- Academic Editor
Background: Device-related thrombosis (DRT) after successful closure
implantation on left atrial appendage (LAA) was considered as a major challenge
and optimal strategy on antithrombotic therapy remains to be solved. This study
was performed to compare the clinical effectiveness and safety of reduced
rivaroxaban dose (RRD) and dual antiplatelet therapy (DAPT) after left atrial
appendage closure (LAAC) implantation with the Watchman device. Methods:
After successful LAAC, consecutive participants were medicated with a standard
DAPT or RRD. The primary endpoints included DRT, thrombosis events (TE), and
bleeding events that were documented during a 12-month follow-up period.
Results: 767 patients (DAPT: n = 140; RRD: n = 627) were initially
included. After propensity score matching (PSM), 140 patients treated with DAPT
and 280 patients with RRD were included in each group with similar baseline
information, thromboembolic and bleeding risk factors, cardiovascular risk
factors and concomitant medication. In the RRD group, 193 patients were on
rivaroxaban 15 mg (R
Left atrial appendage closure (LAAC) has been currently proven to be effective and safe in stroke prevention among patients with non-valvular atrial fibrillation (NVAF) [1, 2]. Long-term follow-up revealed that LAAC significantly reduced the mortality of cardiovascular disease and all-cause mortality [3]. LAAC was regarded as an effective and safe alternative to oral anticoagulation (OAC) in thromboembolic (TE) prevention related to NVAF among patients contraindicated to long-term anticoagulation [4, 5]. Nowadays, thrombus development on the device after successful device insertion was considered as a major challenge with a reported incidence ranging from 3% to 5% of cases, which was considered as an increased thrombotic risk [6, 7].
Several antithrombotic strategies had been adopted for thrombus prevention after LAAC while endothelialization of the device is achieved. Current guidelines recommended a 45-day period of anticoagulation with a direct oral anticoagulant (DOAC) or warfarin after LAAC followed by dual antiplatelet therapy (DAPT) up to 6 months and then aspirin (100 mg qd) alone for life [8]. Nonetheless, concerns regarding bleeding risks and delayed device-related thrombosis (DRT) have prompted interest in exploring alternative antithrombotic regimens [9, 10].
Reduced rivaroxaban dose (RRD) has gained increasing attention as a potential alternative anticoagulation strategy for patients undergoing LAAC [11]. RRD, which involves a lower dose of rivaroxaban than typically used for anticoagulation, has shown promise as a potential alternative for reduction of DRT and thrombosis events (TE) without increasing bleeding risks [12, 13]. Clinical trials indicated that RRD has been proposed as a potentially effective approach to reduce the incidence of DRT without compromising safety [14, 15]. In the sub-analysis of J-ROCKET AF, the thrombotic and bleeding occurrence of RRD (10 mg) was consistent in patients with preserved renal function and moderate renal impairment, which confirmed the validity of RRD (10 mg) once daily for east Asia population with moderate renal impairment [16]. However, the efficacy and safety of RRD as a post-LAAC anticoagulation strategy have not been well studied.
Therefore, the main purpose of this study was to investigate the effectiveness and safety of RRD as a post-LAAC anticoagulation strategy for DRT and TE prevention without an increased bleeding risk during 1-year follow-up. The findings of this study may provide insights into the potential benefits and limitations of RRD as an antithrombotic regimen for LAAC.
