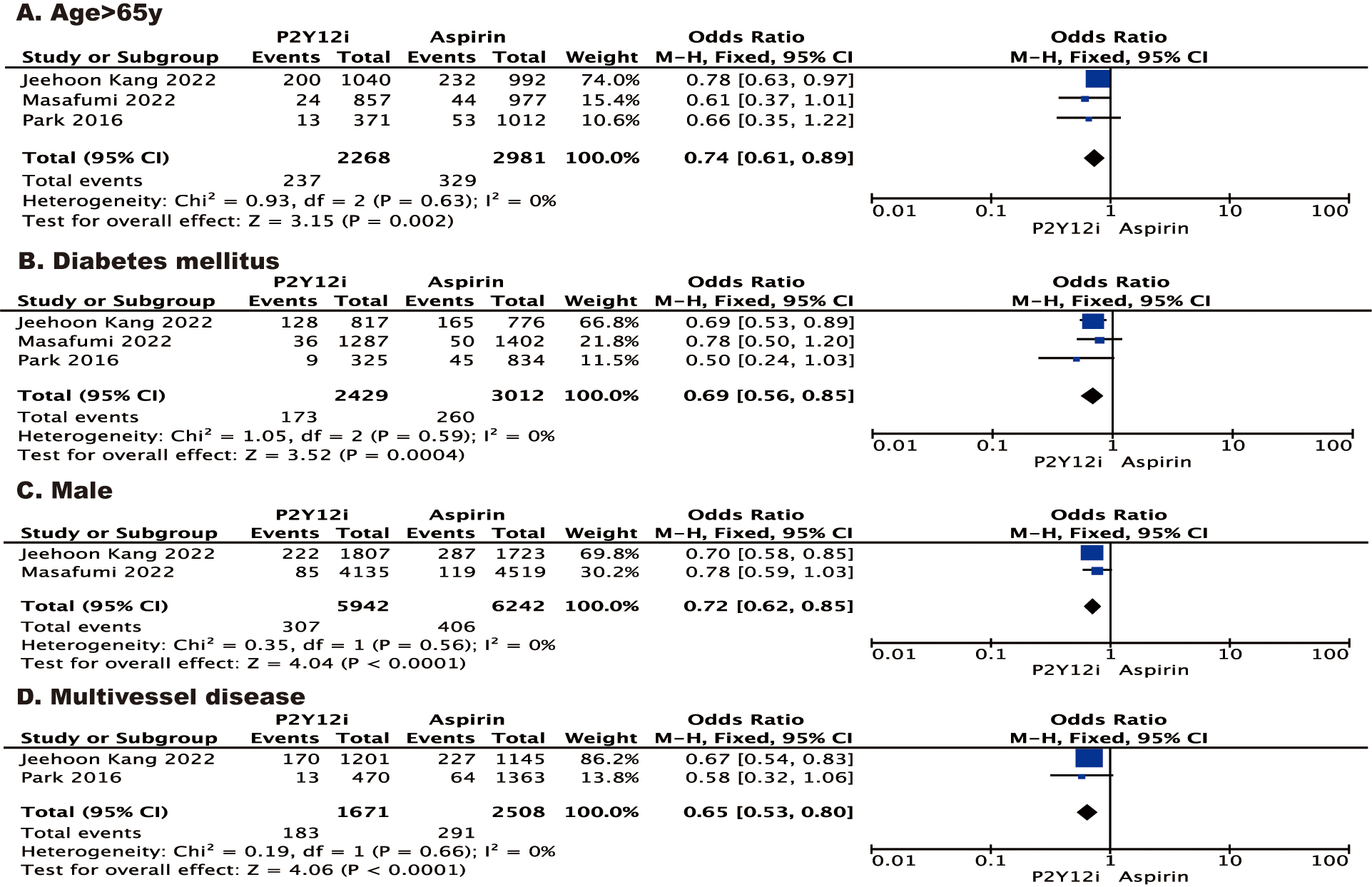 Fig. 3.
Fig. 3.
