1. Introduction
Secondary mitral regurgitation (SMR) is a common valvular heart disease that
affects heart failure symptoms and clinical outcomes [1, 2, 3]. According to the
current guidelines, two-dimensional (2D) echocardiographic parameters, including
vena contracta width (VCW) and effective regurgitant orifice area by the proximal
isovelocity surface area method (EROA), are
recommended to determine SMR severity; however, the severity may be
underestimated using VCW and EROA if regurgitant orifice area is
elliptical [4, 5, 6].
Vena contracta area (VCA) hydrodynamically corresponds to the regurgitant
orifice area [7]. Kahlert et al. [8] primarily reported direct planimetry
of VCA (VCA) based on three-dimensional transesophageal echocardiography
(3D-TEE), and VCA was subsequently validated using an in vitro
model and cardiac magnetic resonance imaging [9, 10]. Furthermore, Goebel
et al. [11] reported that compared with EROA, VCA is a
robust parameter for discriminating severe SMR. Moreover, previous studies have
suggested that VCA is elliptical in cases of SMR based on several vena contracta (VC)
parameters, including anteroposterior VCW (VCW), mediolateral VCW
(VCW), average of VCW and VCW (VCW), and VCA
calculated as an ellipse (VCA). These studies have also reported that
the ellipticity consequently limited the ability of VCW and EROA
to accurately classify SMR severity [8, 12]. However, these were relatively
small-scale studies, and there is little information available regarding the best
cutoff values of VC parameters for severe SMR.
Thus, we hypothesized that parameters that considered the elliptical shape of
the mitral regurgitant orifice, including VCA and VCA,
are better surrogate markers for severe SMR based on VCA than
EROA. This study also investigated the best cutoff values of these VC
parameters for severe SMR. Furthermore, we reassessed the true SMR severity using
the cutoff values of VC parameters to avoid underestimating SMR based on
EROA.
2. Methods
2.1 Patient Population
Patient characteristics and echocardiographic data were collected from the
medical records and echocardiography reports. The study protocol was approved by
the Institutional Review Board of New Tokyo Hospital and was in accordance with
the guidelines of the Declaration of Helsinki. The requirement for informed
consent was waived because of the retrospective nature of this study. Based on
integrative methods using qualitative, semiquantitative, and quantitative
approaches, 154 patients with at least mild SMR were identified via a review of
echocardiography databases at New Tokyo Hospital between January 2018 and March
2021. These patients underwent 3D-TEE based on clinical indications and
transthoracic echocardiography (TTE) within 1 month of 3D-TEE at our center [4].
SMR was defined as incomplete mitral leaflet closure because of regional
myocardial dysfunction, global left ventricular remodeling, apical tethering of
the mitral valve (MV), or annular dilation in the presence of an anatomically
normal valve apparatus [4, 13]. Of 172 patients, those with multiple or
nonholosystolic SMR jet (6 patients), previous MV intervention (7 patients),
concomitant mitral stenosis (2 patients) [14], and mitral annular calcification
(3 patients) were excluded from this study.
Overall, 19 of 154 patients were excluded because the quality of 3D imaging was
inadequate for VCA analysis, and 7 patients were excluded because of
incomplete data for the quantitative assessment of SMR; hence, 128 patients were
included in the final analysis.
2.2 Echocardiographic Parameters
Echocardiographic examinations were performed using iE33 system (Philips
Healthcare, Andover, MA, USA) and EPIQ7 system (Philips Healthcare, Andover, MA,
USA) equipped with a matrix-array transducer for transthoracic (X5-1) and
transesophageal echocardiography (X7-2t and X8-2t), according to the guidelines
for the clinical application of echocardiography [4, 14, 15, 16, 17, 18]. For offline
analysis, echocardiographic data were stored in a computer at a dedicated
workstation.
Regarding two-dimensional TTE (2D-TTE) parameters, left ventricular
end-diastolic and -systolic volumes, left ventricular ejection fraction (LVEF),
and left atrial volume were estimated using the biplane Simpson disk method via
transthoracic echocardiography.
