- Academic Editors
†These authors contributed equally.
Background: Recommendations for drug treatment of left ventricular
thrombus (LVT) are based on the ST-segment elevation myocardial infarction
(STEMI) guidelines; however, the etiology of LVT has changed. Due to the lack of
evidence regarding LVT treatment in the heart failure population, current heart
failure guidelines do not cover LVT treatment. We sought to
review the etiology of LVT and changes in antithrombotic therapy over the
previous 12 years and explore the impact of anticoagulation treatment from a
single center’s experience. Methods: From January 2009 to June 2021, we
studied 1675 patients with a discharge diagnosis of LVT at a single center to
investigate the clinical characteristics, incidence of all-cause death,
cardiovascular death, ischemic stroke, major adverse cardiac and cerebrovascular
events (MACCE), systemic embolism (SE), and major bleeding events. Patients were
divided into an anticoagulant group and a non-anticoagulant group according to
whether they received oral anticoagulant therapy at discharge. Results:
The study included 909 patients (anticoagulation, 510; no anticoagulation, 399).
While overall antiplatelet therapy dramatically decreased, more patients with LVT
received oral anticoagulation in 2021 (74.0%) than in 2009 (29.6%). In
addition, more than half of the patients had heart failure with reduced ejection
fraction (HFrEF) each year. The all-cause mortality was 17.3% during 3.8 years
of follow-up. The incidences of cardiovascular death, stroke, MACCE, SE, and
major bleeding were 16.0%, 3.3%, 19.8%, 5.1%, and 1.7%, respectively. The
anticoagulation group had a significantly higher proportion of dilated
cardiomyopathy than the non-anticoagulation group (24.7% vs. 5.5%, p
Left ventricular thrombus (LVT), a severe consequence of ventricular
dysfunction, is linked to a significant risk of major adverse cardiac and
cerebrovascular events (MACCE) [1]. The temporal incidence of LVT after myocardial
infarction may be decreasing due to improved percutaneous coronary intervention
(PCI) [1, 2], however the risk of LVT cannot be overlooked [3]. Recommendations
for drug treatment of LVT are based on evidence from the ST-segment elevation
myocardial infarction (STEMI) guidelines in acute myocardial infarction (AMI)
patients [4, 5]. However, the etiology of LVT has changed—heart failure is now
the most common cause of LVT [6]. A recent scientific statement [7] recommended
anticoagulation in patients with LVT in the presence of dilated cardiomyopathy
for at least 3–6 months, with discontinuation if the ejection fraction improves
to
All 1675 consecutive patients who were hospitalized with LVT at the Fuwai Hospital between January 2009 and June 2021 were included. Preliminary screening was performed using discharge diagnosis.
Medical reports were used to gather all patient baseline data, including long-term anticoagulant treatment before an LVT diagnosis. The type of anticoagulant used and its relationship to antiplatelet therapy received were also documented. The pattern of follow-up was consistent with Robinson et al. [12]. The interquartile range (IQR) for the follow-up period was 1.9–6.6 years (median, 3.8 years). The overall follow-up rate was 91.1% (1526/1675).
Patients were diagnosed with LVT using transthoracic echocardiography, contrast-enhanced computed tomography, or cardiac magnetic resonance (CMR) imaging. A ventricular cavity with an abnormal echo mass or intensity, whose margin was distinct from the ventricular endocardium, was recognized as a ventricular thrombus. Morphological information of the thrombus including location, range of motion, mural or protruding, number and density were also recorded.
The endpoints were all-cause death, cardiovascular death, ischemic stroke, MACCE, systemic embolism (SE), and major bleeding. MACCE was a composite of end points, including cardiovascular death, ischemic stroke, and AMI. SE included ischemic stroke, AMI, or acute peripheral artery emboli (limb, renal, or digestive arteries). The occurrence of a Bleeding Academic Research Consortium (BARC) type 2, 3, or 5 bleeding was defined as major bleeding [13].