This was a prospective, observational and single center study including
consecutive eligible participants following percutaneous LAAC between September
2016 and September 2020. Ethics approval of antithrombotic protocols was granted
by the Ethics Committee of Zhongshan Hospital, Fudan University. Patients
eligible for Watchman (Boston Scientific, Natick, MA, USA) implantation met the
following inclusion criteria: (1) age
There is currently concern regarding post-LAAC antithrombotic regimens. However,
there is limited evidence on DRT and bleeding prevention. Therefore, this
observational study sought to provide further data regarding the antithrombotic
strategy among Chinese patients following LAAC. The study was not randomized;
instead, the antithrombotic protocol was determined by the implanting physicians’
judgment. Subsequently, the patients were divided into three groups based on
their prescribed antithrombotic plans, which were at the discretion of the
physician. The RRD group comprised participants in the rivaroxaban 10 mg
(R
The LAAC device implantation had been described in detail [17]. The procedure
was performed under general anesthesia with fluoroscopy and intracardiac
echocardiography guidance. The post-implant anti-thrombotic regimen was
individualized, and left to physician discretion. Participants were discharged
after observation with no periprocedural complication. A routine TEE examination
was performed 45 days after device implantation to determine the presence of
significant residual flow (
Detailed demographic and baseline clinical parameters were recorded from
hospital information systems (HIS). CHA
Routine outpatient follow-ups performed for each enrolled participant included up to 3 repeated TEE examinations scheduled approximately 45 days, 180 days and yearly post procedure for the presence of DRT. Out-patient visits and trans-telephonic clinical evaluations were conducted every 3 months during the 1-year follow-up. All the follow-up TEE images and recordings were reviewed by one physician and participants who did not complete the follow-up examination were excluded from the final analysis.
The primary clinical endpoint was a composite of effective and safety characteristics of each strategy. The efficacy endpoints were as followings: (1) DRT defined as a well-circumscribed and uniformly echo-dense mass lying on the closure, measured by TEEs, (2) TE events including stroke or transient ischemic attack (TIA) determined on magnetic resonance imaging (MRI) or computed tomography (CT), peripheral thromboembolism, pulmonary embolism and venous thromboembolism. The safety endpoints included clinical major and non-major bleeding complications defined according to the guidance of the International Society on Thrombosis and Haemostasis [18]. The definition of major bleeding in the study involved a decrease in the hemoglobin level of no less than 20 g/L, transfusion of two or more units of blood, or symptomatic bleeding that affected a critical organ. Clinically significant non-major bleeding was defined as bleeding that necessitated medical attention from a healthcare professional, a higher level of intensive care, or an on-site evaluation.
The sample size was calculated based on the lower hospitalization rate of
confirmed DRT and TE with long-term RRD compared with a standard antiplatelet
therapy. Using PASS statistical software (version 11.0; NCSS, LLC. Kaysville,
UT, USA), a class I error rate (
Continuous variables were presented as mean
The baseline characteristics comparison between groups was conducted using
appropriate statistical tests, such as t-tests and
The primary efficacy and safety variables were the cumulative occurrence of confirmed DRT, TE, and bleeding complications, for each enrolled patient during the follow-up period. Kaplan-Meier curves were performed to illustrate the time-to-first thrombosis or bleeding, and log-rank tests were used to compare these curves. Statistical analysis was performed using SPSS software (version 22.0; IBM Corp., Armonk, NY, USA), and a p value of 0.05 was considered statistically significant.
This cohort study initially enrolled 779 patients following successful Watchman
implantation between December 2017 and December 2021. A total of 12 participants
(DAPT group: n = 6 [0.8%]; RRD group: n = 6 [0.8%]) were finally excluded from
the study because their follow-up TEEs were completed at a different clinical
institution and no images were provided for review. Ultimately, 767 patients
(DAPT group: n = 140; RRD group: n = 627; Mean age 68.0
 Fig. 1.
Fig. 1.Enrollment flow chart of patients. LAAC, left atrial appendage
closure; TEE, trans-esophageal echocardiography; RRD, reduced rivaroxaban doses;
DAPT, dual antiplatelet therapy; R
Baseline information, cardiovascular risk factors, potential thromboembolic and bleeding risks, and concomitant medication are presented in Table 1. There was a higher percentage of higher HAS-BLED score in participants taking DAPT. After propensity score matching (PSM) with 1:2 ratio (140 patients for DAPT and 280 patients for RRD), the two subgroups were not significantly different in baseline information, cardiovascular risk factors, laboratory indicators and predetermined stroke and bleeding risk.