Regarding TEE parameters, EROA and regurgitant volume (RV)
were estimated using the proximal isovelocity surface area method [4]. A
continuous wave Doppler cursor was aligned parallel to the SMR jet for obtaining
peak velocity and velocity–time integral at a Nyquist limit of 50–70 cm/s, with
the gain set to a level immediately below the threshold for noise. EROA
was derived using a color Doppler in a four-chamber view at an aliasing velocity
of 30–40 cm/s. Moreover, during systole, proximal isovelocity surface area (PISA) radius and flow velocity
parameters were obtained at similar time points for calculating EROA. To
determine VC parameters, 3D color Doppler datasets were acquired from an
intercommissural view using full volume for each patient. The quantification of
VCA was performed via multiplanar reconstruction using dedicated software
(Philips QLAB Versions 9.0, Philips Healthcare, Andover, MA, USA) (Fig. 1) [4].
The cropping plane was moved along the direction of the jet until the smallest
jet cross-sectional area became visible at the level of VC. Subsequently,
VCA was measured using manual planimetry of the color Doppler flow signal.
VCW and VCW were also measured as anteroposterior and mediolateral
VCWs, respectively, in reconstructed 2D planes from the 3D-TEE dataset;
VCW and VCW were obtained in the left ventricular outflow tract and
intercommissural views (or views that were close to intercommissural views),
respectively [8]. VCW was calculated as (VCW + VCW)/2,
VCA was calculated as (VCW/2)
(VCW/2) [8], and VCA shape index was calculated as
VCW/VCW. In patients with irregular rhythm (i.e., atrial
fibrillation or flutter not requiring constant ventricular pacing for
bradycardia), these parameters were calculated as the mean of 3–5 parameters
performed by avoiding remarkable irregular RR intervals. EROA and VC
parameters were performed by one observer (H.O.).
 Fig. 1.
Fig. 1.
Assessment of vena contracta using 3D-TEE. A case of an
84-year-old woman with dilated cardiomyopathy and secondary mitral regurgitation.
(A) Vena contracta described by multiplanar reconstruction of 3D color Doppler
datasets. (B) VCA measured using manual planimetry of the vena contracta
was 0.42 cm. VCW and VCA measured as the narrow and wide VCWs
in the anteroposterior and mediolateral directions were 0.43 and 1.21 cm,
respectively. VCW, calculated as (VCW + VCW)/2, was
0.82 cm. VCA, calculated as (VCW/2)
(VCW/2), was 0.41 cm. IC, intercommissural; LVOT, left ventricular outflow tract;
3D-TEE, three-dimensional transesophageal echocardiography; VCA, three-dimensional vena contracta
area; VCW, anteroposterior vena contracta width; VCW, mediolateral
vena contracta width; VCW, average of anteroposterior and
mediolateral vena contracta widths; VCA, vena contracta area as an
ellipse.
VCA of 0.39 cm was used as a reference standard of severe
SMR in the current study, considering that the severity of SMR may be
underestimated using EROA and that VCA is a more robust parameter
for distinguishing severe SMR than EROA [4, 11].
2.3 Statistical Analysis
Categorical variables were presented as frequencies and analyzed using
chi-square, Fisher’s exact, or Cochran–Armitage test, as appropriate. Continuous
variables were presented as mean standard deviation or median with
interquartile range and were compared using Mann–Whitney U or
Jonckheere–Terpstra test, as appropriate. The overall rates of correct SMR
severity classifications based on VCA were statistically compared using
McNemar’s test in 2 2 tables. Correlations between different
parameters were determined using Pearson’s test and linear regression analysis.
Receiver operating characteristic (ROC) curve analyses were performed to assess
the ability of each parameter to identify severe SMR based on VCA. The
Youden index was used to determine the best cutoff value for severe SMR based on
VCA considering optimal sensitivity and specificity. Discrimination of
severe SMR based on VCA was assessed using the C-statistic. All
statistical tests were two-tailed, and a two-sided p-value of 0.05
was considered to indicate statistical significance. Data analysis was performed
using EZR software version 1.50 (Saitama Medical Center, Jichi Medical
University, Saitama, Japan) [19].
3. Results
3.1 Patient Characteristics
The mean age of the patients was 77.0 8.9 years, and 78 (60.9%)
patients were men (Table 1). Regarding echocardiographic data, the mean LVEF was
37.5% 13.4%, with an LVEF of 50% in 95 (74.2%) patients (Table 2).