All continuous variables were expressed by median and IQR, whereas categorical
variables are presented as numbers and percentages. Categorical variables were
compared using the
Competing-risk analysis was performed in line with previous studies [14, 15]. Heterogeneity of the treatment effect for MACCE was evaluated by assessing treatment interactions across subgroups, including age, sex, BMI, LVEF, diabetes, and eGFR.
Propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) were performed as sensitivity analysis. Information about matching and weighting is described in detail in the Supplementary Methods.
Among 1675 patients extracted from an electronic database according to International Classification of Diseases (ICD)
codes, 909 were included in final analysis (Fig. 1). The baseline characteristics
of the patients are listed in Table 1. The cohort had a median age of 55.0
(45.0–64.0) years and a high prevalence of coronary artery disease (77.2%, n =
702). One in four patients (23.9%, n = 217) had a STEMI. More than 50% of the
population (60.8%, n = 553) had a reduced LVEF (LVEF
 Fig. 1.
Fig. 1.Study flow chart. VKA, vitamin K antagonists; DOAC, direct oral anticoagulants; LVT, left ventricular thrombus.
| Overall | Without anticoagulation | With anticoagulation | p value | ||
| n | 909 | 399 | 510 | ||
| Demographic | |||||
| Age | 55.0 [45.0, 64.0] | 57.0 [48.0, 67.0] | 53.0 [42.0, 62.0] | ||
| Male | 753 (82.8) | 337 (84.5) | 416 (81.6) | 0.290 | |
| Body mass index/kg/m |
25.00 [22.72, 27.47] | 24.97 [22.85, 27.30] | 25.01 [22.61, 27.76] | 0.519 | |
| Past medical history | |||||
| Hypertension | 445 (49.0) | 205 (51.4) | 240 (47.1) | 0.220 | |
| Diabetes mellitus | 366 (40.3) | 159 (39.8) | 207 (40.6) | 0.875 | |
| eGFR |
133 (14.6) | 54 (13.5) | 79 (15.5) | 0.463 | |
| Peripheral artery disease | 68 (7.5) | 26 (6.5) | 42 (8.2) | 0.395 | |
| Prior stroke | 149 (16.4) | 63 (15.8) | 86 (16.9) | 0.731 | |
| Prior MI | 472 (51.9) | 248 (62.2) | 224 (43.9) | ||
| Prior CABG | 19 (2.1) | 11 (2.8) | 8 (1.6) | 0.313 | |
| Prior PCI | 138 (15.2) | 66 (16.5) | 72 (14.1) | 0.359 | |
| Prior cerebral hemorrhage | 6 (0.7) | 4 (1.0) | 2 (0.4) | 0.475 | |
| Atrial fibrillation | 71 (7.8) | 25 (6.3) | 46 (9.0) | 0.158 | |
| Underlying disease | |||||
| Coronary artery disease | 702 (77.2) | 368 (92.2) | 334 (65.5) | ||
| STEMI | 217 (23.9) | 125 (31.3) | 92 (18.0) | ||
| NSTEMI | 39 (4.3) | 20 (5.0) | 19 (3.7) | 0.432 | |
| Dilated cardiomyopathy | 148 (16.3) | 22 (5.5) | 126 (24.7) | ||
| Hypertrophic cardiomyopathy | 19 (2.1) | 3 (0.8) | 16 (3.1) | 0.024 | |
| ARVD with associated LV impairment | 4 (0.4) | 1 (0.3) | 3 (0.6) | 0.796 | |
| Perinatal cardiomyopathy | 12 (1.3) | 1 (0.3) | 11 (2.2) | 0.027 | |
| Restrictive cardiomyopathy | 4 (0.4) | 0 (0.0) | 4 (0.8) | 0.205 | |
| Alcoholic cardiomyopathy | 11 (1.2) | 0 (0.0) | 11 (2.2) | 0.008 | |
| Myocarditis | 6 (0.7) | 2 (0.5) | 4 (0.8) | 0.912 | |