| Variables | Before matching | After matching | |||||||
| All (N = 767) | RRD (n = 627) | DAPT (n = 140) | p value | All (N = 420) | RRD (n = 280) | DAPT (n = 140) | p value | ||
| Age, y | 68.0 |
67.9 |
68.3 |
0.639 | 68.8 |
69.0 |
68.3 |
0.418 | |
| Male | 478 (62.3) | 391 (62.4) | 87 (62.1) | 0.962 | 267 (63.6) | 180 (64.3) | 87 (62.1) | 0.667 | |
| CHA |
3.5 |
3.5 |
3.7 |
0.355 | 3.7 |
3.7 |
3.7 |
0.844 | |
| 372 (48.5) | 309 (49.3) | 63 (45.0) | 0.359 | 190 (45.2) | 127 (45.4) | 63 (45.0) | 0.945 | ||
| 4 | 169 (22.0) | 141 (22.5) | 28 (20.0) | 0.521 | 86 (20.5) | 58 (20.7) | 28 (20.0) | 0.864 | |
| 226 (29.5) | 177 (28.2) | 49 (35.0) | 0.112 | 144 (34.3) | 95 (33.9) | 49 (35.0) | 0.827 | ||
| HAS-BLED score | 2.5 |
2.4 |
3.2 |
0.001 | 3.1 |
3.0 |
3.2 |
0.092 | |
| 391 (51.0) | 352 (56.1) | 39 (27.9) | 0.001 | 115 (27.4) | 76 (27.1) | 39 (27.9) | 0.877 | ||
| 3 | 217 (28.3) | 170 (27.1) | 47 (33.6) | 0.125 | 154 (36.7) | 107 (38.2) | 47 (33.6) | 0.352 | |
| 4 | 121 (15.8) | 81 (12.9) | 40 (28.6) | 0.001 | 113 (26.9) | 73 (26.1) | 40 (28.6) | 0.586 | |
| 38 (5.0) | 224 (3.8) | 14 (10.0) | 0.002 | 38 (9.0) | 24 (8.6) | 14 (10.0) | 0.630 | ||
| Risk factors for stroke and bleeding | |||||||||
| CHF | 10 (1.3) | 8 (1.3) | 2 (1.4) | 1 | 5 (1.2) | 3 (1.1) | 2 (1.4) | 1.000 | |
| Hypertension | 488 (63.6) | 398 (63.5) | 90 (64.3) | 0.857 | 293 (69.8) | 203 (72.5) | 90 (64.3) | 0.084 | |
| 179 (23.3) | 144 (23.0) | 35 (25.0) | 0.607 | 104 (24.8) | 69 (24.6) | 35 (25.0) | 0.936 | ||
| 65–74 years of age | 345 (45.0) | 283 (45.1) | 62 (44.3) | 0.855 | 202 (48.1) | 140 (50.0) | 62 (44.3) | 0.269 | |
| Diabetes mellitus | 160 (20.9) | 124 (19.8) | 36 (25.7) | 0.118 | 79 (18.8) | 50 (17.9) | 29 (20.7) | 0.480 | |
| History of stroke/TIA | 326 (42.5) | 266 (42.4) | 60 (42.9) | 0.925 | 186 (44.3) | 126 (45.0) | 60 (42.9) | 0.677 | |
| Stroke | 282 (36.8) | 223 (35.6) | 59 (42.1) | 0.145 | 179 (42.6) | 120 (42.9) | 59 (42.1) | 0.889 | |