The mean tenting height of MV was 0.88 0.34 cm. Regarding SMR
quantification, EROA and RV were 0.26 0.12 cm and
40.6 17.3 mL, respectively, with severe SMR based on EROA of
0.40 cm (according to the current guidelines) in 16 (12.5%)
patients [4]. VCA was 0.46 0.26 cm, with severe SMR based on
VCA in 75 (58.6%) patients. VCW and VCA were 0.84
0.26 cm and 0.49 0.28 cm, respectively.
Table 1.Patient demographics.
| Variables |
All patients (n = 128) |
| Age, years |
77.0 8.9 |
| Men, n |
78 (60.9) |
| Body surface area, m |
1.57 0.17 |
| Hypertension, n |
66 (51.6) |
| Diabetes mellitus, n |
42 (32.8) |
| Dyslipidemia, n |
59 (46.1) |
| Smoking, n |
71 (55.5) |
| Chronic kidney disease (eGFR 60 mL/min/1.73 m), n |
108 (84.4) |
| Paroxysmal atrial fibrillation/flutter, n |
32 (25.0) |
| Persistent atrial fibrillation/flutter, n |
64 (50.0) |
| Irregular rhythm, n |
54 (42.2) |
| Previous myocardial infarction, n |
35 (27.3) |
| Pacemaker, n |
16 (12.5) |
| Implantable cardioverter defibrillator, n |
16 (12.5) |
| Cardiac resynchronization therapy, n |
7 (5.5) |
| NYHA functional class |
2.1 0.6 |
|
I, n |
19 (14.8) |
|
II, n |
85 (66.4) |
|
III, n |
23 (18.0) |
|
IV, n |
1 (0.8) |
Continuous data are presented as means standard deviations, except brain
natriuretic peptide (median and interquartile range); categorical data are given
as the counts (percentages).
eGFR, estimated glomerular filtration rate; NYHA, New York Heart Association.
Table 2.Echocardiographic data.
| Variables |
All patients (n = 128) |
| Measurements on two-dimensional transthoracic echocardiography |
|
LVEDV index, mL/m |
120.6 50.0 |
|
LVESV index, mL/m |
83.9 47.9 |
|
LVEF, % |
37.5 13.4 |
|
LVEF 50%, n |
95 (74.2) |
|
Interventricular septum thickness, mm |
9.3 1.9 |
|
Posterior wall thickness, mm |
9.2 1.8 |
|
Left atrial volume index, mL/m |
119.8 72.6 |
|
PASP, mmHg |
41.6 14.0 |
|
Severe aortic stenosis, n |
0 (0.0) |
|
Severe aortic regurgitation, n |
3 (2.3) |
|
Severe mitral stenosis, n |
0 (0.0) |
|
Severe tricuspid regurgitation, n |
32 (25.0) |
|
Severe pulmonary regurgitation, n |
0 (0.0) |
|
Atrial septal defect, n |
5 (3.9) |
| Measurements in mitral valve on three-dimensional transesophageal echocardiography |
|
Heart rate, bpm |
70.0 10.3 |
|
Heart rate in 54 patients with irregular rhythm, bpm |
71.9 10.4 |
|
Anterior mitral leaflet pseudoprolapse, n |
42 (33.0) |
|
Tenting height, cm |
0.88 0.34 |
|
Anteroposterior annulus diameter, cm |
3.28 0.43 |
|
Mediolateral annulus diameter, cm |
3.49 0.43 |
|
EROA, cm |
0.26 0.12 |
|
RV, mL |
40.6 17.3 |
|
Severe SMR based on EROA of 0.40 cm, n |
16 (12.5) |
|
VCW, cm |
0.49 0.14 |
|
VCW, cm |
1.19 0.44 |
|
VCA, cm |
0.46 0.26 |
|
Severe SMR based on VCA of 0.39 cm, n |
75 (58.6) |
|
VCW, cm |
0.84 0.26 |
|
Severe SMR based on VCA of 0.78 cm, n |
72 (56.3) |
|
VCA, cm |
0.49 0.28 |
|
Severe SMR based on VCA of 0.42 cm, n |
70 (54.7) |
|
VCA shape index |
2.47 0.84 |
|
Frame rate in VCA measurements, Hz |
18.4 6.1 |
|
Frame rate in VCA measurements in 54 patients with irregular rhythm, Hz |
18.8 5.4 |
|
Continuous data are presented as means standard deviations; categorical
data are given as the counts (percentages).
LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular
end-systolic volume; LVEF, left ventricular ejection fraction; PASP, pulmonary
artery systolic pressure; EROA, effective regurgitant orifice area
by the proximal isovelocity surface area method; RV, regurgitant volume
based on proximal isovelocity surface area method; SMR, secondary mitral
regurgitation; VCW, anteroposterior vena contracta width; VCW,
mediolateral vena contracta width; VCA, vena contracta area based on
three-dimensional echocardiographic data; VCW, averaged vena
contracta width; VCA, elliptical vena contracta area.
3.2 Associations of EROA with VCA
EROA showed a strong correlation with VCA (r = 0.801, p 0.001) (Fig. 2A). ROC curve analysis revealed that EROA showed good
discrimination of severe SMR based on VCA (C-statistic, 0.910; 95%
confidence interval [CI], 0.859–0.961; p 0.001), with the best
cutoff value of 0.21 cm (Fig. 2B). The sensitivity and specificity of
EROA for severe SMR based on VCA were as follows: EROA
of 0.20 cm, 92.0% and 73.6%; EROA of 0.30 cm, 49.3% and
94.3%; and EROA of 0.40 cm, 22.6% and 100.0%; respectively. In
addition, VCA and SMR incidence were significantly lower (p
0.001) in patients with nonsevere SMR based on EROA of 0.40 cm
(according to the current guidelines) than in those with severe SMR based on
EROA of 0.40 cm (Fig. 2C,D) [4]. Notably, among 112
patients with nonsevere SMR based on EROA of 0.40 cm, 59
(52.7%) had discordantly severe SMR based on VCA. SMR severity based on
VCA was not correctly reclassified as severe SMR by EROA