| NVM | 20 (2.2) | 5 (1.3) | 15 (2.9) | 0.135 | |
| Medications | |||||
| Aspirin | 547 (60.2) | 359 (90.0) | 188 (36.9) | ||
| Clopidogrel | 452 (49.7) | 269 (67.4) | 183 (35.9) | ||
| Ticagrelor | 38 (4.2) | 34 (8.5) | 4 (0.8) | ||
| DAPT | 405 (44.6) | 293 (73.4) | 112 (22.0) | ||
| VKA | 295 (32.5) | 0 (0.0) | 295 (57.8) | ||
| Rivaroxaban | 198 (21.8) | 0 (0.0) | 198 (38.8) | ||
| Dabigatran | 17 (1.9) | 0 (0.0) | 17 (3.3) | 0.001 | |
| DOAC | 215 (23.7) | 0 (0.0) | 215 (42.2) | ||
| Antiplatelet therapy only | 369 (40.6) | 369 (92.5) | 0 (0.0) | ||
| Anticoagulation only | 247 (27.2) | 0 (0.0) | 247 (48.4) | ||
| Anticoagulation status | |||||
| Dabigatran 110 mg BID | 16 (1.8) | 0 (0.0) | 16 (3.1) | ||
| Rivaroxaban 2.5 mg QD | 9 (1.0) | 0 (0.0) | 9 (1.8) | ||
| Rivaroxaban 5 mg QD | 6 (0.7) | 0 (0.0) | 6 (1.2) | ||
| Rivaroxaban 10 mg QD | 18 (2.0) | 0 (0.0) | 18 (3.5) | ||
| Rivaroxaban 15 mg QD | 57 (6.3) | 0 (0.0) | 57 (11.2) | ||
| Rivaroxaban 15 mg BID | 25 (2.8) | 0 (0.0) | 25 (4.9) | ||
| Rivaroxaban 20 mg QD | 79 (8.7) | 0 (0.0) | 79 (15.5) | ||
| Aspirin with anticoagulant | 76 (8.4) | 0 (0.0) | 76 (14.9) | ||
| Clopidogrel with anticoagulant | 74 (8.1) | 0 (0.0) | 74 (14.5) | ||
| Anticoagulant with DAPT | 112 (12.3) | 0 (0.0) | 112 (22.0) | ||
| Imaging morphology of LVT | |||||
| LVEDD | 58.0 [53.0, 65.0] | 56.0 [51.0, 60.4] | 60.0 [55.0, 69.0] | ||
| LVEF | 38.0 [29.0, 46.0] | 41.0 [34.0, 48.0] | 34.0 [26.0, 43.0] | ||
| LVEF |
553 (60.8) | 198 (49.6) | 355 (69.6) | ||
| Global hypokinesis | 229 (25.2) | 43 (10.8) | 186 (36.5) | ||
| Hypokinesis | 395 (43.5) | 212 (53.1) | 183 (35.9) | ||
| Akinesis | 562 (61.8) | 289 (72.4) | 273 (53.5) | ||
| Apical LVT | 832 (91.5) | 364 (91.2) | 468 (91.8) | 0.866 | |
| Round LVT | 559 (61.5) | 219 (54.9) | 340 (66.7) | ||
| Mobile LVT | 77 (8.5) | 15 (3.8) | 62 (12.2) | ||
| Multiple LVT | 107 (11.8) | 23 (5.8) | 84 (16.5) | ||
| Calcified LVT | 167 (18.4) | 53 (13.3) | 114 (22.4) | 0.001 | |
| LVT largest diameter/mm | 23.0 [16.0, 31.0] | 23.0 [17.0, 32.0] | 22.0 [16.0, 31.0] | 0.182 | |
| LVT area/mm |
2.9 [1.6, 4.6] | 2.9 [1.7, 4.5] | 2.8 [1.6, 4.6] | 0.906 | |
| Left ventricular aneurysm | 472 (51.9) | 252 (63.2) | 220 (43.1) | ||
Data are n/N (%) or median (IQR). ARVD, arrhythmogenic right ventricular dysplasia; CABG, coronary artery bypass grafting; DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulants; DAPT, dual antiplatelet therapy; eGFR, estimated glomerular filtration rate; LVT, left ventricular thrombus; LVEDD, left ventricular end diastolic dimension; LVEF, left ventricular ejection fraction; LV, left ventricle; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; NVM, noncompaction of the ventricular myocardium; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; VKA, vitamin K antagonists; BID: bis in die (in Latin, twice a day); QD, quaque die (which means, in Latin, once a day).