| TIA | 49 (6.4) | 43 (6.9) | 6 (4.3) | 0.26 | 17 (4.0) | 8 (2.9) | 9 (6.4) | 0.080 | |
| Vascular disease | 407 (53.1) | 329 (52.5) | 78 (55.7) | 0.487 | 228 (54.3) | 150 (53.6) | 78 (55.7) | 0.678 | |
| Renal Dysfunction | 44 (5.7) | 32 (5.1) | 12 (8.6) | 0.111 | 37 (8.8) | 25 (8.9) | 12 (8.6) | 0.903 | |
| Liver Dysfunction | 71 (9.3) | 55 (8.8) | 16 (11.4) | 0.327 | 53 (12.6) | 37 (13.2) | 16 (11.4) | 0.603 | |
| History of major bleeding | 56 (7.3) | 41 (6.5) | 15 (10.7) | 0.086 | 50 (11.9) | 35 (12.5) | 15 (10.7) | 0.594 | |
| Intracranial bleeding | 33 (4.3) | 25 (4.0) | 8 (5.7) | 0.363 | 30 (7.1) | 22 (7.9) | 8 (5.7) | 0.421 | |
| GI bleeding | 13 (1.7) | 10 (1.6) | 3 (2.1) | 0.715 | 10 (2.4) | 7 (2.5) | 3 (2.1) | 1.000 | |
| Other | 11 (1.4) | 7 (1.1) | 4 (2.9) | 0.123 | 11 (2.6) | 7 (2.5) | 4 (2.9) | 1.000 | |
| History of minor bleeding | 21 (2.7) | 14 (2.2) | 7 (5.0) | 0.07 | 17 (4.0) | 10 (3.6) | 7 (5.0) | 0.484 | |
| GI bleeding | 4 (0.5) | 3 (0.5) | 1 (0.7) | 0.554 | 3 (0.7) | 2 (0.7) | 1 (0.7) | 1.000 | |
| Epistaxis | 3 (0.4) | 2 (0.3) | 1 (0.7) | 0.454 | 2 (0.5) | 1 (0.4) | 1 (0.7) | 1.000 | |
| Other | 13 (1.7) | 9 (1.4) | 4 (2.9) | 0.271 | 6 (2.1) | 2 (1.4) | 4 (2.9) | 0.684 | |
| Labile INR | 36 (4.7) | 29 (4.6) | 7 (5.0) | 0.85 | 32 (7.6) | 25 (8.9) | 7 (5.0) | 0.153 | |
| Alcohol | 43 (5.6) | 38 (6.1) | 5 (3.6) | 0.247 | 33 (7.9) | 27 (9.6) | 6 (4.3) | 0.054 | |
| CAD | 122 (15.9) | 93 (14.9) | 29 (20.7) | 0.085 | 68 (16.2) | 39 (13.9) | 29 (20.7) | 0.075 | |
| LVEF, % | 63.1 |
63.1 |
62.7 |
0.471 | 63.0 |
63.1 |
62.7 |
0.575 | |
Values are mean
The RRD group had a 12-month median (interquartile range (IQR): 11–14)
follow-up and the DAPT group had a 13-month (IQR: 11–15) median follow-up. In
the RRD group, 193 were on R
The incidence of DRT was documented in 12 (9.3%) patients in DAPT, 3 (6.3%) in
R
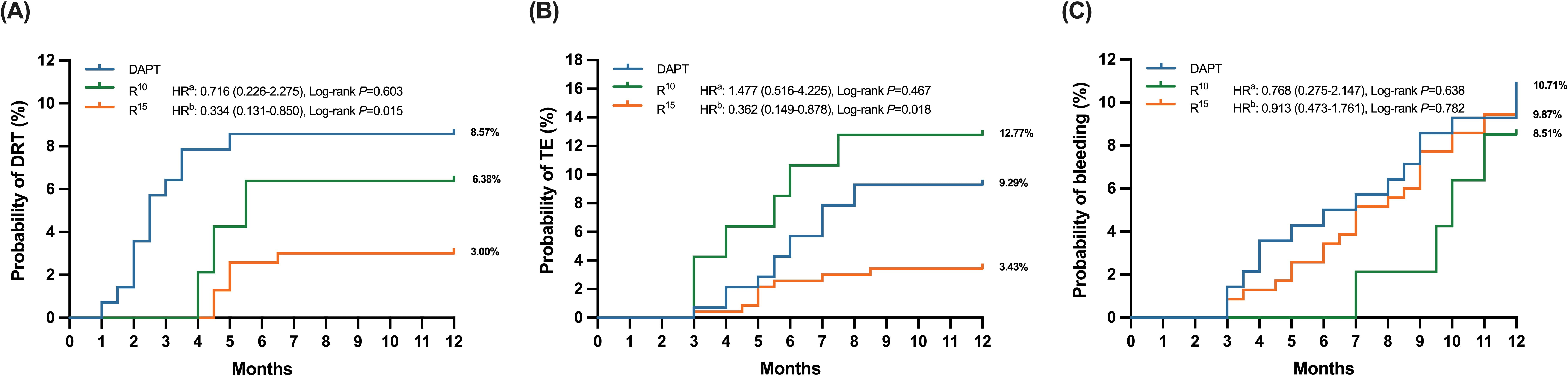 Fig. 2.