(McNemar’s test; p 0.001).
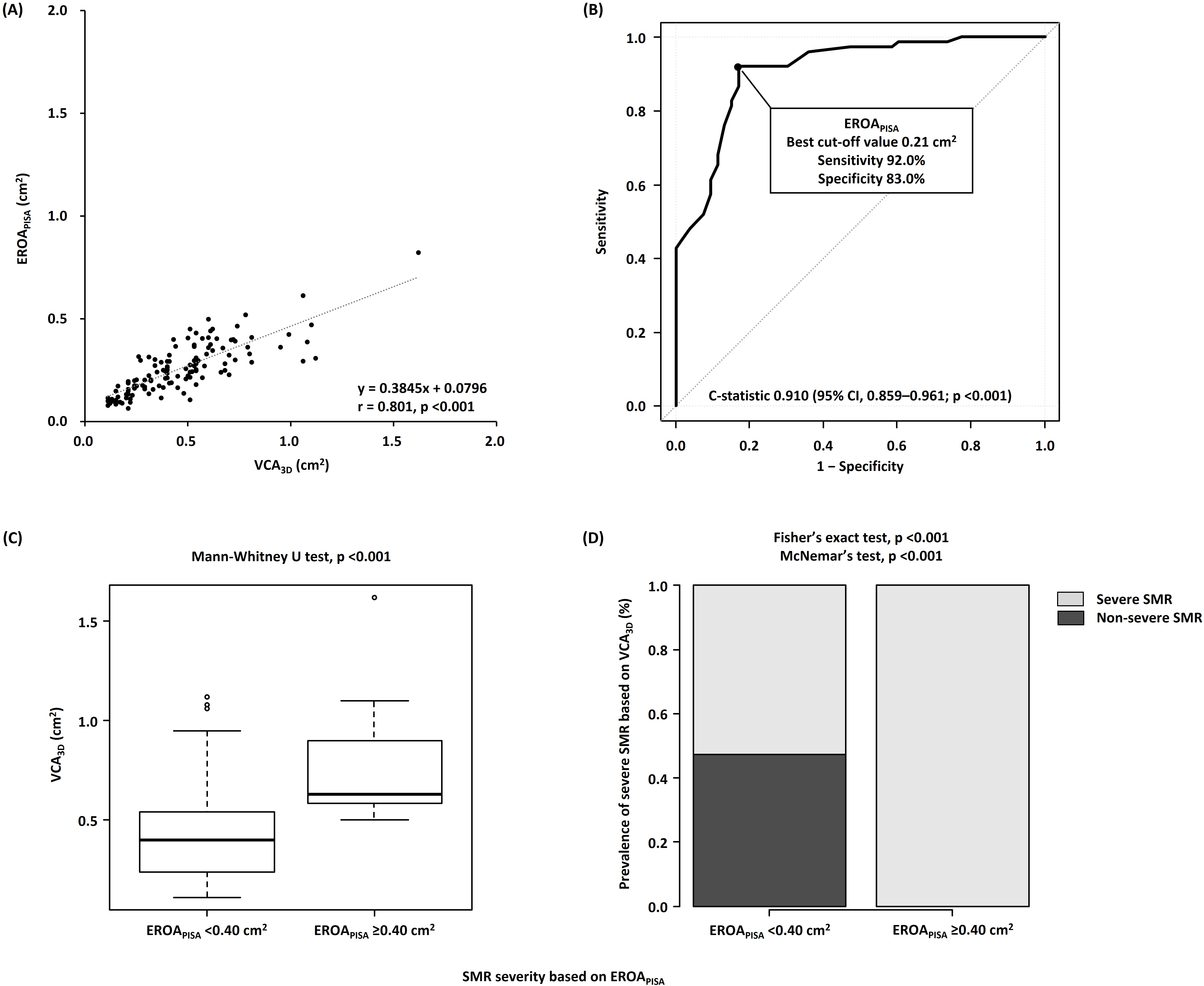 Fig. 2.
Fig. 2.
Associations of VCA with EROA. (A) Correlations
between VCA and EROA. (B) Receiver operating characteristic curve
analyses of EROA to identify severe SMR. (C) Comparison of VCA
between the nonsevere (EROA of 0.40 cm) and severe
(EROA of 0.40 cm) SMR groups. (D) Incidence of severe SMR
based on VCA of 0.39 cm in the nonsevere (EROA of
0.40 cm) and severe (EROA of 0.40 cm) SMR groups.
VCA, three-dimensional vena contracta area; EROA, effective
regurgitant orifice area by proximal isovelocity surface area method; SMR,
secondary mitral regurgitation.
3.3 Associations of VCW with VCA
VCW showed a strong correlation with VCA (r = 0.786, p 0.001). ROC curve analysis indicated that VCW showed relatively good
discrimination of severe SMR based on VCA (C-statistic, 0.874; 95% CI,
0.812–0.936; p 0.001), with the best cutoff value of 0.43 cm.
3.4 Associations of VCW and VCA with
VCA
VCW and VCA had a strong correlation with VCA (r
= 0.940, p 0.001 and r = 0.980, p 0.001, respectively)
(Figs. 3A,4A). According to ROC curve analysis, VCW and
VCA showed fairly good discrimination of severe SMR based on
VCA (C-statistic, 0.981; 95% CI, 0.963–1.000; p 0.001 and
C-statistic, 0.985; 95% CI, 0.970–1.000; p 0.001, respectively),
with the best cutoff values of 0.78 cm and 0.42 cm, respectively (Figs. 3B,4B). Moreover, regarding the comparison of C-statistics, VCW and
VCA showed significantly better discrimination than EROA
(p = 0.007 and p = 0.003, respectively).