Compared with patients without anticoagulation (n = 399), those who received
anticoagulation (n = 510) at discharge were younger (53.0 vs. 57.0, p
In the anticoagulation group, more than half the patients received vitamin K antagonists (VKA) (57.8%, n = 295) and the rest received direct oral anticoagulants (DOAC) (42.2%, n = 215). Rivaroxaban was the most commonly used DOAC (38.8%, n = 198), while dabigatran was used less often (3.3%, n = 17). The doses of rivaroxaban were varied: 79 patients were prescribed 20 mg once daily, 57 were prescribed 15 mg once daily, 25 were prescribed 15 mg twice daily, and 18 were prescribed 10 mg once daily.
While overall antiplatelet therapy has dramatically decreased (Fig. 2A), more
patients with LVT started oral anticoagulation (OAC) in 2021 than in 2009 (74.0%
vs. 29.6%, Fig. 2B). The use of VKA has dropped yearly, from a peak of 44.8% in
2017 to 13.7% in 2021 (Fig. 2C). In contrast, DOAC usage has increased, especially
in the past 6 years, from 11.4% in 2016 to 60.3% in 2021 (Fig. 2D). The
proportion of rivaroxaban has also rapidly increased (Fig. 2E), whereas the use
of dabigatran has been low for over a decade (Fig. 2F). All p values for the
trend except for dabigatran were
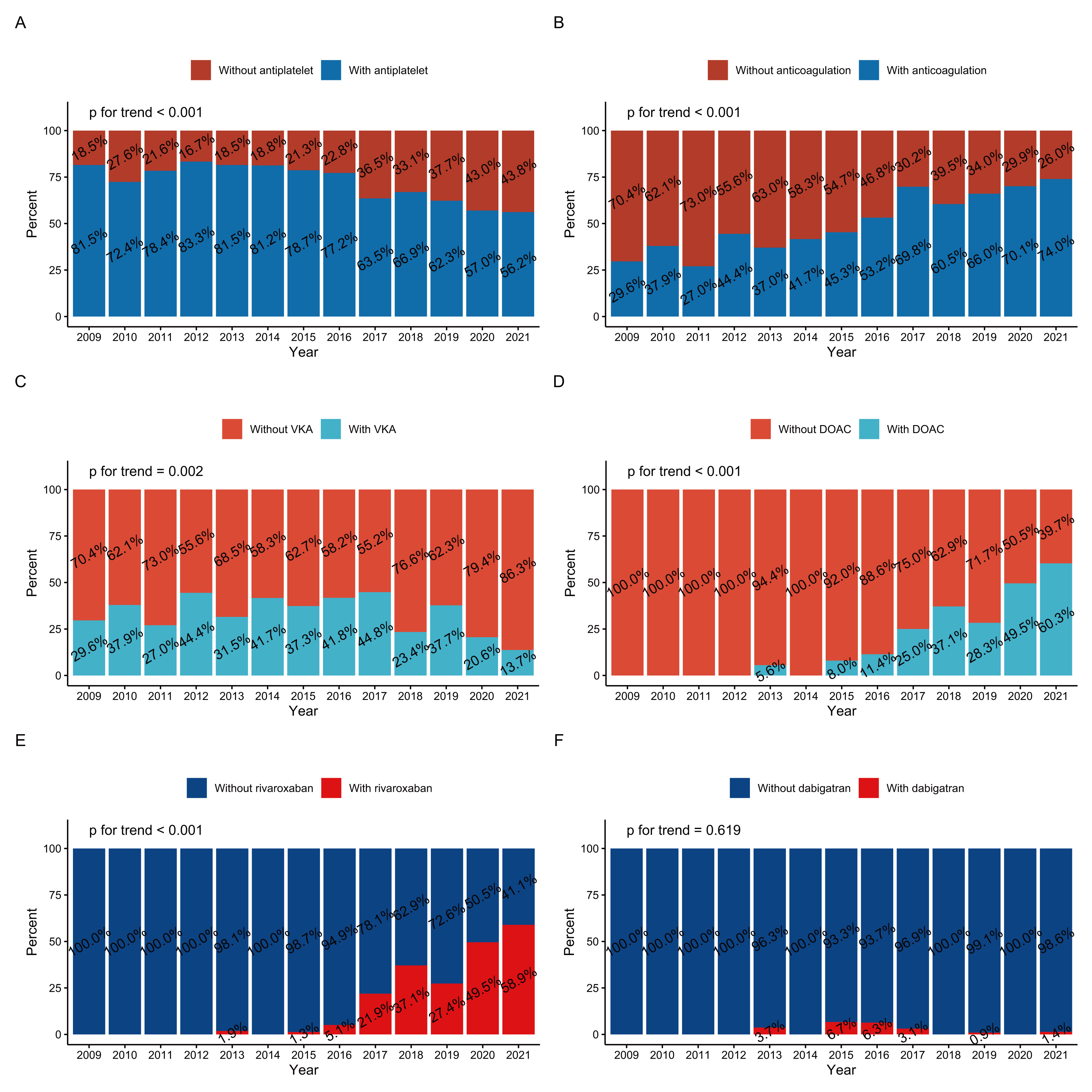 Fig. 2.
Fig. 2.Time trends of therapy among patients with LVT at discharge (2009–2021): (A) antiplatelet therapy, (B) oral anticoagulation therapy, (C) VKA, (D) DOAC, (E) rivaroxaban, and (F) dabigatran. DOAC, direct oral anticoagulants; LVT, left ventricular thrombus; VKA, vitamin K antagonists.
Over 50% of patients with LVT had a LVEF
As shown in Supplementary Fig. 2, patients discharged between 2016 and 2021 had a higher risk of stroke (p = 0.012), SE (p = 0.001), and major bleeding (p = 0.025) than those discharged between 2009 and 2015. There were no significant differences in all-cause mortality (p = 0.414), cardiovascular death (p = 0.561), or MACCE (p = 0.101) between the two groups.