Fig. 2.Time to clinical events in antithrombotic treated patients,
stratified into three subgroups (DAPT, R
In the DAPT group, a total of 14 patients (10.0%) experienced TEs in terms of
ischemic stroke or systemic embolism during the follow-up period compared with 6
patients (12.7%) in the R
The median length of DRT in the R
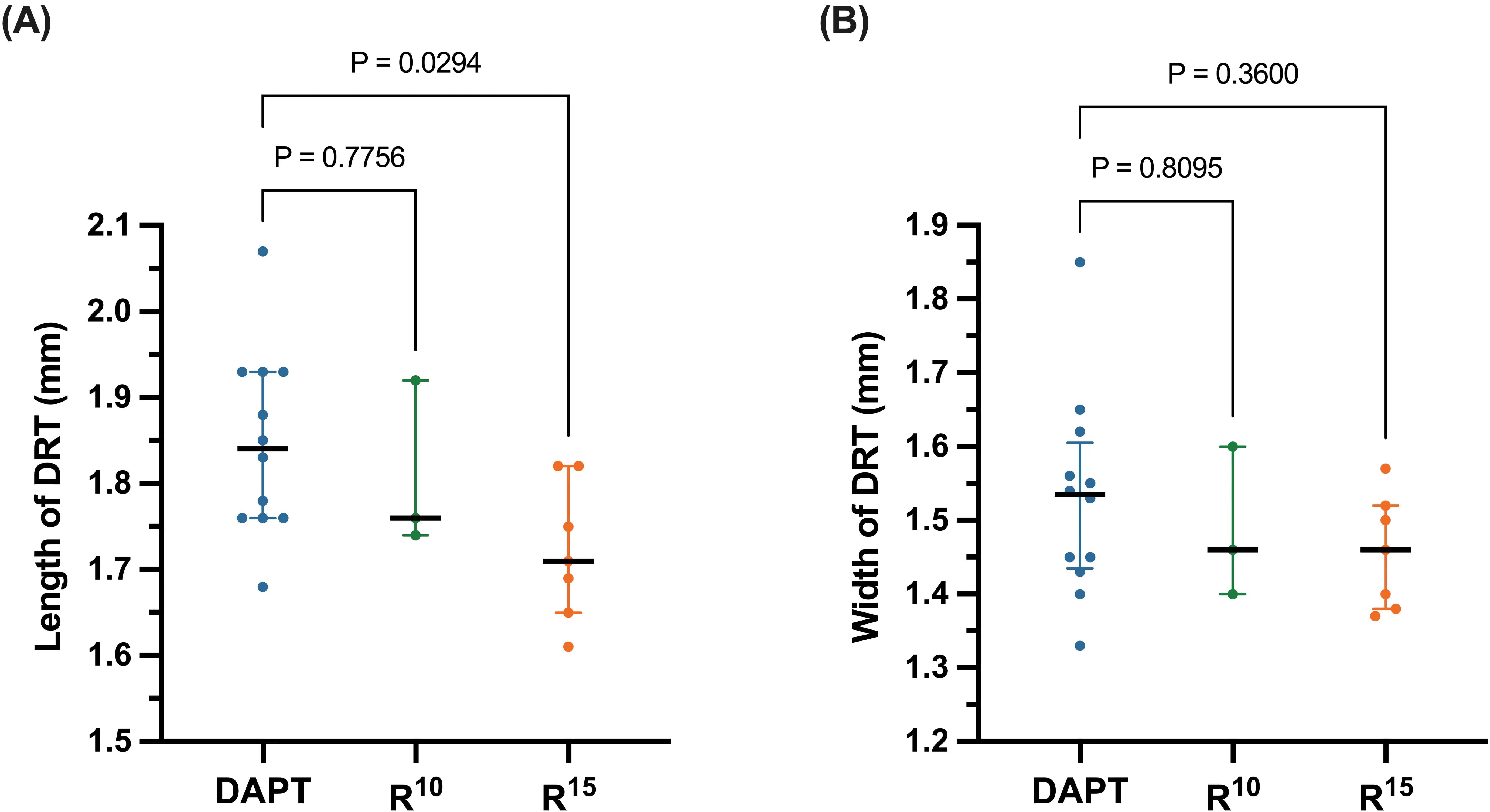 Fig. 3.
