- Academic Editors
†These authors contributed equally.
Background: Increased leisure-time physical activity (LTPA) is linked
with decreased mortality risk, while also with increased left ventricular mass,
which may induce left ventricular hypertrophy (LVH). We investigated whether LVH
modifies the association between higher LTPA and lower mortality risk in
population at high cardiovascular risk. Methods: In a prospective
national cohort, we used the left ventricular mass/body surface area (LVM/BSA)
method to define LVH. Baseline LTPA was self-reported and divided into: low
(
Regular leisure-time physical activity (LTPA) of at least 500 to 1000 metabolic equivalent of task (MET)-minutes per week without upper limits is consistently advised in patients at high cardiovascular risk [1], based on the evidence of its advantages in lowering the incidence and mortality of cardiovascular disease (CVD) [2]. Nevertheless, in this population, left ventricular hypertrophy (LVH) is a prevalent condition related to poor prognosis, and has a complicated relationship with LTPA at different levels [3, 4, 5]. Several studies have shown that moderate LTPA intensity and duration was related to a reduced risk of LVH and might produce regression of LVH among high-cardiovascular risk patients [6, 7, 8]. In contrast, some studies found that strenuous physical activity was linked with increased left ventricular mass, which may induce hypertrophy, moreover increase the risk of death [9, 10, 11, 12]. In healthy individuals, exercise is commonly linked with benign and reversible cardiac remodeling [9]. Nonetheless, in individuals with certain predisposing risk factors such as electrical cardiac abnormalities and pathological hypertrophy, etc., high-intensity exercise might be linked with an unfavorable prognosis and increased risk of mortality [13]. High-risk cardiovascular populations exhibited multiple risk factors, including hypertension, diabetes, etc., which might contribute to electrical cardiac remodeling, myocardial fibrosis, and pathological hypertrophy [14].
In this case, the influence of LVH on the dose-response association between LTPA and mortality risk in high-risk cardiovascular populations remains unknown. In other words, whether the benefits of moderate to high volumes of LTPA regarding mortality risk could be modified by the presence of LVH has not been extensively studied. This is critical for providing tailored prevention measures, particularly in high cardiovascular risk population.
Therefore, we analyzed the data from a national population-based cohort, with standardized echocardiography conducted in individuals with high cardiovascular risk at baseline. We aimed to evaluate whether the existence of LVH alters the associations between LTPA levels and all-cause and cardiovascular mortality risks.
The China PEACE (Patient-centered Evaluative Assessment of Cardiac Events) Million People Project, a government-funded public health initiative, was launched in 2014. The project design has been previously explained in full [15]. Research sites were chosen from each of 31 provinces in China to ensure a diversified geographical distribution, population structure, exposure to risk variables, and disease trends (Supplementary Methods).
In this project, community residents aged 35 to 75 years old with a residency history of at least six out of the preceding twelve months were recruited. Questionnaire interviews, physical measurements, and laboratory testing were conducted to obtain information on socioeconomic characteristics, health status, risk factors, and drug use. In individuals with high CVD risk defined based on their medical history and risk factor profile, echocardiography and carotid ultrasound were also performed.
In the present study, we included individuals with an assessed 10-year CVD risk of greater than 10% (according to factors including age, gender, blood pressure, diabetes, total cholesterol, body mass index (BMI), and smoking status), based on the 2019 CVD risk charts of the World Health Organization [16] (Supplementary Methods), and those who underwent routine echocardiography. The individuals with previous episodes of CVD or stroke were excluded.
The research was approved by China’s National Center for Cardiovascular Diseases Ethics Council, which is located in Beijing. All participants provided their written informed consent.
LTPA was evaluated with a verified questionnaire [17], which included questions
to collect information about the type, frequency, and duration of activity
performed during the past 12 months. Tai Chi/qigong/leisure walking, swimming,
jogging/aerobic exercise, brisk walking/gymnastics/folk dance, ball games,
mountain hiking, home exercise, and rope jumping were presented examples of
various activities. Based on the 2011 Compendium of Physical Activity, each LTPA
was allocated a unique metabolic equivalent of task (MET) [18]. Frequency,
intensity, and duration were added together to get the energy expenditure
(MET-min/week) associated with LTPA. Low (
All echocardiography ultrasound examinations were performed according to a standardized protocol by certificated ultrasound physicians. Before the implementation of this project, a quality control team comprised of senior ultrasound physicians from the National Center for Cardiovascular Diseases had monitored compliance and quality of ultrasound examinations at each study site. During the implementation, echocardiograms ultrasound images were transmitted to the National Center for Cardiovascular Diseases in DICOM format (JPG and AVI from rural sites where DICOM files were not available) for central adjudication.