As shown in Table 2, all-cause mortality was 17.3% (n = 157). The incidence of
cardiovascular death and stroke was 16.0% (n = 145) and 3.3% (n = 30),
respectively. Moreover, MACCE and SE occurred in 19.8% (n = 180) and 5.1% (n =
46) of patients, respectively, while major bleeding (BARC
| Overall (n = 909) | Without anticoagulation (n = 399) | With anticoagulation (n = 510) | Multivariable analysis | PSM analysis | IPTW analysis | ||||
| Hazard ratio (95% CI) | p values | Hazard ratio (95% CI) | p values | Hazard ratio (95% CI) | p values | ||||
| All-cause death | 157 (17.3%) | 77 (19.3) | 80 (15.7) | 0.96 (0.67–1.38) | 0.800 | 0.71 (0.45–1.12) | 0.140 | 0.83 (0.59–1.18) | 0.307 |
| Cardiovascular death | 145 (16.0%) | 65 (16.3) | 80 (15.7) | 1.05 (0.72–1.53) | 0.800 | 0.74 (0.47–1.18) | 0.200 | 0.91 (0.63–1.31) | 0.616 |
| Stroke | 30 (3.3%) | 13 (3.3) | 17 (3.3) | 1.62 (0.76–3.45) | 0.200 | 1.72 (0.66–4.47) | 0.300 | 1.59 (0.74–3.41) | 0.232 |
| MACCE | 180 (19.8%) | 81 (20.3) | 99 (19.4) | 1.15 (0.82–1.61) | 0.400 | 0.88 (0.59–1.32) | 0.500 | 1.03 (0.74–1.44) | 0.846 |
| Systemic embolism | 46 (5.1%) | 19 (4.8) | 27 (5.3) | 1.69 (0.92–3.13) | 0.093 | 1.40 (0.66–2.97) | 0.400 | 1.81 (0.97–3.39) | 0.062 |
| Major bleeding | 15 (1.7%) | 9 (2.3) | 6 (1.2) | 0.77 (0.29–2.03) | 0.600 | 0.49 (0.10–2.48) | 0.400 | 0.78 (0.26–2.34) | 0.663 |
Values are n (%). MACCE, major adverse cardiac and cerebrovascular events; CI, confidence interval; PSM, propensity score matching; IPTW, inverse probability of treatment weighting.
 Fig. 3.
Fig. 3.Time-to-event curves according to anticoagulation treatment. AC, anticoagulation; MACCE, major adverse cardiac and cerebrovascular events.
After 1:1 PSM, 514 patients (anticoagulation, 257; no anticoagulation, 257) were
selected (Supplementary Table 1). Ultimately, 905 patients
(anticoagulation, 510.38; no anticoagulation, 394.62) were chosen after IPTW
(Supplementary Table 2). All baseline variables included in the matching
and weighting process became balanced (standardized mean difference (SMD)
Multivariable analysis concludes diabetes mellitus (hazard ratio (HR), 1.42; 95% confidence interval (CI),
