1 School of Medicine, South China University of Technology, 510006 Guangzhou, Guangdong, China
2 Department of Cardiology, Guangdong Provincial People's Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, 510080 Guangzhou, Guangdong, China
3 Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, 510080 Guangzhou, Guangdong, China
4 The Second School of Clinical Medicine, Southern Medical University, 510515 Guangzhou, Guangdong, China
5 Guangdong Provincial Geriatrics Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, 510080 Guangzhou, Guangdong, China
6 Global Health Research Center, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Science, 510100 Guangzhou, Guangdong, China
†These authors contributed equally.
Abstract
Background: Hyperglycemia has been associated with an adverse prognosis
in patients with premature coronary artery disease (CAD). However, whether the
intermediate hyperglycemia status affects the risk of mortality in premature CAD
patients treated with percutaneous coronary intervention (PCI), remains unclear.
Methods: We retrospectively included 14,585 premature CAD patients
undergoing PCI from 2007 to 2020. Patients were divided into normal glycemia
(
Keywords
- premature coronary artery disease
- intermediate hyperglycemia
- mortality
- percutaneous coronary intervention
Coronary artery disease (CAD) represents one of the principal causes of death and morbidity globally and the most important cause of premature death in the developed world [1]. Young CAD patients with symptoms (i.e., premature CAD) accepting percutaneous coronary intervention (PCI), were more likely to have acute myocardial infarction, unstable angina, out-of-hospital cardiac arrest, and cardiac shock compared with elderly patients [2]. The long-term morbidity or mortality of premature CAD patients has not improved over the previous decades, accompanied by an increasing burden of cardiovascular risk factors [3]. In addition, current studies revealed that premature CAD is often accompanied by ischemic recurrence and has a high proportion of cardiovascular risk factors, which were modifiable but frequently failed to control [4]. The control of risk factors is therefore particularly important in premature CAD patients to improve their prognosis.
It was reported that 36% of premature CAD patients develop recurrent major adverse cardiovascular events (MACE) at least twice, while diabetes is a major exposure factor for the recurrence of MACE [5]. 