Forest plot of stratified analysis of MACE according to the
characteristics of patients. Forest plot reporting the odds ratios of P2Y
inhibitor vs aspirin: (A) age 65 y; (B) diabetes mellitus; (C) male; (D)
multivessel disease. y, years.
The results of the risk of bias assessment with the Newcastle-Ottawa Scale for
cohort studies and the RoB2 of randomized control trials are presented in
Supplementary Tables 2,3. Five
studies had a lower risk of overall bias.
Stata 14.0 was used to investigate the impact of a single study on the overall
pooled estimate for each predefined outcome. It was observed that deleting any of
the studies did not affect the following results (Supplementary Fig. 1): MACE, all-cause death, repeat revascularization, stent
thrombosis, myocardial infarction and any stroke. Cardio death and major bleeding
may be affected by trial Jeehoon Kang [12]. Fortunately, we can find in Revman’s
results that I and p values of cardio death meet the fixed effect
condition. As for the bleeding results, we will further discuss the subgroup
analysis according to the different P2Y inhibitors.
4. Discussion
In this study, we compared monotherapy with aspirin versus a P2Y receptor
inhibitor for secondary prevention in patients with ischemic heart disease after
PCI following DAPT. The main findings of the present study are: (1) The risk of
MACE is lower in patients receiving a P2Y receptor inhibitor compared with
those receiving aspirin, which is driven by repeated revascularization and
stroke. (2) Clopidogrel does not increase the risk of major bleeding, however,
ticagrelor showed an increased risk of major bleeding.
Following a routine duration of DAPT, the patients may have the option of
aspirin or P2Y receptor inhibitor for long-term SAPT for secondary
prevention of cardiovascular events. Aspirin is classically considered the SAPT
of choice following DAPT discontinuation after PCI. Notably, most randomized
trials for secondary prevention assessing long-term aspirin therapy to establish
its cornerstone role in the secondary prevention of cardiovascular disease were
done decades ago [17]. P2Y inhibitors are the most commonly used
antiplatelet drugs as an alternative to aspirin and are especially suitable for
patients who are intolerant or allergic to aspirin [18, 19]. Previous studies
have shown that P2Y inhibitors could at least provide similar protective
effects in patients with established atherosclerosis, compared to aspirin [5].
The pharmacodynamics of P2Y inhibitors endows them with more profound
platelet inhibition than aspirin [20]. Furthermore, a previous study found that
clopidogrel was actually more effective than aspirin in atherosclerotic cardiovascular disease (ASCVD) secondary
prevention, with a reduced risk of MACE, but with similar safety results [21]. As
for more focused patients who received PCI, HOST-EXAM Extended (Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis–Extended Antiplatelet Monotherapy) Study indicated
clopidogrel monotherapy as compared with aspirin monotherapy showed lower rates
of the composite net clinical outcome after PCI with drug-eluting stent (DES) [12]. Given these
promising results, we conducted this meta-analysis intending to provide more
evidence for the optimal long-term antiplatelet strategy after standard DAPT.
Our present meta-analysis includes 5 studies (3 observational studies and 2
RCTs), and the results indicate that P2Y inhibitor significantly reduced
MACE compared to aspirin. Of note, this benefit of reduction in MACE was
primarily derived from a significant reduction in repeat revascularization and
any stroke. As for endpoint of all-cause death, cardiac death, myocardial
infarction and stent thrombosis, no obvious benefit was observed. For the safety
endpoint, the incidence of major bleeding was found to be no different between
the P2Y inhibitor group and the aspirin group. These results were similar
to that reported in the study of Tan et al. [13]. What’s different from
their findings is thatwe found a reduction in the risk of repeat
revascularization. This may be due to the large sample size and the longer
follow-up time. To our interest, no reduction in risk of myocardial infarction
was found and this was similar to previous studies [13, 17]. However, this
finding differs from that in the study of Andò et al. [9]. What
needs to be pointed out is that reduction in myocardial infarction between the
two monotherapies does not convert into a decreased risk of cardiovascular death.