1.04–1.93; p = 0.027), estimated glomerular filtration rate (eGFR)
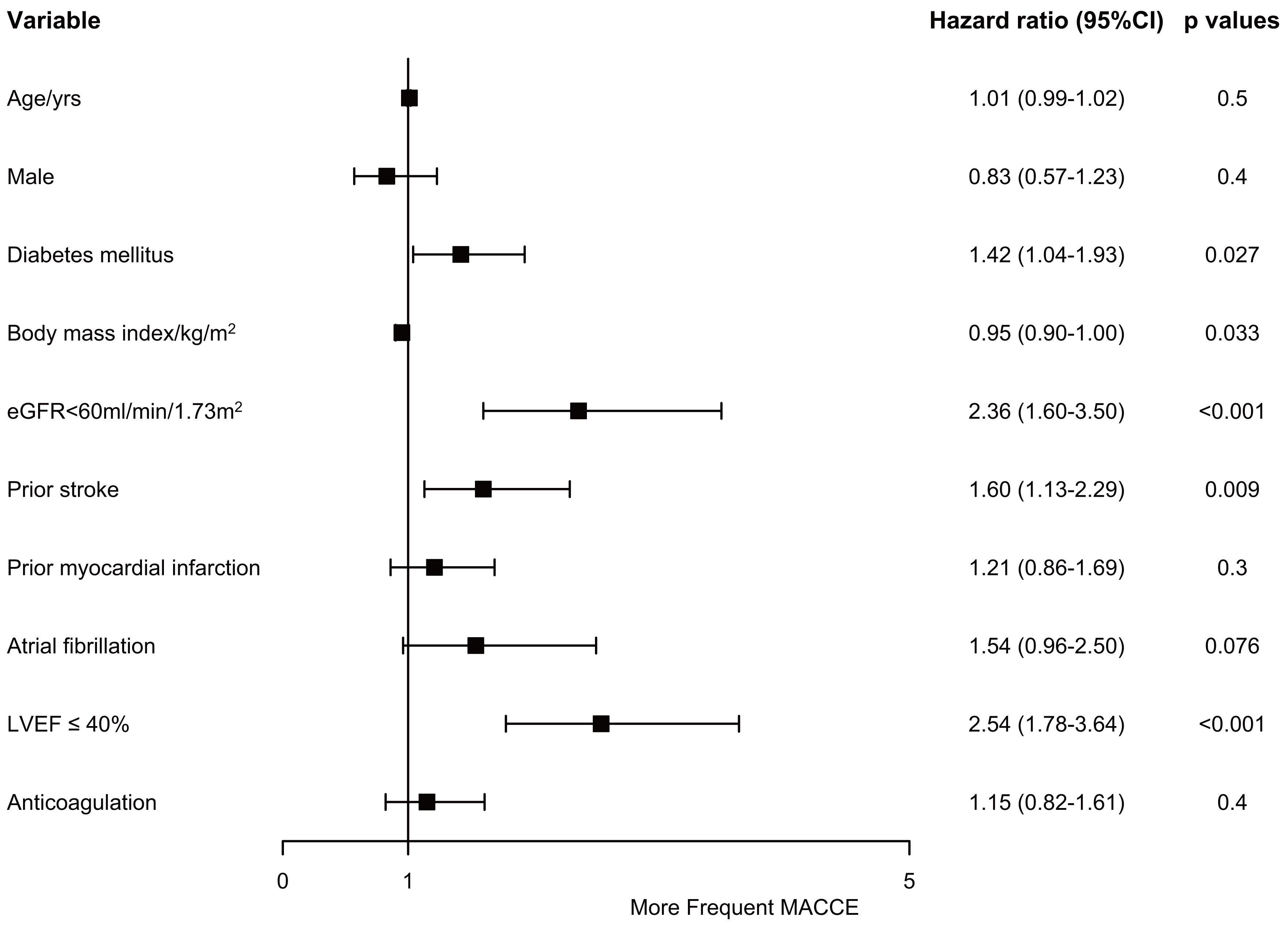 Fig. 4.
Fig. 4.Risk factors of major adverse cardiac and cerebrovascular events. CI, confidence interval; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiac and cerebrovascular events.
The results were in line with the overall efficacy finding across all subgroups
(Fig. 5). None of the interactions were significant. Surprisingly, in patients
with LVEF
 Fig. 5.
Fig. 5.Relationship between anticoagulation therapy and risk of major adverse cardiac and cerebrovascular events in subgroup analyses. CI, confidence interval; LVEF, left ventricular ejection fraction; eGFR, estimated glomerular filtration rate.
As shown in Fig. 6, the direct oral anticoagulants (DOAC) group had a higher risk of SE than the other two groups (p = 0.021).
 Fig. 6.
Fig. 6.Time-to-event curves according to anticoagulation treatment (None vs. VKA vs. DOAC). VKA, Vitamin K antagonists; DOAC, direct oral anticoagulants; MACCE, major adverse cardiac and cerebrovascular events.
To the best of our knowledge, our study is the largest LVT cohort. The study’s
key conclusions include the following: (i) heart failure with reduced ejection
fraction overtook AMI as the leading cause of LVT; (ii) currently, an increasing
proportion of patients with LVT are receiving anticoagulant therapy at discharge;
(iii) since 2020, rivaroxaban has displaced warfarin as the most popular OAC for
LVT patients; (iv) a trend towards better prognosis in the no anticoagulation
group can be noted from survival curves; (v) from multivariable, matching and
weighting analysis, no improvement in prognosis was observed with anticoagulant
therapy; (vi) diabetes mellitus, eGFR
Previous research has often added the qualifier of AMI to LVT [1, 2, 16, 17] while
ignoring
Lemaitre et al. [19] found all-cause mortality occurred in 10% of
patients with LVT and heart failure, while symptomatic emboli occurred in 15%
and major bleeding occurred in 5% during a median follow-up of 8.7 years. This
differs from the findings in our current study. In our cohort, the risk of SE and
major bleeding was lower, while the risk of all-cause mortality was higher.