2019 European Society of Cardiology (ESC) Guidelines highlighted the prevention and control of risk factors as of great significance for people suffering CAD, especially for patients accompanied with diabetes requiring active control of hemoglobin A1c (HbA1c) [6]. However, the target of glycemic control for premature CAD patients remains undetermined. On the other hand, previous studies have shown that intensive glycemic control to keep HbA1c below 6.5% can reduce macrovascular and microvascular events by 10% [7]. Patients with intermediate hyperglycemia of HbA1c ranging from 6.0 to 6.5% were defined as prediabetic according to the World Health Organization (WHO) [8]. Compared with normoglycemic individuals, prediabetes is related to a high atherosclerotic burden and the number of vessels affected in Ahmed’s study [9]. Another study illustrated that patients with prediabetes have a significantly increased long-term risk of cardiovascular disease after PCI [10]. However, Juan Francisco Cueva-Recalde et al. [11] reported that prediabetes shows no association with higher mortality or adverse cardiovascular outcomes in CAD patients undergoing PCI. Whether the intermediate hyperglycemia of HbA1c range from 6.0–6.5% should be treated in premature CAD patients with or without diabetes is unclear yet.
Given the paucity of data on the association between the glycemic control target and prognosis among patients, the study aims to assess the prevalence of intermediate hyperglycemia and its effect on long-term all-cause death in premature CAD patients treated with PCI.
The research conducted was a retrospective, observational investigation
utilizing data from the registry of Cardiorenal ImprovemeNt II (CIN-II,
NCT05050877) cohort, which enrolled consecutive patients undergoing coronary
angiography at five tertiary teaching hospitals in southern China from January
2007 to December 2020. A total of 21,774 patients diagnosed with premature CAD
undergoing PCI were included, in which patients diagnosed with stenosis
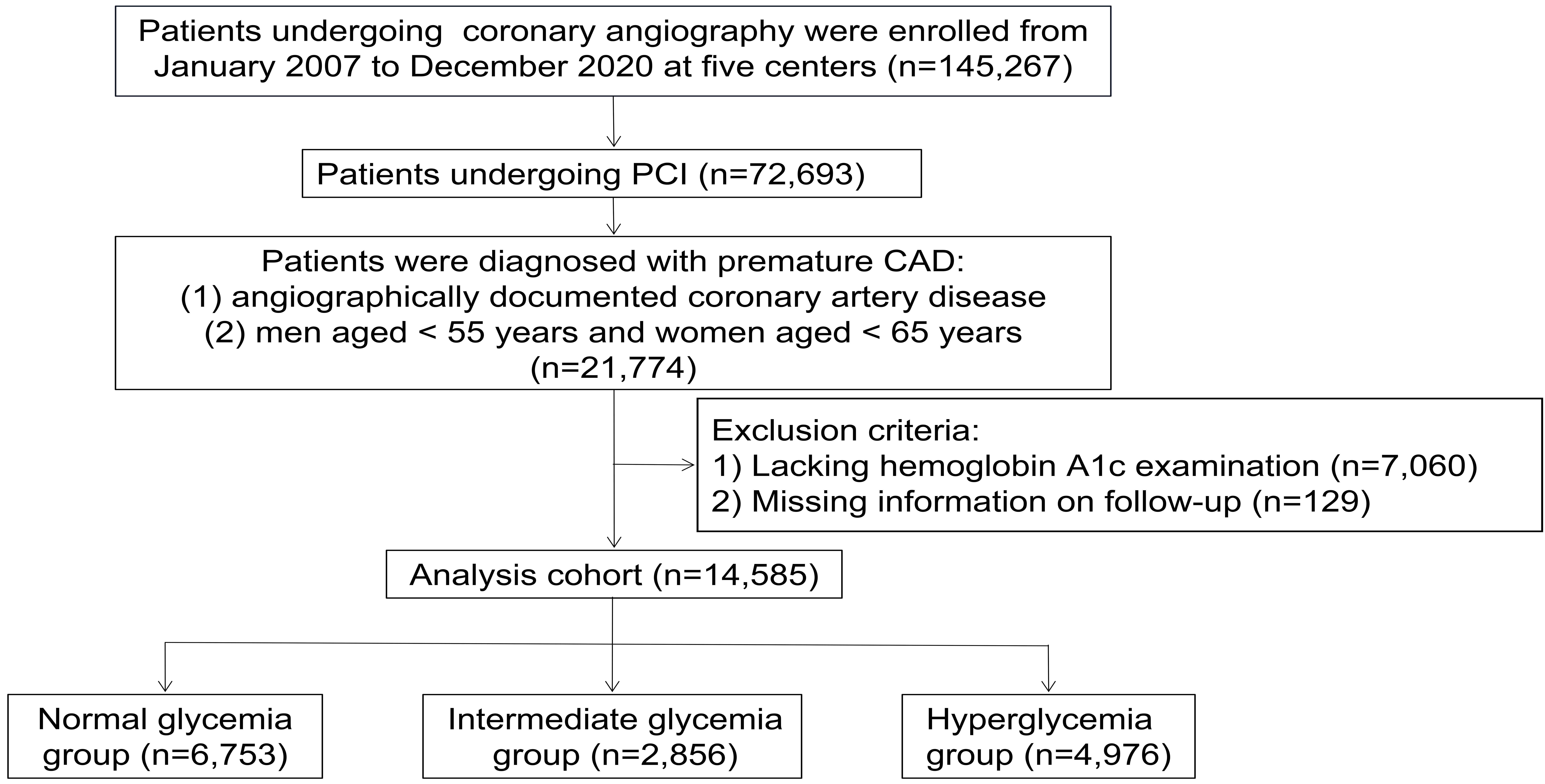
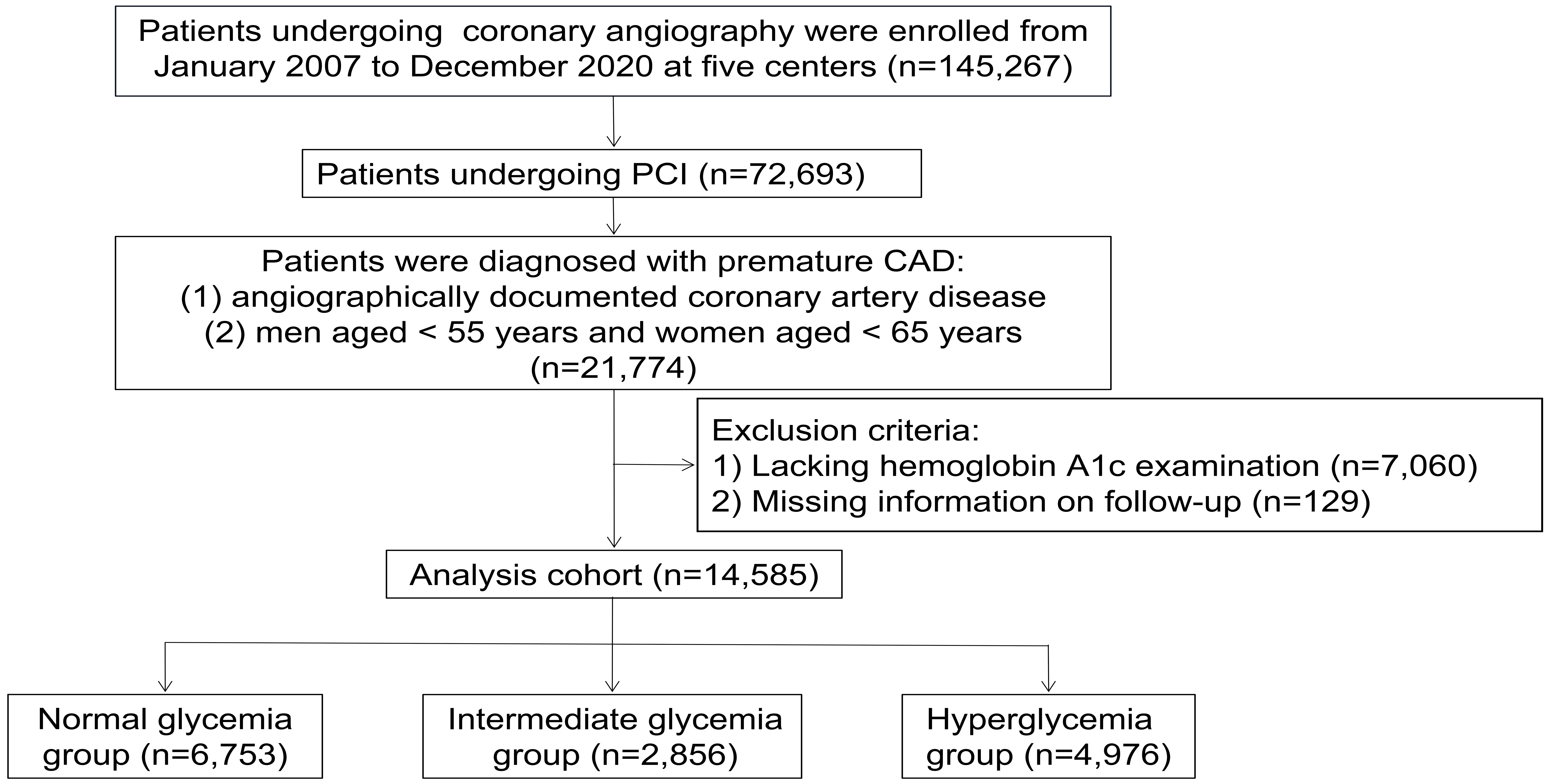 Fig. 1.
Fig. 1.The flow of participants through the trial. PCI, percutaneous coronary intervention; CAD, coronary artery disease.
The data for the study were sourced from the Electronic Clinical Management System utilized by the hospitals involved in the research. The baseline information mainly included demographic characteristics, medical history, medications at discharge, laboratory examination and other clinical characteristics.
Per the criteria established by the International Diabetes Expert Committee (in
accordance with the World Health Organization guidelines), glycemic status was
categorized as follows: [8]. (i) normal HbA1c, less than 6.0%; (ii) prediabetes,
ranging from 6.0% to 6.4%; and (iii) diabetes, equal to or greater than 6.5%.
Based on the baseline HbA1c levels in whole blood instead of disease status, the
patients were classified into three distinct groups: (i) normal glycemia group
[HbA1c level was
The primary endpoint was long-term all-cause mortality. The secondary endpoint was cardiac mortality, defined as any death due to any cardiac cause. The follow-up information was acquired by matching the survival data from the Centers for Disease Control and Prevention.