End-diastolic ultrasound measurements included the thickness of the posterior
wall, end-diastolic diameter, and septal wall thickness of the left ventricle.
Using the Devereux formula, the left ventricular mass index (LVMI) was determined
by dividing the anatomic mass by the body surface [20]. According to American
Society of Echocardiography (ASE)/European Association for Cardiovascular Imaging (EACI)
guidelines, LVH was defined as LVMI of 115 g/m
All analyses utilized data accessible through December 31, 2021. Using the National Mortality Surveillance System and Vital Registration of China’s Center for Disease Prevention and Control, we determined each participant’s vital status and cause of death. We obtained confirmation of death from local residential, medical, and health insurance records. The 10th Edition of the International Classification of Diseases (ICD) was used to categorize the underlying causes of death. All-cause and cardiovascular mortality were the primary health outcomes (Supplementary Methods).
Continuous variables were reported as mean (standard deviation, SD) or medians
(inter quartile range, IQR), and categorical variables as number (percent).
One-way ANOVA was used to evaluate continuous variables. Categorical variables
were compared using the
The analyses were performed by SAS 9.4 (SAS Institute Inc., Cary, NC, USA) and
statistical significance was defined as a two-sided
p-value
The data of 195,567 participants with a high cardiovascular risk and available echocardiograms were analysed. After excluding those with missing information on LTPA (3584, 1.8%), and those with a history of CVDs (28,977, 15.1%), 163,006 were included (Supplementary Fig. 1). At baseline, the mean (SD) age was 62.4 (7.4) years, and 90078 (55.3%) were females (Table 1). In total, 89.4% had a history of hypertension, 35.0% diabetes, 55.9% dyslipidemia, and 41.3% obesity; while 21.5% and 20.4% were current tobacco smokers and alcohol drinkers, respectively. In addition, 65.2% of the population resided in rural areas, 14.1% had a family income of above 7000 USD per year, and 17.2% had at least a high school diploma.
| Variables | Overall | Non-LVH | LVH | p-value* | |
| (n = 163,006) | (n = 120,234) | (n = 42,772) | |||
| Age (years), mean (SD) | 62.4 (7.4) | 58.6 (8.8) | 63.8 (6.6) | ||
| Age (years), n (%) | |||||
| 35–44 | 3719 (2.3) | 3370 (2.8) | 349 (0.8) | ||
| 45–54 | 20,815 (12.8) | 17,238 (14.3) | 3577 (8.4) | ||
| 55–64 | 69,015 (42.4) | 51,357 (42.8) | 17,658 (41.2) | ||
| 65–75 | 69,457 (42.6) | 48,269 (40.1) | 21,188 (49.5) | ||