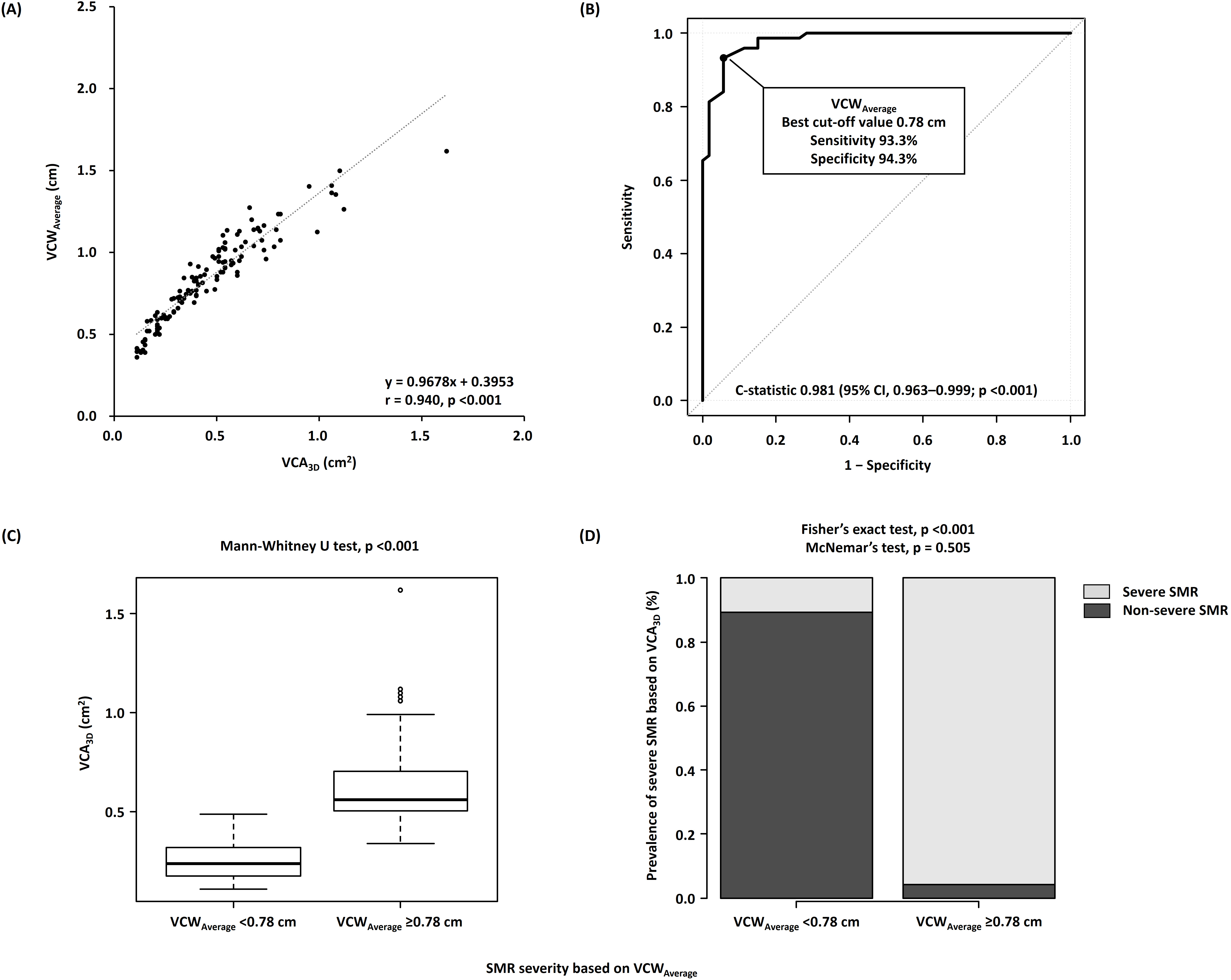 Fig. 3.
Fig. 3.
Associations of VCA with VCW. (A)
Correlations between VCA and VCW. (B) Receiver operating
characteristic curve analyses of VCW to identify severe SMR. (C)
Comparison of VCA between the nonsevere (VCW of 0.78 cm)
and severe (VCW of 0.78 cm) SMR groups. (D) Incidence of
severe SMR based on VCA of 0.39 cm in the nonsevere
(VCW of 0.78 cm) and severe (VCW of 0.78 cm)
SMR groups. VCA, three-dimensional vena contracta area; VCW,
average of anteroposterior and mediolateral vena contracta widths; SMR, secondary
mitral regurgitation.
 Fig. 4.
Fig. 4.