Compared with the study findings of Lattuca et al. [18], LVEF was
similar in our population (31.9% vs. 38.0%), but the proportion of mobile LVT
was lower (34.6% vs. 8.5%), and the proportion of calcified LVT (1.3% vs.
18.4%) was higher. The risk of embolism in patients with LVT is closely related
to two morphologic features: movement and protrusion [2]. Differences in LVT
characteristics may be a possible explanation for the lower risk of embolism in
our population. The major bleeding was higher in the no anticoagulation arm
(2.3% vs. 1.2%). This may be due to the fact that the proportion of dual
antiplatelet therapy in the no-anticoagulation group was much higher than that in
the anticoagulation group (73.4% vs. 22.0%, p
Our cohort is the largest LVT cohort reported, allowing us to perform some
sensitivity analyses. With a prevalence inversely associated with LVEF [23], LVT
has been documented in 11–44% of patients with heart failure [24].
Anticoagulation failed to produce positive results, even in patients with LVEF
This study included the largest cohort of LVT patients to date and was a retrospective observational analysis. However, this study had several limitations. The main limitation of this study is that it is a retrospective study. We attempted to collect various factors that might influence prognosis for multivariate analysis. However, data on medication adherence and switching medications were not collected during the follow-up. In addition, imaging follow-up data on patients were difficult to obtain, so we did not conduct a relevant analysis. Our results should be viewed as exploratory because we realize that the comparison between the two groups was constrained by the disparate baseline characteristics. We cannot conclude whether anticoagulation improves cardiovascular outcomes for patients with LVT. Our study only suggests that the management of anticoagulation should be strengthened to achieve better results. Not all of the patients in our study who had LVT as determined by different imaging tests had a CMR. This may introduce heterogeneity issues. Finally, the low incidence of embolism and bleeding in our cohort may limit the validity of the findings in other races. Asian populations have a different risk of bleeding and ischemia, as demonstrated in the coronary heart disease population [27].
In this retrospective analysis, we examined the temporal patterns in the causes, courses of therapy, and results among Asian LVT patients. We found that heart failure, rather than AMI, was the primary cause of LVT. Additionally, there has been an increase in OAC utilization among patients discharged with LVT. Moreover, rivaroxaban has surpassed warfarin as the most popular first-line anticoagulant among patients with LVT since 2020. A trend towards better prognosis in the no anticoagulation group was noted from the survival curves. From multivariable, matching and weighting analysis, no improvement in prognosis was observed with anticoagulant therapy. Our study does not negate the efficacy of anticoagulation but suggests the need to strengthen the management of anticoagulation in order to achieve better efficacy.
AMI, acute myocardial infarction; DOAC, direct oral anticoagulants; LVT, left ventricular thrombus; LVEDD, left ventricular end diastolic dimension; LVEF, left ventricular ejection fraction; MACCE, major adverse cardiac and cerebrovascular events; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; VKA, vitamin K antagonists.
The data used in our study is not publicly available but is available from corresponding author on reasonable request.
BS, YS, LM, LJ, DY, HW, WF, KFD, and WS—study design and interpretation of results. BS—data collection. BS, YS, LM, XT, JL, RZ, CS—data analysis. BS, YS, LM, XT, JL, RZ, CS, LJ, DY, HW—manuscript preparation. BS, YS, LM, XT, JL, RZ, CS, LJ, DY, HW, WF, WS, KFD—manuscript revision. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study protocol was approved by the Fuwai Hospital Ethics Committee (2021-1644) and was conducted according to the Declaration of Helsinki. Written consent was waived due to the low patient risk. Oral consent was obtained during the telephonic interview.
Not applicable.
This work was supported by Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences [2021-I2M-1-008 and 2020-I2M-C&T-B-056].
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