Fig. 3.(A) Length and (B) width of device-related thrombus (DRT)
evaluated with transesophageal echocardiography. The solid black lines medians of
each subgroup, while the error bars represent the interquartile range. DAPT,
dual antiplatelet therapy; R
Compared with the unadjusted estimated rates of ischemic events for patients
with similar CHA
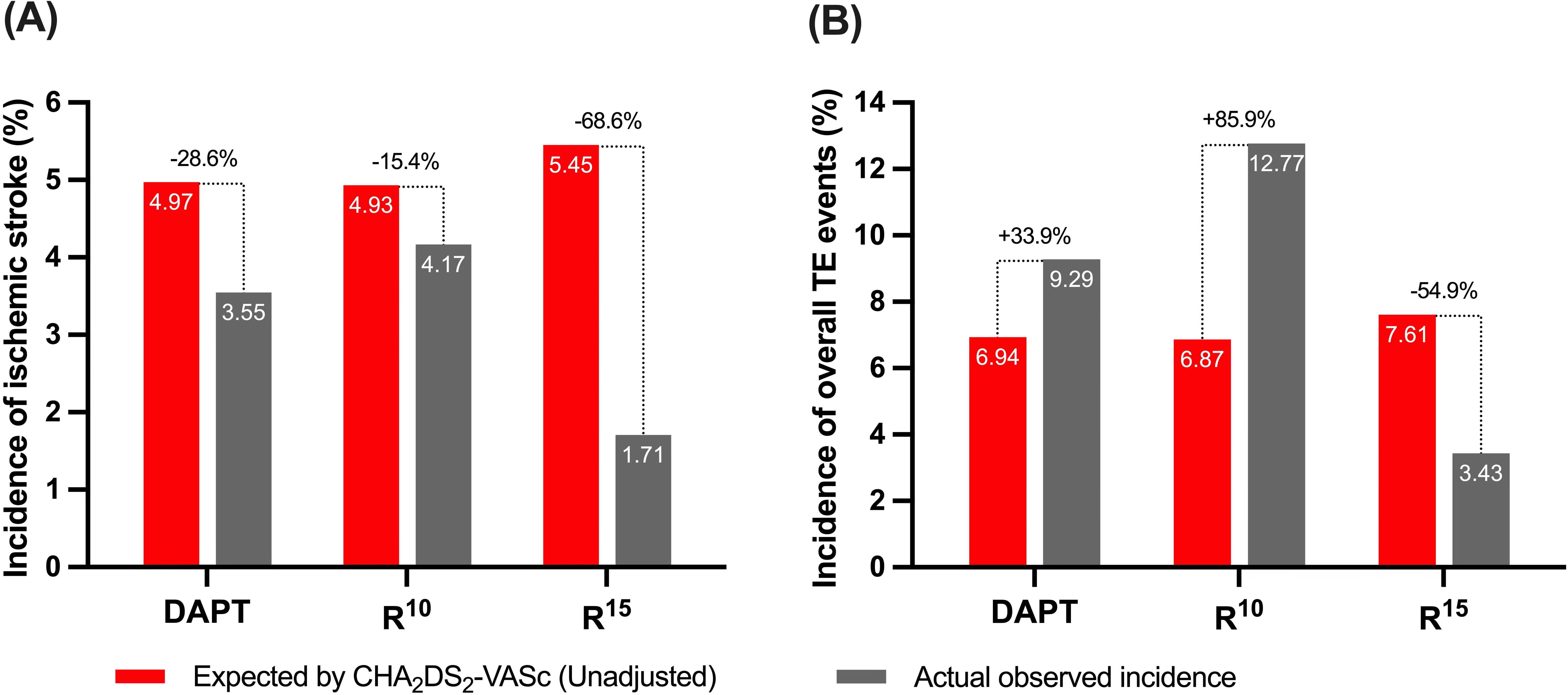 Fig. 4.
