- Academic Editors
Background: A common functional variant (c.-1306A
Peripheral artery disease (PAD) is a condition where the flow of blood to the muscles and other tissues in the legs is reduced, typically due to atherosclerosis in the arteries of the lower legs [1, 2].
While major risk factors such as smoking, diabetes, hypertension, and hypercholesterolemia are known to increase the risk for PAD, there is also evidence to suggest that independent genetic factors may contribute to its development. Studies conducted on families have indicated that even after accounting for conventional atherosclerosis risk factors, the heritability of PAD susceptibility is estimated to be around 20% [3, 4, 5].
Erythropoietin, a hormone produced in the kidney, is a key regulator of erythropoiesis and angiogenesis [6, 7, 8]. Angiogenesis is initiated by proliferation and migration of endothelial cells, which can lead to the development of a collateral circulation system, which can function as “endogenous bypass vessels”. The system of collateral blood vessels can be beneficial in mitigating the symptoms and progression of PAD and may result in later onset of symptomatic PAD [9, 10, 11]. Increased serum erythropoietin has been proposed as a useful biomarker and positive predictor for coronary collateral development among patients with chronic coronary artery occlusion [12].
A variant in the promoter region of the gene encoding erythropoietin
(EPO c.-1306A
In a study by Amanzada and coworkers [14] among chronic hepatitis C patients undergoing antiviral treatment, individuals with the EPO rs1617640 CC genotype had a weaker rise of erythropoietin levels and a higher likelihood of needing blood transfusions. Furthermore, a recent genome-wide association study across different ethnic groups found that the EPO rs1617640 gene variation was significantly associated with red blood cell (RBC) count [15].
However, these findings are in contrast with a previous study by Fan and coworkers [16] who found that the EPO C-variant was linked to elevated erythropoietin levels in a dose-dependent manner. Another study reported a higher frequency of the EPO C variant in blood donors with elevated hematocrit levels [17]. Additionally, Kästner and coworkers [18] presented data showing that the C-allele was linked to higher activity of the EPO gene promoter. These conflicting studies suggest that the role of the EPO rs1617640 gene variation in erythropoietin expression may vary depending on the underlying physiological and pathological conditions.
We have previously observed an association of the EPO rs1617640 variant with hematocrit, hemoglobin levels and RBC count in PAD patients [19]. Aim of the present study was to analyze the potential role of this genetic variant in long-term survival of PAD patients.
The current study included 951 patients with PAD who were recruited at the
Division of Angiology at the Department of Internal Medicine, University Hospital
Graz, Austria, between 1997 and 2000 [19]. To be eligible, patients had to have
an ankle-brachial index of less than 0.9 and/or
The identification of cardiovascular risk factors and cardiovascular disease was done through a combination of medical records from the University Hospital Graz, medical records provided by general practitioners, and self-reported medical and medication history. Measurement of ankle pressure and calculation of the ankle-brachial index was done according to the method described by Sanchez and Veith [20]. In-person interviews were conducted to obtain information on smoking habits and age at first onset of PAD. Diagnosis of diabetes was based on the criteria established by the World Health Organization [21]. Blood specimens were collected in the morning after an overnight fast. Laboratory measurements of RBC count, hematocrit values and hemoglobin were available for 887 (93.9%) subjects.
DNA was extracted from whole blood using a MagNA Pure LC system (Roche, Vienna, Austria). EPO genotypes were analyzed using the 5’-exonuclease (TaqMan) method [22]. To ensure the accuracy of the genotyping process, a subset of 96 samples was analyzed twice, and no discrepancies were detected.
Survival follow-up was analysed using electronic medical records from the Medical University of Graz.