Continuous variables were presented as mean
The association between glycemia status and long-term all-cause mortality as well as cardiac mortality was evaluated by the Cox proportional-hazards model, presented as hazard ratio (HR) and 95% confidence interval (CI). Adjusted covariates included age, sex, CKD, hypertension, CHF, AF, AMI, cholesterol, and low-density lipoprotein cholesterol (LDL-C) according to the significance in the univariate analysis, clinical practice and previous studies. The time-to-endpoint data were presented graphically using Kaplan-Meier (K-M) curves, and we used restricted cubic splines (RCS) to explore the linear relationship between HbA1C and hazard ratios (HRs) for all-cause mortality in premature CAD with PCI.
Among the 14,585 premature CAD patients undergoing PCI, the majority were male
(71.9%), and the mean age was 50.4
| Overall | Normal glycemia | Intermediate hyperglycemia | Hyperglycemia | p-value | |
| No. of participants | 14,585 | 6753 | 2856 | 4976 | |
| Age, years | 50.40 ( |
49.07 ( |
51.11 ( |
51.78 ( |
|
| Female, % | 4101 (28.1) | 1468 (21.7) | 855 (29.9) | 1778 (35.7) | |
| Hypertension, % | 6928 (47.6) | 2799 (41.6) | 1408 (49.5) | 2721 (54.8) | |
| Diabetes mellitus, % | 4577 (34.1) | 332 (4.9) | 429 (15.0) | 3816 (100.0) | |
| Congestive heart failure, % | 2044 (14.1) | 843 (12.5) | 376 (13.2) | 825 (16.6) | |
| Atrial fibrillation, % | 202 (1.4) | 71 (1.1) | 44 (1.5) | 87 (1.8) | 0.005 |
| Acute myocardial infraction, % | 5047 (34.7) | 2482 (36.9) | 882 (31.0) | 1683 (33.9) | |
| Multi-vessel disease, % | 4598 (36.3) | 1862 (32.4) | 950 (36.9) | 1786 (41.3) | |
| 2-vessel disease, (%) | 993 (6.9) | 468 (7.0) | 187 (6.6) | 338 (7.0) | 0.826 |
| 3-vessel disease, (%) | 3605 (28.5) | 1394 (24.2) | 763 (29.6) | 1448 (33.5) | |
| Chronic kidney disease, % | 1317 (9.0) | 448 (6.6) | 244 (8.5) | 625 (12.6) | |
| Stroke, % | 88 (0.6) | 32 (0.5) | 28 (1.0) | 28 (0.6) | 0.012 |
| Anemia, % | 2946 (20.5) | 1202 (18.1) | 569 (20.2) | 1175 (24.0) | |
| FBG, mmol/L | 5.99 ( |
4.91 ( |
6.50 ( |
10.17 ( |
|
| Album, g/L | 38.37 ( |
38.70 ( |
38.43 ( |
37.89 ( |
|
| Triglyceride, mmol/L | 2.03 ( |
1.81 ( |
1.96 ( |
2.36 ( |
|
| Cholesterol, mmol/L | 4.83 ( |
4.78 ( |
4.87 ( |
4.87 ( |
0.001 |
| HDL-C, mmol/L | 0.98 ( |
1.00 ( |
0.99 ( |
0.96 ( |
|
| LDL-C, mmol/L | 3.07 ( |
3.06 ( |
3.11 ( |
3.05 ( |
0.056 |
| Hemoglobin A1c, % | 6.59 ( |
5.49 ( |
6.17 ( |
8.34 ( |
|
| eGFR, mL/min/1.73 m |
88.85 [75.45, 104.37] | 89.10 [76.87, 103.34] | 88.01 [75.55, 102.42] | 89.15 [72.94, 107.06] | 0.095 |
| Uric acid, mmol/L | 389.79 ( |
391.05 ( |
396.98 ( |
383.95 ( |
|
| Neutrophil counts, 10 |
5.72 ( |
5.77 ( |
5.46 ( |
5.80 ( |
|
| Lymphocyte counts, 10 |
2.09 ( |
2.03 ( |
2.16 ( |
2.14 ( |
|
| Statins, % | 13705 (96.2) | 6351 (96.3) | 2737 (97.0) | 4617 (95.6) | 0.005 |
| ACEI, % | 6952 (48.8) | 3303 (50.1) | 1412 (50.1) | 2237 (46.3) | |
| ARB, % | 3392 (23.8) | 1366 (20.7) | 658 (23.3) | 1368 (28.3) | |
| Aspirin, % | 13943 (97.9) | 6458 (98.0) | 2758 (97.8) | 4727 (97.9) | 0.815 |
| 12299 (86.4) | 5621 (85.3) | 2458 (87.1) | 4220 (87.4) | 0.002 | |
| Calcium channel blocker, % | 2965 (20.8) | 1350 (20.5) | 550 (19.5) | 1065 (22.0) | 0.019 |
| Diuretics, % | 1335 (9.4) | 496 (7.5) | 211 (7.5) | 628 (13.0) | |
| Oral antidiabetic drug, % | 2974 (20.9) | 133 (2.0) | 266 (9.4) | 2575 (53.3) | |
| Insulin, % | 670 (4.7) | 99 (1.5) | 36 (1.3) | 535 (11.1) |
Values are mean
Abbreviation: FBG, fasting blood glucose; HDL-C, high-density lipoprotein
cholesterol; LDL-C, low-density lipoprotein cholesterol; eGFR, estimated
glomerular filtration rate; ACEI, angiotensin-converting enzyme inhibitors; ARB,
angiotensin II receptor blocker; SD, standard deviation.
