1 Cardiology Department, Hospital Universitari Doctor Josep Trueta and Hospital Santa Caterina, 17007 Girona, Spain
2 Medical Science Department, School of Medicine, University of Girona, 17003 Girona, Spain
3 Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV), 28029 Madrid, Spain
4 Dirección Territorial de Radiologia y Medicina Nuclear de Girona, Insititut de Diagnòstic per la Imatge (IDI), 17007 Girona, Spain
5 Radiation Oncology Department, Institut Català d’Oncologia, 17007 Girona, Spain
6 Radiation Oncology Department, Institut Català d’Oncologia, L'Hospitalet de Llobregat, 08908 Barcelona, Spain
7 Center for Cardiovascular Genetics, Biomedical Research Institute of Girona, 17190 Girona, Spain
Abstract
Cancer and its treatments affect cardiovascular (CV) health, including an increased risk of CV death, decreased cardiorespiratory fitness (CRF), and cardiac dysfunction. Moreover, cancer-related fatigue and worse quality of life (QoL) are highly prevalent adverse effects experienced by patients during treatment and can persist years after therapy ends. Physical exercise has been proposed as a strategy to improve different aspects of life of cancer patients, and is recommended as a therapy in cardio-oncology guidelines. Exercise interventions reduce fatigue and improve QoL in patients with both solid tumors and hematological malignancies, although there is a lack of awareness of exercise recommendations, timing, and referral to such programs. New evidence indicates that physical activities improve CRF, which can lead to a reduction in CV mortality. Furthermore, cardiac dysfunction is a side effect of many oncological treatments, which may be mitigated by exercise interventions according to preclinical studies and recent publications. Nevertheless, specific physical exercise programs are not widely used in cancer patients. Thus, the goal of this review was to describe the current evidence on the benefits of exercise in cancer patients, the gaps that remain, and an approach to exercise prescription.
Keywords
- cardiotoxicity
- cardiorespiratory fitness
- cardio-oncology
- cardiovascular health
The effects of cancer and its treatments including chemotherapy, radiation, hormonal, and/or biological therapies can affect cardiovascular (CV) health, causing worsening of the CV profile, prognosis, cardiac function, cachexia, fatigue, and quality of life (QoL). CV mortality is over 20% in patients with breast, endometrial, and thyroid cancers and is almost 30% in those with prostate cancer [1, 2]. Over half of cancer patients report moderate-to-severe fatigue during treatment, of which 25% have persistent fatigue more than 5 years after treatment completion, and 5–26% of cases have a decline in cardiorespiratory fitness (CRF) [3, 4]. Moreover, many current cancer therapies cause significant adverse CV events, such as decreased cardiac function or heart failure. Furthermore, CV disease (CVD) and cancer share risk factors such as obesity, hyperglycemia, hypertension, and hypertriglyceridemia-induced inflammation, promoting carcinogenesis and tumor progression [5, 6].
Physical exercise has been positioned as a strategy to improve different aspects of life of cancer patients (Table 1, Ref. [7, 8]), and is recommended as a therapy in cardio-oncology guidelines. Different studies have shown the benefits of exercise in decreasing CV mortality, and improving CRF and QoL. Accurate exercise prescription is mandatory to obtain the expected benefits of reducing CV risk and mortality [7, 8].
| Difficulties [7, 8] | Benefits [7, 8] |
|---|---|
| Limited access to resources to support exercise | Improves cardiorespiratory fitness |
| Limited financial coverage | Ameliorates quality of life |
| Difficulty of adherence and motivation | Reduces fatigue |
| Improves the cardiovascular profile | |
| Reduces cardiovascular mortality |
Here, we provide an overview of the current evidence on the benefits of exercise interventions in cancer patients in terms of mortality and CRF, primary prevention of cardiotoxicity, fatigue, and QoL as well as the optimal timing of physical exercise and prescription recommendations.
Cancer survivors have an increased risk of long-term CV mortality compared to the general population, either because of unhealthy lifestyles or the toxicities of their treatment. The risk of death from CVD differs depending on the type of cancer and is estimated to be between 3% and 5% for brain and liver cancers, but can increase to 30–40% for prostate and bladder malignancies [1, 9, 10, 11]. Furthermore, significant and marked impairment of CRF has been demonstrated along the entire disease continuum, which may not recover after the completion of treatment. An inversely proportional correlation between CRF and CV mortality has been described, leading to a significant increase in mortality risk with decreasing CRF levels. This relationship is independent of traditional CV risk factors [4, 12, 13, 14, 15].