Statistical analysis was done with IBM SPSS Statistics release 28 (Chicago, IL,
USA). The genotype distribution was tested for Hardy-Weinberg equilibrium using a
chi-square test. Categorical variables were compared by chi-square test or
Kruskal-Wallis test and summarized as percentages. Continuous variables were
analyzed by ANOVA and summarized as means
An overview of demographic and EPO genotype data of the study cohort is presented in Table 1. Determination of EPO genotypes was successful in 946 (99.2%) PAD patients and showed no deviation from the Hardy–Weinberg equilibrium. All further analyses were based upon the 946 patients with valid EPO genotype.
| PAD patients (n = 946) | ||
| Age, years | 68.4 | |
| Age at onset of PAD, years | 64.8 | |
| Male sex | 585 (61.9%) | |
| Type 2 diabetes | 455 (48.1%) | |
| Smoker (former or current) | 591 (62.5%) | |
| Hypercholesteremia | 653 (69.1%) | |
| Arterial hypertension | 635 (67.2%) | |
| EPO genotype | ||
| AA | 356 (37.7%) | |
| AC | 433 (45.8%) | |
| CC | 156 (16.5%) | |
| EPO C allele frequency | 0.394 | |
EPO, erythropoietin.
Twenty years after recruitment of the cohort, 752 (79.5%) patients were dead, 103 (10.9%) were still alive, and 91 (9.6%) were lost-to-follow up. In a Kaplan-Meier analysis, EPO genotype was not associated with survival (Fig. 1). Median survival was 80.1 years for the AA genotype, 80.7 years for the AC genotype, and 81.2 years for the CC genotype (p = 0.98, Log rank test).
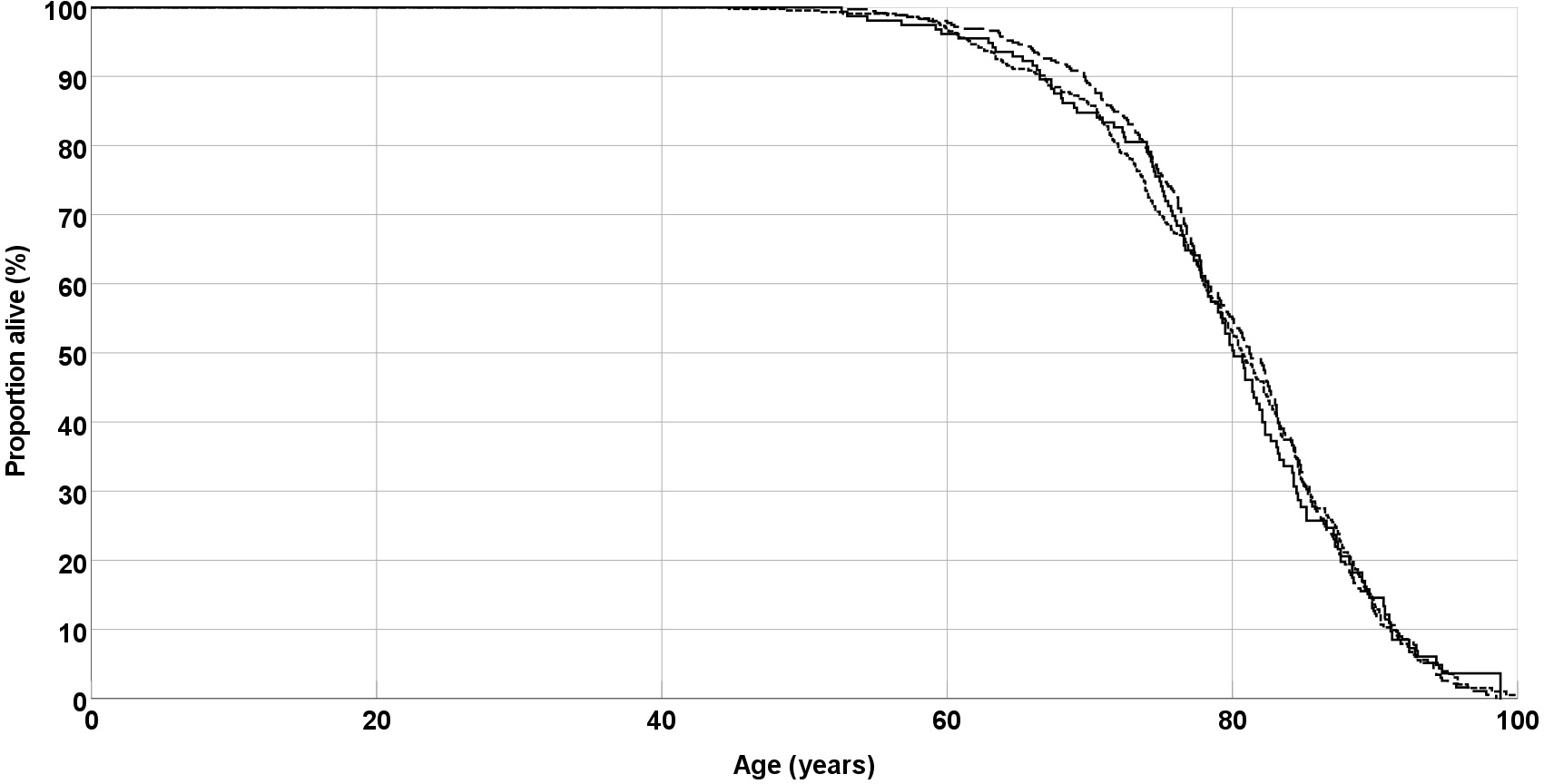 Fig. 1.
Fig. 1.Overall survival of peripheral arterial disease (PAD) patients. Lines are separate for EPO genotypes AA (solid line), AC (short dashes) and CC (long dashes). EPO, erythropoietin.
Similarly, in a multivariate Cox regression analysis, which including sex, smoking habit, type-2 diabetes, hypercholesterolemia and arterial hypertension, EPO genotypes were not associated with overall survival (Hazard ratio 0.63; 95% confidence interval 0.88–1.08, p = 0.63).
We have previously reported that a variant in the promoter region of the EPO gene was associated with elevated hematocrit, hemoglobin levels, and RBC count in patients with PAD, as well as age at onset of the disease, making it a candidate genetic risk factor for the disease [19]. In a follow-up analysis 20 years after recruitment of the patients, this EPO gene polymorphism showed no association with overall survival.