Over a median follow-up of 4.62 years (interquartile range 2.72–7.19 years),
1231 (8.44%) patients died. The all-cause mortality of overall patients in the
intermediate hyperglycemia group and hyperglycemia group were significantly
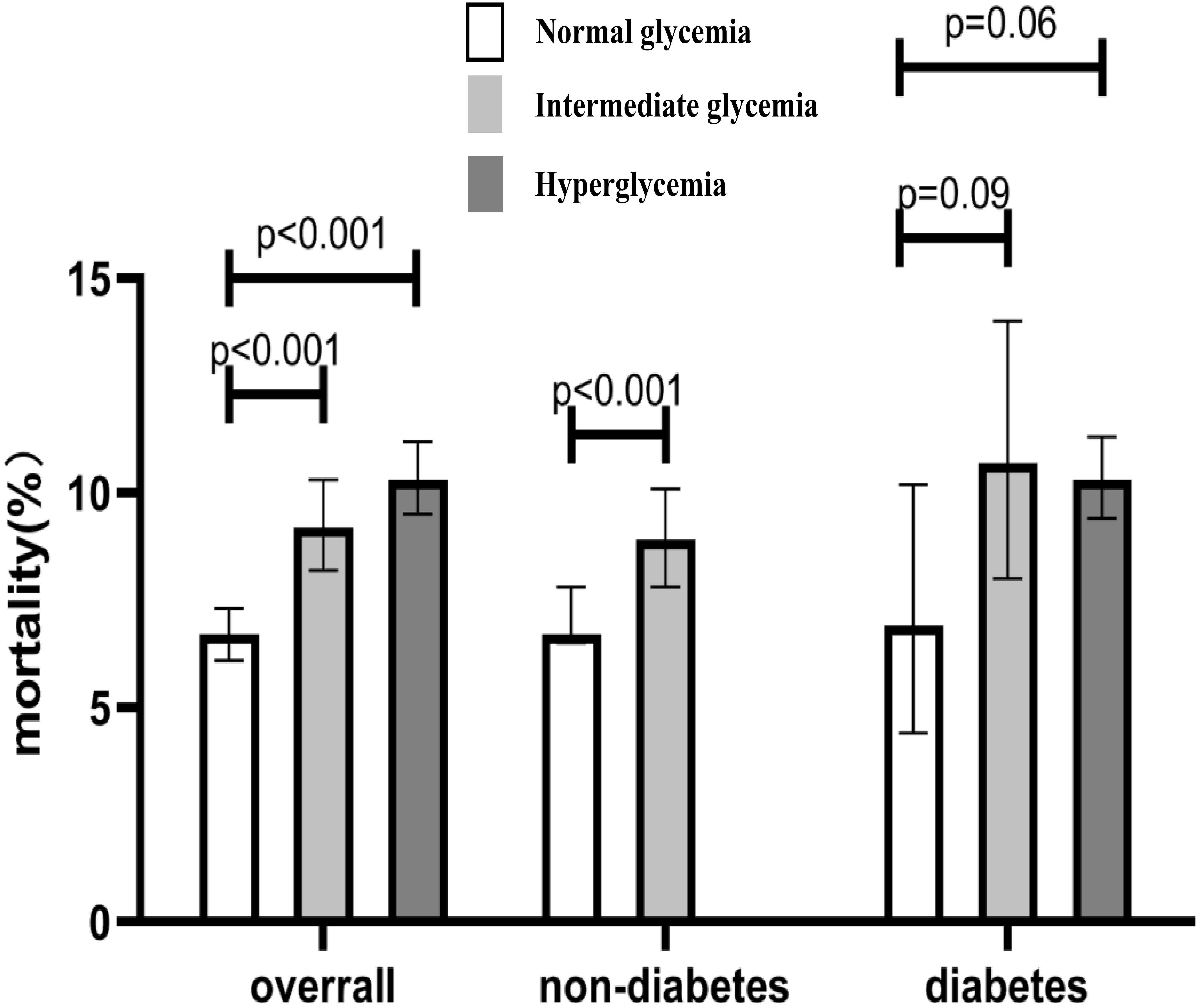
higher than in the normal glycemia group (p
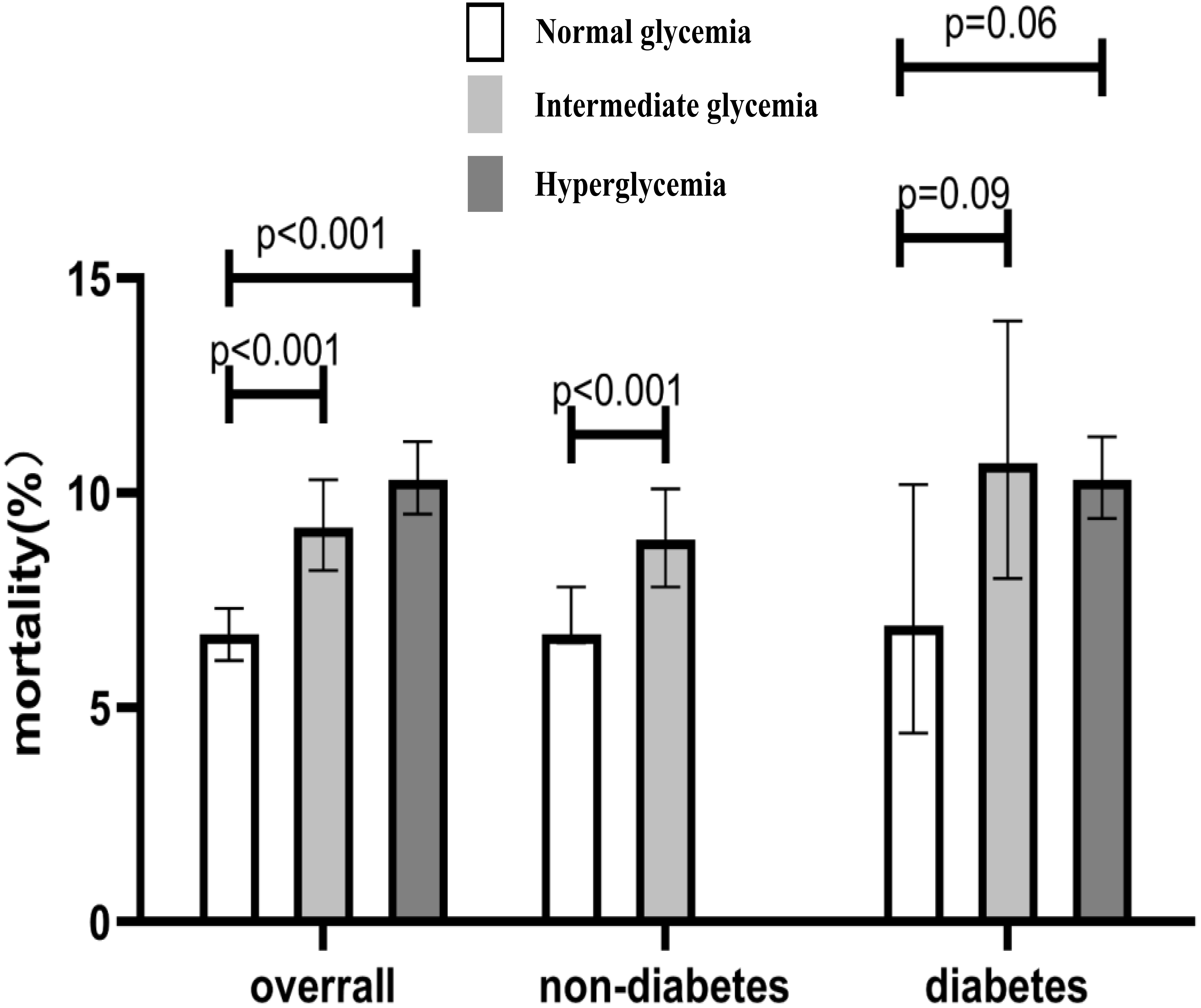 Fig. 2.