Fig. 4.(A) Annualized ischemic stroke and (B) TE event rates after
implantation vs expected (unadjusted) rates estimated based on the
CHA
Details of the bleeding events in the entire patient cohort are reported in
Table 2. No major bleeding was documented throughout the 12-month follow-up
period. The rate of overall minor bleeding was not significantly different
amongst the groups (8.5% among R
| Bleeding complications, n (%) | R |
R1 |
DAPT | p value | |
| Overall bleeding events | 4 (8.5%) | 23 (9.9%) | 15 (10.7%) | 0.944 | |
| GI bleeding | 1 (2.1%) | 5 (2.1%) | 3 (2.1%) | 1.000 | |
| Hematuria | 1 (2.1%) | 4 (1.7%) | 1 (0.7%) | 0.586 | |
| Operation site hemorrhage | 0 (0.0%) | 4 (1.7%) | 2 (1.4%) | 1.000 | |
| Bleeding gums | 1 (2.1%) | 6 (2.6%) | 4 (2.9%) | 1.000 | |
| Skin ecchymosis | 1 (2.1%) | 4 (1.7%) | 5 (3.6%) | 0.520 | |
| PLT |
4 (8.5%) | 10 (8.1%) | 15 (10.7%) | 0.745 | |
| Male: Hb |
5 (10.6%) | 11 (7.3%) | 16 (11.4%) | 0.748 | |
| Female: Hb | |||||
| PT |
12 (25.5%) | 62 (26.6%) | 32 (22.8%) | 0.856 | |
GI, Gastrointestinal; PLT, platelet; Hb, hemoglobin; PT, prothrombin time;
DAPT, dual antiplatelet therapy; R
Table 2 shows the accumulated anticoagulation-related complications and
coagulation function tests among the groups. There was no significant reduction
in levels of platelet (PLT), hemoglobin (Hb), or prothrombin time (PT) among
R
In this study, we prospectively investigated the clinical efficacy and safety
between RRD and DAPT after successful LAAC implantation. The following are the
main findings of the study. First, anticoagulation long term RRD led to a
significant reduction of DRT and TE compared to DAPT. Second, long-term
rivaroxaban provided more effective and safer thrombus prevention, when compared
with unadjusted, estimated rates of ischemic stroke and TE for patients with
similar CHA
Similar to all medical devices implanted into the body, a longer implantation time beyond 90 days is expected to enable complete endothelialization for occluders post LAAC when exposed to circulating blood [19, 20]. Thrombosis formation might occur on the exposed device and adequate antithrombotic regimens are required for DRT prevention. Currently, pharmacological regimens following successful LAAC implantation were mainly dependent on strategies from recent clinical studies [21, 22]. Previous studies indicated short-term DAPT adoption followed by long-term aspirin could prevent DRT and TE events [23, 24]. Although antiplatelet therapy has confirmed the efficacy for thrombosis prevention after stent implantation, substantial variation still remains for the selection of the appropriate antithrombotic strategy for LAAC implantation. In this study we sought to compare the clinical efficacy and safety between RRD and standard antiplatelet strategy following LAAC. Some novel observations could be made based on the data derived from this study.
It is important to identify anticoagulants that can prevent the occurrence of DRT and TE. Currently, there is limited data regarding the correlation between antithrombotic strategies and thrombus on closure devices. In this study, the scheduled out-patient visits and trans-telephonic clinical evaluations were frequently conducted 6 months after discharge to help reduce the occurrence of thromboembolic events. Our results documented a lower DRT rate in RRD as compared to DAPT, which was similar to those in previously published studies from other groups [14, 25]. In one propensity matched comparison with Watchman closure implantation patients, the 6-month cumulative DRT occurrence was lower in DAPT as compared to half-Dose DOAC (3.4% vs. 0.0%), which was similar with our findings [14]. Another multicenter study with patients undergoing LAAC implantation indicated that DOACs proved to be a feasible and safe alternative antithrombotic regimen to warfarin for DRT and thromboembolic prevention after LAAC implantation, without increasing the risk of bleeding [14].