| Female sex, n (%) | 90,078 (55.3) | 56,655 (47.1) | 33,423 (78.1) | ||
| Education, n (%) | |||||
| Primary school or lower | 88,157 (54.1) | 60,028 (49.9) | 28,129 (65.8) | ||
| Middle school | 46,803 (28.7) | 37,012 (30.8) | 9791 (22.9) | ||
| High school | 20,609 (12.6) | 16,807 (14.0) | 3802 (8.9) | ||
| College or above | 7437 (4.6) | 6387 (5.3) | 1050 (2.5) | ||
| Household income (USD/year), n (%) | |||||
| 41,593 (25.5) | 28,350 (23.6) | 13,243 (31.0) | |||
| 1400–7000 | 98,441 (60.4) | 74,042 (61.6) | 24,399 (57.0) | ||
| 22,972 (14.1) | 17,842 (14.8) | 5130 (12.0) | |||
| Urbanity, n (%) | |||||
| Rural | 108,656 (65.2) | 79,303 (64.4) | 29,353 (67.5) | ||
| Urban | 54,349 (34.8) | 40,932 (35.6) | 13,419 (32.5) | ||
| Metabolic factors, n (%) | |||||
| Hypertension |
145,665 (89.4) | 105,750 (88.0) | 39,975 (93.3) | ||
| Diabetes |
57,111 (35.0) | 42,516 (35.4) | 14,595 (34.1) | ||
| Dyslipidemia |
91,122 (55.9) | 67,758 (56.4) | 23,364 (54.6) | ||
| Obesity |
67,283 (41.3) | 54,475 (45.3) | 12,808 (29.9) | ||
| Lifestyle, n (%) | |||||
| Tobacco smoker | 35,091 (21.5) | 30,552 (25.4) | 4539 (10.6) | ||
| Alcohol drinker | 33,189 (20.4) | 28,503 (23.7) | 4686 (11.0) | ||
| LTPA, n (%) | |||||
| Low LTPA | 107,407 (65.9) | 77,663 (64.6) | 29,744 (69.5) | ||
| Moderate LTPA | 36,047 (22.1) | 27,763 (23.1) | 8284 (19.4) | ||
| High LTPA | 19,552 (12.0) | 14,808 (12.3) | 4744 (11.1) | ||
| LVMI, g/m |
88.3 (75.1, 103.3) | 81.5 (71.1, 91.2) | 113.8 (102.7, 124.9) | ||
Data presented as number n proportion (%) or mean (SD). Abbreviations: LVH,
left ventricular hypertrophy; LVMI, left ventricular mass index; LTPA, leisure
time physical activity; MET, metabolic equivalent of task; Low LTPA,
Overall, LVH was present in 26.2% of the study population. Compared to those
without LVH, individuals with LVH were more prone to be female, older, and rural
residents, and had less advanced education and income. In addition, the LVH group
has higher frequency of hypertension (93.3% versus 88.0%, p-value
Using a multivariable restricted cubic spline regression, the curvilinear
pattern was observed in the association of volumes of LTPA and LVMI after
adjusting for age, sex, BMI, hypertension, dyslipidemia, diabetes, smoking,
drinking, education, income, and medication use (Fig. 1). In comparison with low
LTPA, there was an incrementally lower LVMI until leveling occurred at
approximately 2000 in the whole population, and LVMI showed a slow increase after
a threshold of approximately 2000, which indicated that the high LTPA group
(
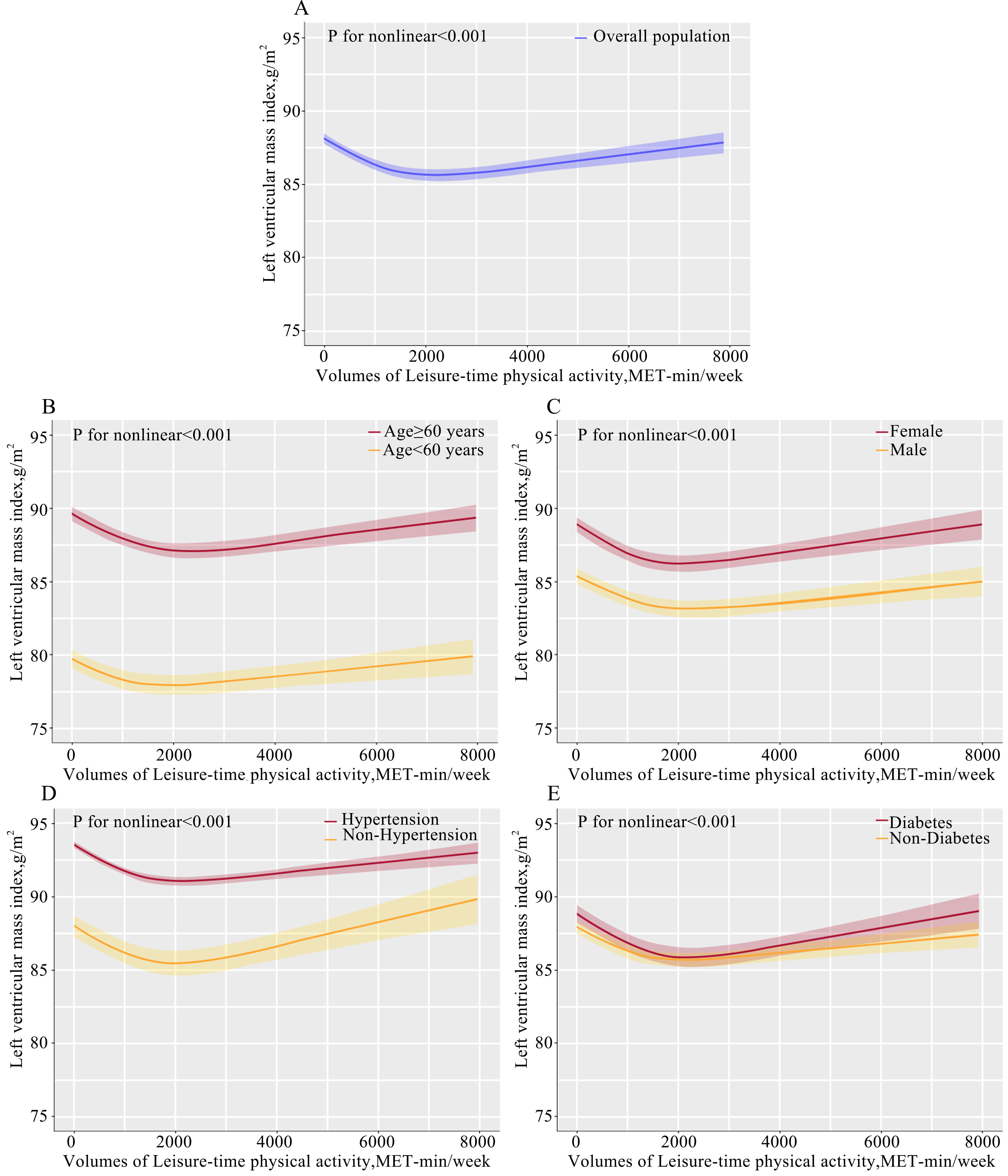 Fig. 1.