Erythropoietin is known to be an important regulator of angiogenesis and higher erythropoietin levels in the blood were a positive predictor of collateral formation in patients suffering from coronary artery occlusion [13, 23]. It is possible that the potential favourable angiogenic effects of higher EPO expression may have been outweighed by increased erythropoiesis, leading to higher viscosity of the blood and an elevated risk for microvascular complications. It has furthermore to be kept in mind that the observed differences of hematocrit, hemoglobin levels and RBC count between different EPO rs1617640 genotype groups were small and did not necessarily indicate severe clinical pathological consequences.
To reduce the chance of false positive results, analyses of EPO gene variations was restricted to the rs1617640 polymorphism, which was previously associated with markers of erythropoiesis in PAD patients [19]. Including other non-functional EPO variants would inevitably have resulted in a strong decrease of prior probability of association, leading to a higher risk of false positive findings (type I error) [24].
Available medical records included only date of death, but no further information on cause of death. No separate analyses for different causes of death, such as cardiovascular death or cancer-realted death, could be performed.
Furthermore, a part of the initial cohort (n = 91) were lost-to-follow-up with unknown outcome. It is likely that the majority of these patients died before the wide-spread launch of electronic medical records. Due to ethical reasons, survival analysis was restricted to the use of medical records and we were not allowed to approach the patients’ relatives for further survival data.
In summary, the results of the present study indicate that the functional EPO rs1617640 gene polymorphism, irrespective of its association with markers of erythropoiesis and age at onset of the disease, does not affect overall survival of PAD patients.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
WR and TL designed the research study. WR and TL performed the research. UL provided help and advice on data management and analysis. WR analyzed the data. WR, UL and TL wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work. All authors read and approved the final manuscript.
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethical Committee of the Medical University of Graz (ethical vote 09-124 ex 98/99). Informed consent was obtained from all subjects involved in the study.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