This paradox is hard to explain. It was multifactorial and may include the
influence of competing risks due to insufficient follow-up time, or variability
in patient selection in the trials [22]. As for the specific type of P2Y
inhibitor, ticagrelor seems more promising. Ticagrelor monotherapy was associated
with a reduced risk of myocardial infarction (MI) compared to aspirin monotherapy, which is mainly
derived from the results of GLOBAL LEADERS trial [14] (shown in Fig. 4). Due to
the different pharmacokinetic and pharmacodynamic properties of clopidogrel and
ticagrelor, ticagrelor may have more rapid and effective platelet inhibition. The
PLATO (the Study of Platelet Inhibition and Patient Outcomes) trial indicated that ticagrelor proved to be superior to clopidogrel in ACS
patients [23]. In theory, adequate antiplatelet therapy such as using ticagrelor
would be more effective in patients with high-ischemic risk, such as patients
undergoing complex PCI or those with ACS [24, 25]. For a safety endpoint, major
bleeding was analyzed. Our findings found no difference in the risk of major
bleeding between patients treated with P2Y inhibitors and those treated
with aspirin. This is mainly because clopidogrel has a significantly lower risk
of major bleeding than aspirin. Taken alone, however, patients who received
ticagrelor had an increased risk of major bleeding compared to those who received
aspirin (shown in Fig. 4). Therefore, this means that aspirin monotherapy may be
better than ticagrelor monotherapy to avoid unnecessarily increased risk of
severe bleeding, especially in those patients who are at high risk of bleeding.
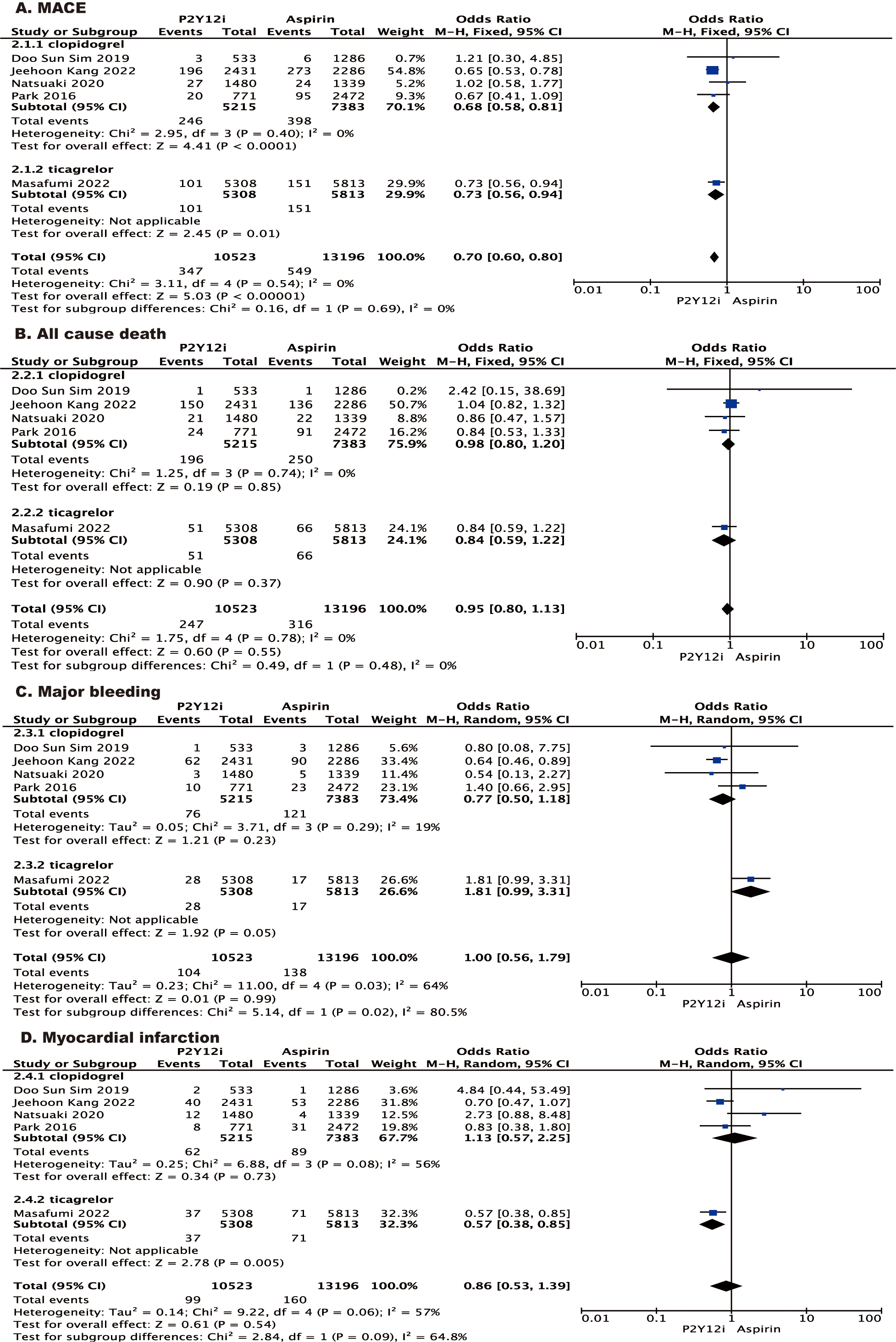 Fig. 4.