Fig. 2.All-cause death rates for patients with different glycemic status.
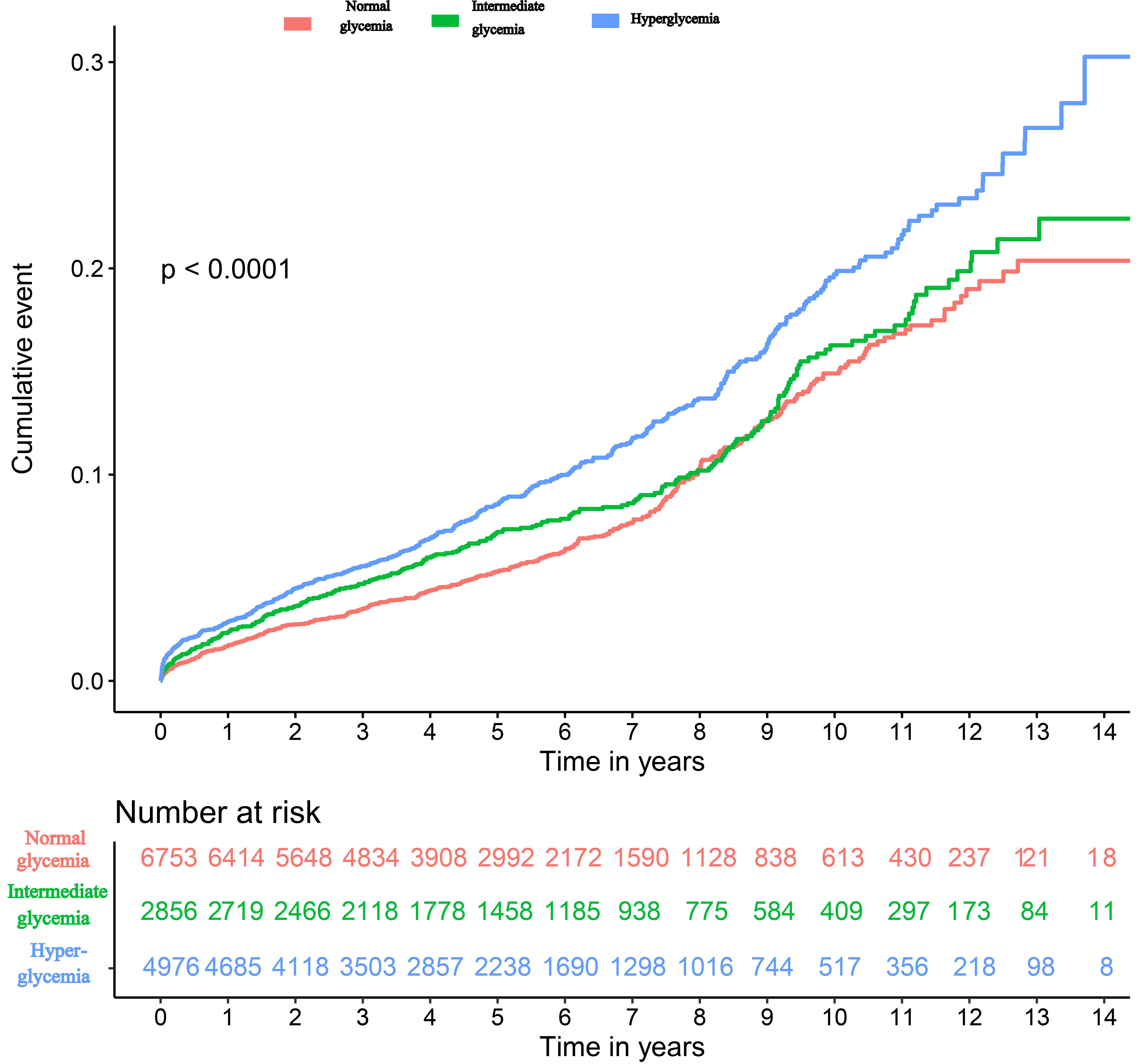
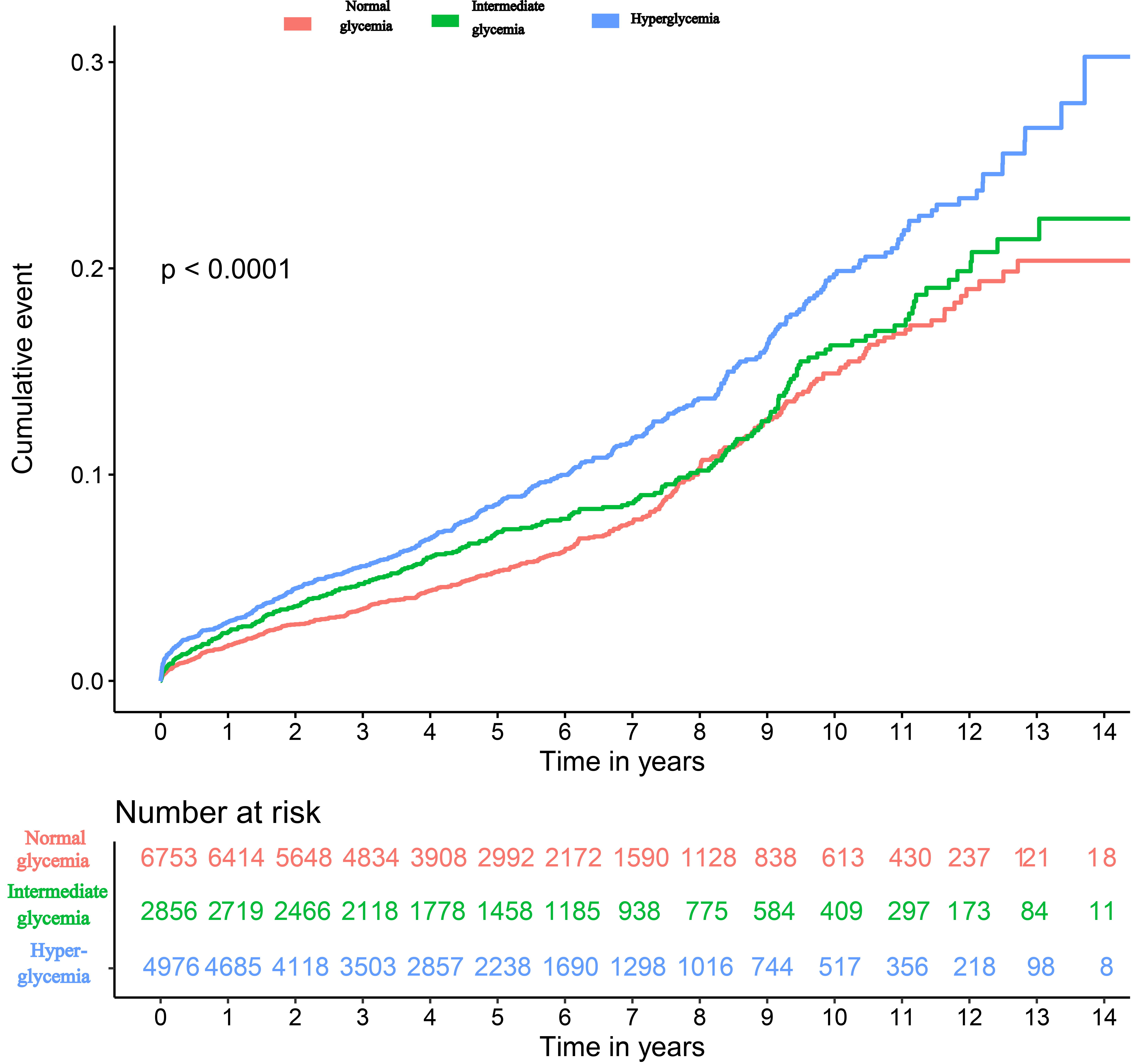 Fig. 3.
Fig. 3.Kaplan-Meier curves of long-term all-cause mortality.
When adjusted for confounders, the Cox regression model indicated that premature
CAD patients undergoing PCI with intermediate hyperglycemia were related to a
17% elevated risk of all-cause mortality, while hyperglycemia was associated
with a 35% increased risk of all-cause mortality (adjusted hazard ratio for
intermediate hyperglycemia group and hyperglycemia group, respectively: 1.169
[95% confidence interval (CI): 1.001–1.364], p = 0.049; and 1.352
[95% CI: 1.185–1.543], p
| Model 1 | Model 2 | Model 3 | ||||
| HR | p-value | HR | p-value | HR | p-value | |
| Normal glycemia | 1 (ref) | - | 1 (ref) | - | 1 (ref) | - |
| Intermediate hyperglycemia | 1.174 (1.008–1.367) | 0.039 | 1.167 (1.002–1.360) | 0.047 | 1.169 (1.001–1.364) | 0.049 |
| Hyperglycemia | 1.483 (1.307–1.683) | 1.478 (1.300–1.680) | 1.352 (1.185–1.543) | |||