Cachexia is a severe complication of cancer that negatively affects QoL, response to chemotherapy, and survival; it affects more than 50% of cancer patients and accounts for up to 20% of cancer-related deaths. Cachexia is considered a multi-organ disease that involves different tissues and organs, including the heart. Cardiac cachexia has been observed in some cancer types, such as lung, pancreatic, and gastrointestinal cancers, and occurs primarily as a consequence of cardiac protein loss [16, 17].
Cancer therapy can also adversely affect cardiac structure and/or function, resulting in ventricular dysfunction that may be asymptomatic or symptomatic, manifesting as heart failure. This condition has been termed “cancer therapy-related cardiac dysfunction” (CTRCD) and occurs with many cancer therapies. The incidences of CTRCD induced by the commonly administered therapies anthracyclines (ACs), human epidermal growth factor receptor 2 (HER2)-targeted agents (e.g., trastuzumab), and immune checkpoint inhibitors are 5–40% (depending on dose and duration of exposure), 18%, and 15% [18, 19, 20, 21], respectively. Moreover, cardiac diastolic function may be impaired even with a low dose of AC as well as with new cancer therapies, which some authors consider a precursor to cardiovascular events [22, 23, 24].
Both cancer survivors and patients undergoing active treatment report fatigue in 62–85% of cases, as well as impairment of health-related QoL (HRQoL) (Fig. 1). The mechanisms underlying cancer-related fatigue suggest the involvement of complex multifactorial processes linked to a range of molecular/physiological processes such as inflammation, as well as the cumulative effects of cancer treatment and psychological factors [25].
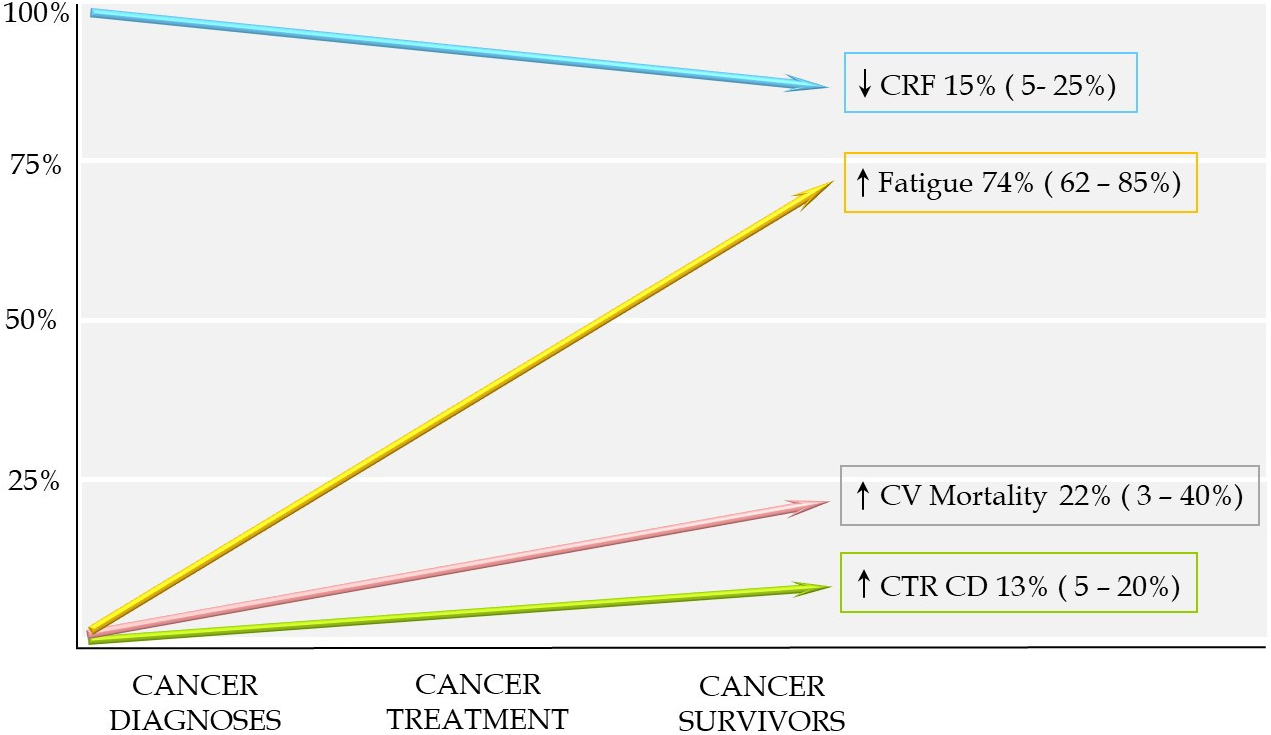 Fig. 1.