Fig. 4.
Forest plot of stratified analysis according to type of
P2Y inhibitor. Forest plot reporting the odds ratios of P2Y
inhibitor vs aspirin: (A) MACE; (B) all-cause death; (C) major bleeding; (D)
myocardial infarction. MACE, major adverse cardiovascular events.
There were several limitations to be mentioned. Firstly, this meta-analysis was
derived from the study-level data but not individual patient-level data. This was
the inherited drawback of meta-analysis. Secondly, only available data from
published literature were used, while some outcomes were not reported. Of note,
only one study involving the comparison between ticagrelor and aspirin could be
obtained. More studies are warranted to verify the association between P2Y
receptor inhibitors and their outcomes. Thirdly, the population is heterogeneous.
Most studies have focused on Asian patients, while only one study was added with
Ticagrelor in a European population. There were only 2 available randomized
controlled trials that directly compared the two monotherapy treatments after
discontinuation of DAPT after PCI, and their limited statistical power provided a
theoretical basis for our meta-analysis. However, we conducted sensitivity
analysis and the final results were consistent.
5. Conclusions
P2Y inhibitor monotherapy following DAPT discontinuation after PCI showed
a reduced risk for MACE, repeat revascularization and stroke compared with
aspirin monotherapy. There was a similar risk for all-cause death, cardiac death
and major bleeding. Our meta-analysis indicates that P2Y inhibitor
monotherapy is potentially superior to aspirin for secondary prevention in the
post-PCI population without an increased risk of major bleeding, but ticagrelor
was associated with an increased risk of bleeding events compared to aspirin
monotherapy.
Abbreviations
PCI, percutaneous coronary intervention; post-PCI, post percutaneous coronary
intervention; DAPT, dual antiplatelet therapy; MACE, major adverse cardiovascular
events; CCS, chronic coronary syndrome; ACS, acute coronary syndrome; SAPT,
single antiplatelet therapy; MI, myocardial infarction; RCTs, randomized
controlled trials; ASCVD, Atherosclerotic Cardiovascular Disease; DES, drug
eluting stent.
Availability of Data and Materials
The data used and analyzed during the current study are available from the
corresponding author on reasonable request.
Author Contributions
TG, CM and YW searched the scientific literature and drafted the manuscript. LB
and SL helped to collect the data and performed statistic analysis. YG, PZ
contributed to the conception, design, data interpretation, manuscript revision
for critical intellectual content, and supervision of the study. All authors
contributed to editorial changes in the manuscript. All authors read and approved
the final manuscript. All authors have participated sufficiently in the work and
agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Not applicable.
Acknowledgment
We would like to thank all the peer reviewers for their opinion and suggestions.
Funding
The work was supported by the Beijing Municipal Administration of Hospitals’
Ascent Plan (Code: DFL20190902), Tsinghua University Spring Breeze Fund, and the
Beijing Tsinghua Changgung Hospital Fund (Grant No. 12019C1009).
Conflict of Interest
The authors declare no conflict of interest.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4.