Model 1: unadjusted.
Model 2: adjusted for age, gender.
Model 3: adjusted for age, gender, cholesterol, low-density lipoprotein
cholesterol, acute myocardial infraction, congestive heart failure, atrial
fibrillation, hypertension, chronic kidney disease.
HbA1c, hemoglobin A1c; HR, hazard ratio.
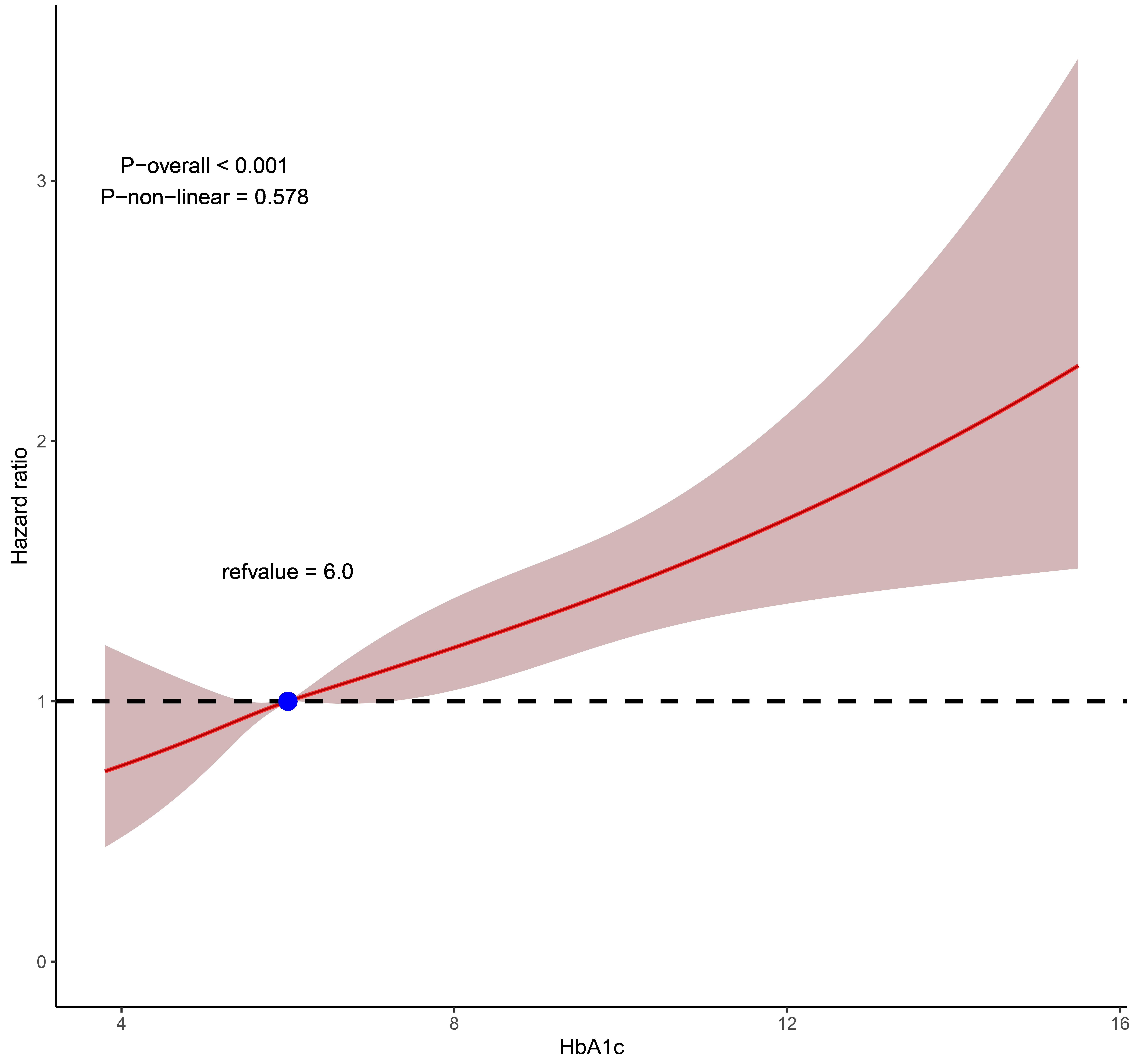
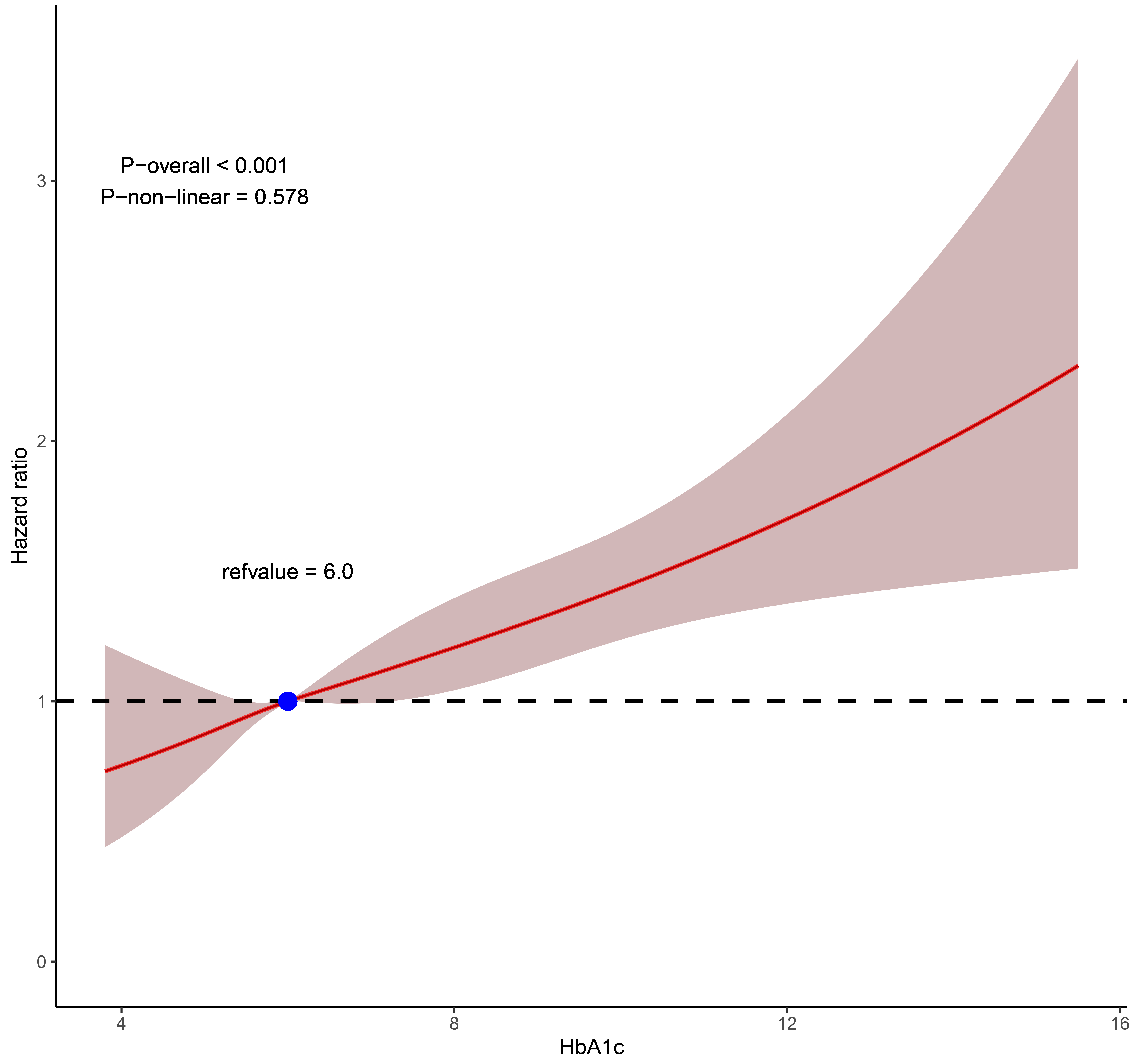 Fig. 4.
Fig. 4.Restricted cubic splines for the relationship between HbA1c and all-cause mortality. HbA1c, hemoglobin A1c.
Overall, in this multi-center retrospective study, we observed that one-third of patients had hyperglycemia, and intermediate hyperglycemia was present in 19.6% of our cohort. According to our results, we found that among premature CAD patients treated with PCI, intermediate hyperglycemia status is associated with a 17% increased risk of all-cause mortality. The finding reinforces the importance of strictly controlling HbA1c among individuals who present with CAD at a young age.
Premature CAD represents a large proportion of overall cardiovascular disease, and the rate of mortality has stalled despite an overall declining global cardiovascular mortality rate, probably due to inadequate control of risk factors [3, 13, 14]. Our study discovered a high prevalence of hypertension and DM in the study population and the mean levels of LDL-C were much higher than the guideline-recommended goal [15, 16]. The previous study confirmed that premature CAD, as a majority cardiovascular disease in the young, was significantly and positively associated with some risk factors, including DM, family history of CAD, dyslipidemia, smoking, and hypertension, which could lead to poor prognoses [5, 17]. In addition to hyperglycemia, our study demonstrated that intermediate hyperglycemia was associated with increased mortality in premature CAD patients undergoing PCI as well. Therefore, in parallel to the traditional risk factors like smoking and dyslipidemia, intermediate hyperglycemia may need to be controlled in these young patients imminently.