Fig. 1.Effects of cancer in cardiovascular health. CRF, cardiorespiratory fitness; CTRCD, cancer therapy-related cardiac dysfunction; CV, cardiovascular.
In current guidelines, physical exercise has been proposed to reduce morbidity and mortality in cancer patients [7, 8]. Although randomized controlled trials (RCTs) are lacking, current published meta-analyses and retrospective studies provide reliable evidence to support physical exercise as a strategy to decrease CV mortality (Table 2, Ref. [26, 27, 28, 29, 30]). This benefit can be partly explained by the fact that a considerable proportion of patients are at significant risk of CVD at the time of cancer diagnosis or have a worsened CV profile during treatment. In addition, the risk of CV toxicity is higher in patients with hypertension, hyperlipidemia, smoking, diabetes, or obesity [31]. Exposure to chemotherapy leads to vascular endothelial dysfunction, which contributes to the development of CVD. Current evidence shows that exercise training improves vascular endothelial function and wall thickening, especially in breast and prostate cancer survivors [32, 33, 34]. Thus, a supervised and structured physical exercise program would improve the CV profile of cancer patients and, therefore, potentially reduce CV mortality in the medium and long term.
| Study | Type | Cancer | N | Results |
| Kuiper JG et al. 2012 [26] | Prospective | Colorectal | 1339 | PA of |
| Beasley JM et al. 2016 [27] | Prospective | Breast | 2265 | Reduction in death from any cause and death from breast cancer for women who met the PA of 10 MET-hours/week 18 to 48 months post-diagnosis |
| Cormie et al. 2017 [28] | Meta-analyses | Breast | 68,285 | Trend to reduced risk of mortality in patients with higher exercise behaviours |
| 15 cohort studies | Colorectal | |||
| 4 RCT | Prostate | |||
| Scott et al. 2018 [30] | Meta-analyses | Breast | 3632 | VO |
| 48 RCT | Prostate | 1990 (55%) Ex | ||
| Lung, Hematologic | 1642 (45%) UC | |||
| Colorectal | ||||
| Gastrointestinal | ||||
| Kim et al. 2021 [29] | Retrospective | Breast | 39,775 | All exercise intensities had a lower risk of CVD |
CVD, cardiovascular disease; Ex, exercise; PA, physical activity; RCT,
randomized control trial; UC, usual care; CRF, cardiorespiratory fitness; VO
In addition to the positive impacts of physical exercise on the CV profile, the role of muscle-strengthening physical activity is essential for cancer patients and is associated with a lower risk of cancer mortality. This favorable effect could be explained by the reduction in systemic inflammation or insulin resistance [35, 36]. Recently, a J-shaped relationship has been observed between muscle-strengthening activities and cancer mortality, with the greatest reduction in risk occurring with 30–60 min of activity per week [37].
Cancer cachexia negatively affects survival. Exercise, as a therapeutic approach to decrease skeletal muscle degradation and body weight loss, could be adequate therapy. However, cachexia generally appears late in the disease, and there have been very few robust RCTs to determine the best therapeutic approach using physical activity [16].
Cancer patients present with significant impairment of CRF, which can be
improved by exercise therapy. Scott et al. [30] conducted a
meta-analysis to evaluate the effects of exercise therapy on adult-onset cancer
patients as measured by peak oxygen consumption (VO
Preclinical studies have shown that exercise interventions protect against AC-induced cardiotoxicity in rodents; less doxorubicin accumulation in cardiac tissue may be the key underlying mechanism [40, 41, 42]. Furthermore, an experimental study in rats demonstrated that exercise preserved cardiac function and attenuated the autophagic response in the heart and tumor tissues in cancer-induced cardiac cachexia [43]. In humans, there are only a few studies with a limited sample size that have evaluated the cardioprotective effects of exercise (Table 3, Ref. [44, 45, 46, 47, 48]).