Activation of the coagulation system and enhanced thrombin generation without platelet aggregation were associated with DRT formation within days after LAAC [26]. In our results, persistent elevation of thrombosis size was also detected in the DAPT group after the procedure, which was consistent with the result of previous prospective studies. A randomized pilot study documented that circulating prothrombin fragments and thrombin-antithrombin complex were numerically lower after rivaroxaban treatment than that with DAPT, which might explain the lower rate of thrombosis and TE following medication with rivaroxaban after successful LAAC [27].
Another important factor for consideration is the safety for long-time anticoagulation among different antithrombotic regimens in patients undergoing LAAC. Our results indicated decreased bleeding occurrence after the initial follow-up, which might be related to more scheduled out-patient visits and clinical evaluations 6 months after discharge. Of note, a reduced DOAC dose was associated with lower bleeding for NVAF patients compared with vitamin K antagonists (VKA) in the east Asia population [28]. One large cohort of east Asia patients showed lower post-extraction bleeding rates with DOAC compared with warfarin (HR: 0.84; 95% CI: 0.54–1.31) [29]. In our study, there was no significant difference in coagulation function tests between the RRD and DAPT groups. Furthermore, our findings suggest that RRD may be a safe alternative to DAPT for short-term antithrombotic therapy after LAAC, without a statistically significant difference observed in bleeding events. However, the optimal antithrombotic regimen for long-term anticoagulation after LAAC remains uncertain, and individualized treatment plans should be developed based on patient-specific factors, such as bleeding risk, thromboembolic risk, comorbidities, and medication interactions. Although the overall minor bleeding rate was 10.0% in our study, the risk for postoperative bleeding was increased in these patients who underwent a percutaneous strategy and were exposed to anticoagulation therapy. Given the concern for bleeding and the need for TE prophylaxis, a minimally invasive surgical strategy such as epicardial LAA occlusion with no further anticoagulation is more favorable to patients at higher risk for bleeding and thrombosis. The rationale for this practice is inferred from the Left Atrial Appendage Occlusion Study (LAAOS III) trial, which demonstrated that LAA resection for AF patients during chest cardiac surgery contributed to a reduction of thrombosis and bleeding risks [30, 31]. As for NVAF patients with end-stage renal failure contraindicated to DOAC merging with high bleeding risk, epicardial LAA occlusion for such specific population might offer a clinical benefit [32]. Multi-disciplinary teams involving anesthesiologists, cardiologists and cardiac surgeons will need to determine which patients can reliably and safely undergo epicardial LAA closure in those patients with a contraindication for anticoagulation or those with anatomical abnormalities which are not conducive to percutaneous LAAO.
The present study has many limitations. Firstly, our study is non-randomized and conducted at a single center, which might restrict the generalizability of the results to other cardiovascular centers and healthcare systems. Secondly, the sample size was relatively small and the follow-up duration of only 12 months may not be sufficient to determine the long-term efficacy and safety of different anticoagulation regimens, which potentially led to a low incidence of DRT. The exclusion of 12 participants due to incomplete follow-up TEEs and failure to provide images for review may have introduced selection bias. Thirdly, our study was followed up only by the cardiology department. Finally, the study only enrolled participants receiving RRD and DAPT, which may limit the generalizability of the findings to other anticoagulants. In conclusion, these limitations highlight the need for further studies with larger sample sizes, more frequent monitoring, and more diverse patient populations to confirm the conclusions and establish the clinical efficacy and safety of different antithrombotic strategies.
Based on the evidence presented in this cohort study, antithrombotic therapy with RRD may be a promising option for DAPT for reducing the risk of DRT and composite endpoints in patients following successful Watchman implantation. Further randomized controlled trials conducted at multiple centers are needed to compare the safety and efficacy of different antithrombotic regimens in these patients.
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
XL, YY, QJ, XZ and QL contributed to the conception and design, or acquisition of data, or analysis and interpretation of data, and completed the writing of the paper. XL, QJ, XZ and QL were involved in drafting the manuscript or revising it critically for important intellectual content. XL, YY, XZ and QL were responsible for the revision of the paper. All authors confirmed the final version of the paper. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The studies involving human participants were reviewed and approved by the Institutional Review Committee of Zhongshan Hospital, Fudan University (No: B2020-288R). The patients/participants provided their written informed consent to participate in this study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