HbA1c has been suggested as a dependable tool not only for diagnosing DM but also for assessing the risk of cardiovascular events in both DM and non-DM patients [18, 19]. A previous study concluded that HbA1c exhibited a stronger association with the risks of cardiovascular disease and all-cause mortality when compared to fasting glucose [20]. In Adam’s study, compared with the oral glucose tolerance test (OGTT), HbA1c performed better for the predictive power of cardiovascular disease and chronic kidney disease [21]. Some studies demonstrated that OGTT is more sensitive than HbA1c in detecting hyperglycemia in CAD patients [22, 23]. However, the results of OGTT could be influenced by some factors such as the pre-test carbohydrate diet, physical activity, as well as the severity of the myocardial injury [24]. The 2019 European Society of Cardiology guidelines recommend that screening for diabetes in patients with cardiovascular disease should start with glycosylated hemoglobin [25]. Our study also identified a nearly linear relationship between HbA1c levels and all-cause mortality, suggesting that using HbA1c alone is a viable approach to assess the glycemic status of these patients. Benefiting from the stability of HbA1c and the convenience due to no testing time limitations, routine screening for HbA1c in young patients with CAD may be necessary.
This long-term follow-up study of premature CAD patients undergoing PCI showed that half of the patients’ HbA1c was over 6%, and the HbA1c level of one-third of patients was even more than 6.5%. The 2019 ESC Guidelines suggested monitoring HbA1c in chronic coronary syndrome patients and keeping it below 6.5% [6, 7]. However, as more attention is drawn to prediabetes, studies have found that the risk of cardiovascular events is elevated in the group without diabetes with HbA1c, 5.7–6.4% [26, 27]. In the group without diabetes with HbA1c of 6.0–6.5% (i.e., prediabetes), we found that the all-cause mortality of the group was higher than those with normal glycemia, which is consistent with several meta-analyses [27, 28]. Hence, our study also confirmed the harm of intermediate hyperglycemia in the group without diabetes.
Interestingly, compared with well-controlled persons with diabetes (HbA1c
Despite the accumulating evidence suggesting an association between elevated levels of HbA1c and DM with a poor outcome in premature AMI patients [30, 31, 32], the target of HbA1c in premature CAD patients undergoing PCI has not been well established. Alfredo Caturano et al. [33] reviewed the potential mechanism of strict glycemic control in cardiovascular protection in patients with acute coronary syndrome, mainly including anti-apoptotic actions, anti-inflammatory effects, increasing nitric oxide, reducing plasma level of free fat acid, etc. Considering hypoglycemia, past clinical trials have published contradictory conclusions on whether patients with acute coronary syndrome should strictly control glycemia. However, with the application of Glucagon-like peptide-1 receptor agonists and sodium-glucose cotransporter-2 inhibitors, patients may not need to bear the risk of hypoglycemia and could benefit from their cardiovascular protection [33].
Intermediate hyperglycemia defined by FBG increased all-cause mortality but did not reach statistical significance in supplementary analyses. Still, there are some rationalities to the result. First, the FBG samples should be obtained after at least 8 hours of fasting, which is a considerable practical problem, leading to a large missing rate in our study. Of the 14,585 premature CAD patients undergoing PCI we included, 5571 (38.2%) patients had missing FBG information. Second, FBG may be affected by many factors, including unstable sample, recent exercise, and acute stress. Patients we included were more likely to suffer from acute stress [34]. Third, FBG is less tightly linked to diabetes complications than HbA1C, while CAD is one of the major diabetes complications [35]. Thus, though HbA1C may be affected by anemia, pregnancy, hypertriglyceridemia, and chronic liver disease, it’s still worth using HbA1C to be a prognostic index considering it has very little biological variability.
During 4.62 years of median follow-up, our study showed that intermediate hyperglycemia status, defined as HbA1c 6.0–6.5%, still increases the risk of all-cause mortality in the large cohort of premature CAD patients undergoing PCI with or without DM. Therefore, active lifestyle modification and aggressive treatment should be considered for patients with premature CAD and glycemic disturbances.
Firstly, our results were subject to the limitation of the retrospective design. However, this allowed us to examine a large number of individuals with CAD at a young age in several Chinese regional central tertiary teaching hospitals. Further prospective studies focusing on premature CAD patients are needed. Secondly, despite having controlled for some common confounding factors, there may still be some potential confounding factors, such as the number of vessels treated by PCI that haven’t been fully adjusted for. Thirdly, we chose to focus solely on all-cause mortality as the primary endpoint due to limited data, and therefore, did not include other endpoints such as major adverse cardiovascular events, heart failure, failure of target vessel revascularization, or cardiovascular re-hospitalization in our study. However, all-cause mortality is still worth being the primary endpoint for its accuracy and accessibility. More national studies are necessary to better understand the link between hyperglycemia status and adverse cardiovascular outcomes. Finally, we did not observe the variability of HbA1c in follow-up [36], and identified hyperglycemia based solely on baseline HbA1c, but it is more applicable to routine screening for timely detection to draw the attention of clinical doctors.
In conclusion, we confirmed that compared with normal glycemia, patients with intermediate hyperglycemia are associated with a 17% residual risk of all-cause mortality, while hyperglycemia is associated with a 35% increased risk in premature CAD patients undergoing PCI. Our findings highlight the need for monitoring HbA1c earlier in patients with premature CAD to prevent future adverse prognoses. Active hypoglycemic therapy and lower HbA1c target should be considered in these patients.
CAD, coronary artery disease; PCI, percutaneous coronary intervention; MACE, major adverse cardiovascular events; HbA1C, hemoglobin A1c; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; AMI, acute myocardial infarction; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blocker.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ZZ, LQ, YHL, YH, JC contributed to the study’s conception and design. TC, WG, YH, HL, SY contributed to the literature search, studies screening and data extraction. ZZ, LQ, YHL, YH, TC, WG contributed to the analysis and the first draft of the manuscript. JC, YL, SC, JL contributed to interpretation of data for the work, reviewing manuscript critically, and revising the final versions of the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The study followed the “Declaration of Helsinki” and was approved by the institutional Ethics Research Committee of Guangdong Provincial People’s Hospital (No. GDREC2019-555H-2). All participating sites received institutional review board approval from their own ethics committees. Considering the design of this study is based on a retrospective analysis of the Hospital Information Management System, we have applied to the ethics committee and obtained a waiver of informed consent.
Not applicable.
This work was funded by grants from Guangdong Provincial science and technology project [grant number: 2020B1111170011]; Guangdong Provincial science and technology project [grant number: KJ022021049]; and The National Science Foundation for Young Scientists of China [grant number: 82070360]. The work was not funded by any industry sponsors.
The authors declare no conflict of interest.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.