| Study | Type | Cancer | Cancer Therapy | Trial intervention | Exercise specification | N | Primary outcome measures |
| Haykowsky et al. 2009 [47] | Single group study | Breast Cancer HER2 positive | Trastuzumab | Exercise | 3 days per week during 4 m of trastuzumab therapy | N = 17 | No statistically significant change in LV volumes, mass and ejection fraction |
| 5 min warm-up | |||||||
| 30–60 min cycle at a HR equal to 60% to 90% of peak oxygen consumption. | |||||||
| 5 min cool down | |||||||
| Kirkham et al. 2017 [44] | Two-arm proof-of-concept randomized controlled trial | Breast cancer | Anthracycline | Single bout exercise training vs usual care | Single bout of supervised treadmill exercise: | N = 24 | Change in biomarkers |
| Doxorubicin | 10 min warm-up | 11 = Ex | No change in echocardiographyic paramteres | ||||
| 30 min at 70% of age-predicted HR | 13 = UC | ||||||
| 5 min cool down | |||||||
| Ma Z et al. 2018 [46] | Randomized controlled trial | Breast Cancer | Anthracycline | Exercise training vs usual care | 16-week exercise supervised program | N = 64 | LVEF significantly increased after chemotheraphy in EX group and decrease in control group, statistically significant (p |
| 33 = EX | |||||||
| 31 = UC | |||||||
| Kirkham et al. 2020 [45] | Prospective, non-randomized controlled trial | Breast Cancer | Anthracycline | Exercise training vs usual care | 3 sessions per week | N = 37 | No difference on resting cardiac function |
| 20–30 min of treadmill, elliptical, or cycle ergometer | 26 = EX | ||||||
| aerobic exercise at 50–75% of age-predicted HR | 11 = UC | ||||||
| Hojan et al. 2020 [48] | Randomized controlled trial | Breast Cancer HER2 positive | Trastuzumab | Exercise training vs usual care | Daily sessions 9 w | N = 47 | Statistically significant decrease of the LVEF (p |
| Endurance: | 26 = EX | ||||||
| 2 min warm-up 45 min aerobic activities | 21 = UC | ||||||
| 3 min relaxation period | |||||||
| Strength: 40–45 min |
Min, minutes; EX, exercise; UC, usual care; HR, heart rate; LVEF, left ventricular ejection fraction; HER2, human epidermal growth factor receptor 2; LV, left ventricle.
In patients with breast cancer treated with AC, Kikhram et al. [44, 45] found no differences in resting cardiac function when comparing patients who performed aerobic exercise with the control group. However, an improvement in hemodynamic responses was detected by increasing cardiac output and decreasing systemic vascular resistance, which indicated a positive pathophysiological impact on the CV profile. In addition, Ma et al. [46] reported a positive result in the prevention of ventricular function decline in the physical exercise group.
Pertaining to preventing CTRCD secondary to trastuzumab therapy, discrepancies are found in the medical literature regarding the potential benefits of exercise in this clinical scenario. A prospective study by Haykowsky et al. [47] analyzed HER2-positive breast cancer patients who did aerobic training during the first 4 months of adjuvant trastuzumab, and underwent cardiac magnetic resonances before and after the exercise protocol. Left ventricular cavity dilation and worsening of ejection fraction were observed despite aerobic exercise training. Nonetheless, an RCT conducted by Hojan et al. [48] reported a statistically significant decrease of left ventricular ejection fraction (LVEF) in the control group compared to the intervention group.
Concerning diastolic function, a prospective cohort study of HER2-positive breast cancer women receiving AC and trastuzumab reported that physical activity of moderate-to-vigorous intensity was associated with better diastolic parameters (higher E/A and lower E/e’ ratio) [49]. Preclinical evidence and current clinical data available suggest that physical exercise may prevent the decline of LVEF secondary to cardiotoxic treatments, especially in selected patients; however, larger RCTs are needed to clarify the role of physical exercise in preventing cardiotoxicity in cancer patients.
There is strong evidence for the beneficial effect of exercise interventions on reducing fatigue and improving HRQoL in both solid tumors and hematological malignancies compared to usual care [50, 51, 52].