Fig. 1.Dose-response associations between leisure-time physical activity and LVMI. The dose-association between LVMI and leisure-time physical activity was performed in whole population (A) and in the subgroup stratified by of age (B), sex (C), hypertension (D), and diabetes (E). Abbreviations: LVMI, left ventricular mass index; MET, metabolic equivalent of task; BMI, body mass index. p-value for nonlinear indicates that the dose-relationship between LVMI and leisure-time physical activity using restricted cubic spline curves adjusted for confounders of age, sex, BMI, hypertension, dyslipidemia, diabetes, smoking status, drinking, education levels, income levels, and medication use.
During a median of 4.8 years of follow-up, 6586 (4.0%) all-cause deaths and 3024 (1.9%) cardiovascular deaths occurred in the study population. Specifically, 4773 (4.44%) and 2270 (2.11%) in the low LTPA group, 1173 (3.25%) and 480 (1.33%) in the moderate LTPA group, and 640 (3.27%) and 274 (1.40%) in the high LTPA group, respectively (Table 2, Supplementary Fig. 2).
| Volumes of LTPA (MET-min/week) | All-cause mortality | Cardiovascular mortality | |||||
| Rates, % (N) | Unadjusted | Adjusted | Rates, % (N) | Unadjusted | Adjusted | ||
| HR (95% CI) | HR (95% CI) * | HR (95% CI) | HR (95% CI) * | ||||
| Non-LVH | 3.83% (4601) | 1.67% (2011) | |||||
| Low ( |
4.23% (3286) | Reference | Reference | 1.91% (1485) | Reference | Reference | |
| Moderate (500–1999) | 3.01% (837) | 0.77 (0.71–0.83) | 0.80 (0.73–0.86) | 1.19% (331) | 0.66 (0.59–0.74) | 0.71 (0.63–0.81) | |
| High ( |
3.23% (478) | 0.76 (0.69–0.84) | 0.77 (0.70–0.85) | 1.32% (195) | 0.69 (0.60–0.81) | 0.73 (0.62–0.85) | |
| LVH | 4.64% (1985) | 2.37% (1013) | |||||
| Low ( |
5.00% (1487) | Reference | Reference | 2.64% (785) | Reference | Reference | |
| Moderate (500–1999) | 4.06% (336) | 0.88 (0.78–0.99) | 0.96 (0.84–1.08) | 1.80% (149) | 0.72 (0.60–0.85) | 0.83 (0.69–0.99) | |
| High ( |
3.41% (162) | 0.66 (0.56–0.78) | 0.73 (0.61–0.86) | 1.67% (79) | 0.62 (0.49–0.78) | 0.72 (0.56–0.91) | |
Abbreviations: LVH, left ventricular hypertrophy; LTPA, leisure-time physical activity; MET, metabolic equivalent of task; HR, hazard ratio; CI, confidence interval; BMI, body mass index. * Multivariable-adjusted model was adjusted for age, sex, BMI, hypertension, dyslipidemia, diabetes, smoking, drinking, education levels, income levels, sites, and medication use. The interaction effect of LTPA and LVH was p for interaction = 0.074 for all-cause mortality; p for interaction = 0.581 for cardiovascular mortality.
Among the participants without LVH, those with a high LTPA had lower all-cause mortality [adjusted HR = 0.77 (0.70–0.85)] and cardiovascular mortality [adjusted HR = 0.73 (0.62–0.85)] compared to those with a low LTPA; while for individuals with moderate LTPA, the adjusted HRs were 0.80 (0.73–0.86) and 0.71 (0.63–0.81) for all-cause mortality and cardiovascular mortality, respectively (Table 2, Fig. 2).
 Fig. 2.
Fig. 2.LTPA and the risk of mortality stratified by LVH in males and females. (A) LTPA and the risk of all-cause mortality in overall population and stratified by males and females. (B) LTPA and the risk of cardiovascular mortality in overall population and stratified by males and females. Abbreviations: LVH, left ventricular hypertrophy; LTPA, leisure-time physical activity; HR, hazard ratio (95% CI); CI, confidence interval; MET, metabolic equivalent of task; BMI, body mass index. Multivariable adjusted model was adjusted for age, sex, BMI, hypertension, dyslipidemia, diabetes, smoking, drinking, education levels, income levels, sites, and medication use. The interaction effect of LTPA and LVH on all-cause mortality: p-value for interaction = 0.074 in whole population; p-value for interaction = 0.765 in males; p-value for interaction = 0.006 in females; cardiovascular mortality: p-value for interaction = 0.581 in whole population; p-value for interaction = 0.716 in males; p-value for interaction = 0.531 in females.
In participants with LVH, high LTPA was independently linked with lower mortality, with adjusted HR of 0.73 (0.61–0.86) for all-cause mortality and 0.72 (0.56–0.91) for cardiovascular mortality. However, moderate LTPA showed no significant effect on reducing all-cause mortality (0.96 [0.84–1.08]), but the benefits were observed in cardiovascular mortality (0.83 [0.69–0.99]) (Table 2, Fig. 2). The association was not modified by LVH for all-cause deaths (p-value for interaction = 0.074) or cardiovascular deaths (p-value for interaction = 0.581).
Fig. 3 depicts the dose-response relationship between volumes of LTPA and
mortality risk with and without LVH, adjusting for variables as listed
previously. The risk of death exhibited an inverse nonlinear association with
LTPA in the absence of LVH (p-value for non-linearity
 Fig. 3.
Fig. 3.Dose-response relationship between LTPA (MET-min/week) and mortality with and without LVH. Restricted cubic spline curves were performed to depict the dose-association between volumes of LTPA and all-cause mortality risk with and without LVH. Models were adjusted for age, sex, BMI, hypertension, dyslipidemia, diabetes, smoking status, drinking, education levels, income levels, sites, and medication use including anti-diabetic, anti-hypertensive, and statins. MET, metabolic equivalent of task; LTPA, leisure-time physical activity; LVH, left ventricular hypertrophy; HR, hazard ratio; CI, confidence interval; BMI, body mass index.
In the non-LVH group, moderate and high LTPA were linked with a decreased risk
of deaths from all-causes in both males (moderate: HR 0.85; 95% CI: 0.77–0.94,
high: HR 0.78; 95% CI: 0.69–0.88) and females (moderate: HR 0.71; 95% CI:
0.61–0.81, high: HR 0.77; 95% CI: 0.64–0.91) (p-value for interaction
= 0.187). Similar results were also observed across the subgroups by age (
However, for the individuals with LVH, significant benefits were only observed in high LTPA for females (moderate: HR 1.00; 95% CI: 0.86, 1.17; high: HR 0.67; 95% CI: 0.54, 0.84), rather than males (moderate: HR 0.90; 95% CI: 0.73, 1.12, high: HR 0.86; 95% CI: 0.66, 1.12). Furthermore, in females, the presence of LVH altered the relationships between LTPA and all-cause mortality risk (p-value for interaction = 0.006) (Fig. 2). Regarding cardiovascular mortality and other outcomes, the health benefits of LTPA across subgroups were similar (although not all reached the statistical threshold for significance) (Supplementary Tables 1A–E).