In solid tumors, the benefits of exercise have been reported in several cancer types, such as breast, colorectal cancer (CRC), lung, and prostate cancers. A meta-analysis by Juvet et al. [53] investigated the effects of exercise interventions on women with breast cancer during and after treatment (chemotherapy and/or radiation) and demonstrated that regular exercise decreases fatigue; this benefit persisted at the 6-month follow-up compared to usual care. Exercise programs in patients with CRC have been shown to improve several patient-reported HRQoL outcomes, including physical function, cancer-specific QoL, sleep quality, and fatigue [54]. This benefit is described in different stages of CRC, either pre-surgery, during chemotherapy, or after treatment. Therefore, exercise after CRC diagnosis is feasible and has beneficial effects on HRQoL irrespective of the timing of chemotherapy or surgery [55]. The role of physical activity in anxiety, depression, sleep quality, and fatigue in lung cancer is also favorable. Moreover, structured exercise with or without nutrition therapy has been shown to reduce cancer-related fatigue and improve QoL in patients with prostate cancer [56, 57].
For hematological malignancies, hematopoietic stem cell transplantation (HSCT) is a standard and curative treatment for some of these illnesses, and more than 50,000 HSCTs are performed annually worldwide. A meta-analysis of 10 RCTs of HSCT has demonstrated that exercise interventions have positive effects on reducing fatigue and improving HRQoL and muscle strength, even if initiated before transplant hospitalization (the concept of “pre-hab”) [58]. Based on that data, Mohananey et al. [59] proposed a model of cardiac rehabilitation and exercise in patients undergoing HSCT that included aerobic and strength training.
The role of cardiac rehabilitation in cancer patients is well established in
current cardio-oncology clinical practice guidelines for pre-, active-, and
post-specific treatments [60]. The optimal timing of physical exercise
interventions to obtain the expected benefits of physical activity and mitigate
chemotherapy-induced side effects is not well defined. However, the current
evidence supports the benefit of physical exercise throughout the disease. The
concept of “pre-habilitation” has been considered before HSCT and breast cancer
treatment, with a significant positive impact observed [57, 61]. Physical exercise
to improve HRQoL and diastolic and systolic left ventricular function parameters,
reduce fatigue, and increase VO
Accurate exercise prescription is mandatory to obtain the expected benefits of reducing CV risk and mortality, increasing capacity functional respiratory (CFR), and improving psychosocial well-being. Initially, cancer survivors should receive a comprehensive assessment of all components of health-related physical fitness (e.g., CRF, muscle strength and endurance, body composition, and flexibility), with cancer-specific considerations such as the disease stage and toxicities, in order to individualize the exercise prescription. Moreover, during active cancer therapy, an individual’s ability to tolerate exercise may fluctuate from day to day [65].
For the adequate evaluation of these patients, ergospirometry (cardiopulmonary
exercise testing) provides an assessment of integrative exercise responses
involving pulmonary, CV, neuropsychological, and skeletal muscle systems [66]. It
also involves measurements of VO
Global recommendations in cancer patients include aerobic and resistance exercises, warm-up and cool-down activities, and flexibility stretching exercises [8]. An effective exercise prescription includes moderate-intensity aerobic training at least three times per week for at least 30 min (90–150 min per week), for a minimum of 8–12 weeks. Moderate-intensity aerobic training reduces anxiety, depressive symptoms, improves HRQoL, bone health, and sleep in cancer patients. Moreover, it improves the lipid profile, lowers hypertension, and provides CV benefits [68, 69]. Resistance training added to aerobic exercise should be done two times per week, using no less than two sets of 8–15 repetitions with at least 60% of one repetition maximum [7]. However, apart from physical exercise, it is necessary to provide the patient with a comprehensive long-term service including medical evaluation, prescriptive exercise, and modification of cardiac risk factors and education [70].
The goals of cardio-oncology rehabilitation include increasing functional capacity and reducing morbidity/mortality, attenuating the drop in LVEF, decreasing cancer-related fatigue, and improving QoL and psychosocial well-being (Fig. 2). Multidisciplinary teams supported by cardio-oncology units working with different healthcare specialists and aided by advanced cardiac imaging techniques and ergospirometry are required.