In sensitivity analyses (Supplementary Tables 2,3), the results for all risk estimates were similar when deaths in the first six months of follow-up were excluded, indicating that the reverse causality between LTPA volumes and mortality risk was minor.
In this study involving a large cohort of high CVD risk individuals with or without LVH, we identified significant associations between moderate and high volumes of LTPA and the risk of deaths from all causes or CVD. The correlation patterns appeared distinct between those with LVH and those without. Overall, the associations were not modified by the presence of LVH, despite high LTPA volumes being linked with increased LVMI; while interestingly, the modification of LVH was significant in females regarding all-cause mortality risk.
Both the LVH and LVH-free populations may derive some benefit from moderate and high LTPA, although the dose-relationship of LTPA and mortality risk in the LVH group differs from that of the general population. Our finding was consistent with the results from a prior study including 3078 of the general population, which showed that moderate and high LTPA were linked with a decreased risk of death both in individuals with normal blood pressure and hypertensive patients with a high prevalence of LVH [23]. Moreover, in our study, we extended their result by illustrating the dose-relationship between LTPA and mortality risk in the LVH and non-LVH populations, providing additional evidence that patients with LVH should engage in sufficient volumes of regular physical activity. Notably, in our large-scale study, information on medication and intensity and duration of LTPA was collected, and the latter was quantified as MET-min/week according to the intensity and duration of certain activities, making the results more robust [24]. And this could address the shortcomings of a previous study regarding its small sample size and lack of information on antihypertensive medication information. Additionally, our study focuses on populations at high cardiovascular risk without established CVD and provides fresh insights into the influence of physical exercise on outcomes for this specific group, which had not been accurately characterized in earlier research [23].
High LTPA was more effective than moderate and low LTPA in lowering all-cause mortality risk in the population with LVH. A possible explanation is that high volume LTPA may involve cardiac adaptations which could improve prognosis. Previous studies have demonstrated that long-term strenuous exercise could result in detrimental heart remodeling, including cardiac enlargement, inappropriate myocardial thickening and other abnormalities of diastolic dysfunction [9, 10, 11]. However, these studies focused mostly on athletics and certain specific trainings. Our study indicates that even high volumes of daily physical activity would not increase the risk of mortality in non-athletes, and the increase in volume may be associated with cardiac fitness. Similarly, it is plausible to hypothesize that inappropriate remodeling of the heart may be associated more with the intensity than with the volumes of LTPA. Previous studies have observed that LVH regression could be achieved by pharmacological treatment [25, 26] and modest physical activity (PA) [27, 28], which is consistent with our findings. Additionally, we observed a significantly lower LVMI at about 2000 MET-min/week of LTPA volumes across the subgroups of a high cardiovascular risk population, despite the fact that increased levels of physical activity or exercises were related to a rise in LV mass in patients without LVH at baseline and Asian athletes [11, 23, 29]. The different designs of the study populations might contribute to this contrast. Individuals with high CVD risk factors may be in a different risk category. Therefore, LTPA may have a different effect on their left ventricular mass.
Interestingly, the modification of LVH between LTPA and mortality risk was pronounced in the female population. The PA levels in females were found to be different from those in males both in the healthy and CVD population [30, 31]. Although the precise underlying processes have not been identified, one possible explanation could be females’ particular LV anatomy and high sensitivity to the advantages of LTPA. Females have smaller hearts, which makes the LV more sensitive to myocardial fibrosis [32, 33]. In contrast to males, females tended to have poorer glucose absorption and utilization, higher fatty acid intake and metabolic inefficiency [34], and increased vagal tone due to sex hormones [35]. Additionally, myocardial fibrosis [36], myocardial substrate [37, 38], and autonomic nervous system [39] were the targets of PA on prognosis, which may strengthen the modification of LVH regarding the benefits of LTPA on mortality risk. Overall, the modification of LVH was not significant in the overall population regarding the effects of LTPA on risk of deaths, which could be partly explained by the increased LTPA influence on deaths through its beneficial effect on improving vascular endothelial function [40], lowering blood pressure levels [41], increasing insulin sensitivity, and decreasing CVD risk profiles [42]. These positive effects of LTPA on other established risk factors might affect their interaction, rather than LTPA itself. Nonetheless, the reason for the observed modification is likely to be multi-factorial, which merits further evaluation.
Our study has several clinical implications. First, this study provides new insights into the preventive effects of moderate to high LTPA in LVH patients with high cardiovascular risk. Second, it demonstrates that individuals with LVH tend to acquire lower LTPA levels. In addition to being typically older and suffering from various comorbidities, their myocardial dysfunction impairs their physical performance. Nonetheless, clinicians should emphasize to their patients the value of an active lifestyle. The patients should be encouraged to increase their regular LTPA volume; above 2000 MET-min/week could be considered an applicable requirement for LVH individuals for reducing mortality risk, while volumes above 500 MET-min/week could be beneficial for those without LVH. Third, and more importantly, women are more likely to have LVH, and a regular high LTPA is required to attenuate the mortality risk in LVH individuals. It is probable that women will experience notable advantages, potentially resulting in a decrease in the utilisation of alternative treatments such as pharmaceutical interventions.
Limitations of this study should be acknowledged. First, LTPA was self-reported and whether the LTPA levels changed during the follow-up period was unknown. A large-scale study, however, had verified the accuracy of the PA-related questions [17]. Therefore, we made an assumption that the recollection bias could be minimal. Second, despite rigorous statistical adjustments, it is not possible to exclude the presence of unadjusted confounding factors, such as cardiorespiratory fitness, which was linked to a better prognosis [43]. Notwithstanding these constraints, the majority of participant variables deemed pertinent to mortality risk were incorporated into the models. The findings were robust in sensitivity analysis, thereby enhancing the credibility of the conclusion. However, limited statistical power was observed in some subgroups due to an inadequate sample size.
In conclusion, our findings indicated that LVH does not significantly modify the link between LTPA and mortality risk in the overall high cardiovascular risk population. However, the presence of LVH altered this correlation in females regarding the all-cause mortality risk.
BMI, body mass index; BSA, body surface area; CVD, cardiovascular disease; China PEACE MPP, China Patient-centered Evaluative Assessment of Cardiac Events Million Persons Project; CI, confidence interval; DBP, diastolic blood pressure; FBG, fasting blood glucose; HR, hazard ratio; ICD-10, International Classification of Diseases, 10th edition; LTPA, leisure-time physical activity; LVM, left ventricular mass; LVMI, left ventricular mass index; LVH, left ventricular hypertrophy; MET, metabolic equivalent of task; PA, physical activity; SD, standard deviation; SBP, systolic blood pressure.
The China Patient-centered Evaluative Assessment of Cardiac Events Million Persons Project (China PEACE MPP) data that supported this study are restricted and not publically available. Data are available upon reasonable request and with China PEACE MPP authorization. China PEACE MPP grants conditional data access to qualified researchers with valid requests. Please contact http://cvd-project@nccd.org.cn to seek approval for data access.
XZ, XL and HJJ designed the research study. XL conceived of the China Patient-centered Evaluative Assessment of Cardiac Events Million Persons Project (China PEACE MPP) and takes responsibility for all aspects of it. HD, JLC, BWC, CQW, XYZ, and YW participated in the project operation. XLW, HD, JLC, BWC, CQW, XYZ, AXT and YW made substantial contributions to the data acquisition process. HJJ analyzed the data. BWC performed the validation. HJJ wrote the manuscript, with further contributions from XZ, XL, XLW, AXT, HD, JLC, BWC, CQW, XYZ, and YW. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors contributed to editorial changes in the manuscript and approved the final manuscript.
The study was conducted following the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Fuwai Hospital, Beijing, China (Approval No. 2014-574). All participants provided their informed consent in writing.
We appreciate the multiple contributions made by study teams at the National Center for Cardiovascular Diseases, and the local sites in the collaborative network in the realms of study design and operations.
This research was funded by the CAMS Innovation Fund for Medical Sciences (CIFMS), grant number [2021-I2M-1-011].
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