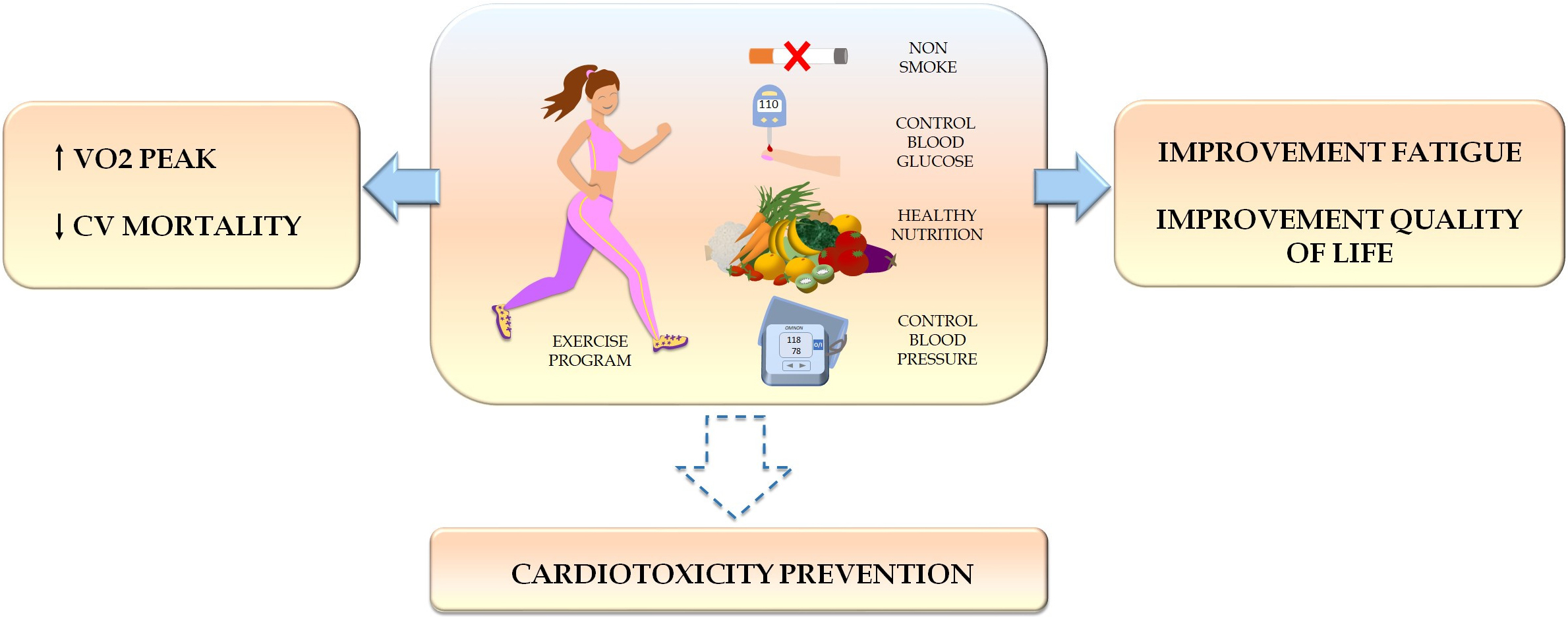 Fig. 2.
Fig. 2.Benefits of physical exercise in cancer patients.
Physical exercise increases functional capacity, reduces cardiovascular
mortality, decreases cancer-related fatigue, and improves quality of life.
Current evidence suggests that exercise prevents cancer therapy-related cardiac
dysfunction, although more evidence is needed. VO
Physical exercise has been demonstrated to provide clinical and emotional benefits to cancer patients. The evidence is reliable for the positive effect of exercise programs in reducing cancer-related fatigue and improving HRQoL, anxiety, depression, and well-being in all cancer populations. The exercise is established as a safe and effective strategy to increase CRF and potentially reduce CV mortality in cancer patients in the medium and long term. Furthermore, preclinical evidence and available clinical data suggest that exercise prevents CTRCD, although more rigorous and extensive RCTs are required, leading to an emerging field of investigation. To obtain the maximum benefits from exercise, this should be individualized according to the patient’s functional capacity, type and stage of cancer, and treatment approach. Moreover, multidisciplinary teams as well as reassessment and continued monitoring of patients during follow-up are critical.
NC and SM designed the research study. NC, MV and EP performed the research. AE and RB provided help and advice on the data analysis. EB assisted in the analysis and interpretation of the obtained data. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research received no external funding.
The author declares no conflict of interest. Sergio Moral is serving as Guest Editor of this journal. We declare that Sergio Moral had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Peter Kokkinos.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.