Associations of VCA with VCA. (A)
Correlations between VCA and VCA. (B) Receiver operating
characteristic curve analyses of VCA to identify severe SMR. (C)
Comparison of VCA between the nonsevere (VCA of 0.42
cm) and severe (VCA of 0.42 cm) SMR groups. (D)
Incidence of severe SMR based on VCA of 0.39 cm in the
nonsevere (VCA of 0.42 cm) and severe (VCA of
0.42 cm) SMR groups. VCA, three-dimensional vena contracta
area; VCA, vena contracta area as an ellipse; SMR, secondary mitral
regurgitation.
In addition, patients with nonsevere SMR, according to VCW of
0.78 cm and VCA of 0.42 cm, showed significantly lower
VCA (p 0.001 for both) and SMR incidence based on VCA
(p 0.001 for both) than those with severe SMR based on
VCW and VCA (Fig. 3C,D and Fig. 4C,D). Notably, SMR
severity based on VCA was correctly reclassified as severe SMR based on
VCW (p = 0.505) and VCA (p = 0.182).
3.5 SMR Severity Based on EROA Considering VCW
and VCA
Our patients were classified into the following three subgroups based on
EROA according to the current guidelines [4]: 88 patients with
EROA of 0.30 cm, 24 patients with EROA of 0.30–0.40
cm, and 16 patients with EROA of 0.40 cm. According
to the incremental EROA, VCA (p 0.001) and SMR
incidence based on VCA (p 0.001) significantly increased
(Fig. 5A,B). Notably, in patients with EROA of 0.30 cm, which
is suggestive of moderate SMR according to the current guidelines, 38 of 88
(43.2%) patients had severe MR based on VCA. However, SMR severity based
on VCA in patients with EROA of 0.30 cm was correctly
reclassified as severe MR based on VCW (p = 0.505) and
VCA (p = 0.182) (Fig. 6A,B).
 Fig. 5.
Fig. 5.
Associations between VCA and EROA among the
three subgroups (EROA of 0.30 cm, EROA of 0.30–0.40
cm, and EROA of 0.40 cm). (A) Increase in
VCA according to the increase in SMR severity. (B) Incidence of severe SMR
based on VCA of 0.39 cm according to the increase in SMR
severity. VCA, three-dimensional vena contracta area; EROA,
effective regurgitant orifice area by proximal isovelocity surface area method;
SMR, secondary mitral regurgitation.
 Fig. 6.
Fig. 6.
Associations of VCA with VCW and
VCA in the EROA 0.30 cm group. (A) Incidence of
severe SMR based on VCA of 0.39 cm between the nonsevere
(VCW of 0.78 cm) and severe (VCW of 0.78 cm)
SMR groups. (B) Incidence of severe SMR based on VCA of 0.39
cm between the nonsevere (VCA of 0.42 cm) and severe
(VCA of 0.42 cm) SMR groups. VCA,
three-dimensional vena contracta area; VCW, average of
anteroposterior and mediolateral vena contracta widths; VCA, vena
contracta area as an ellipse; EROA, effective regurgitant orifice area
determined by the proximal isovelocity surface area method; SMR, secondary mitral
regurgitation.
4. Discussion
The current study revealed the following findings: (1) VCW and
VCA had a fairly strong correlation with VCA, with the best
cutoff values of 0.78 cm and 0.42 cm, respectively, and (2) VCW
of 0.78 cm and VCA of 0.42 cm might be useful
in identifying severe SMR based on VCA, particularly in patients with
EROA of 0.30 cm, corresponding to moderate SMR according to the
current guidelines, who are at potential risk of underestimation of SMR severity
because of the ellipticity of regurgitant orifice area [4].
4.1 Usefulness of VCW and VCA in Identifying
Severe SMR
Although VCW was shown to be a reliable semiquantitative parameter for
evaluating SMR severity according to the current guidelines, VCW
evaluation is not routinely used as a stand-alone parameter [4]. However,
according to a previous study by Kahlert et al. [8], VCW was more
strongly correlated with VCA than with VCW. Furthermore,
VCW is strongly correlated with VCA [8]. To accurately
identify severe SMR, the current guidelines recommend calculating VCW
with a cutoff value of 0.80 cm for severe SMR if the regurgitant orifice area is
elliptical [4]. However, there is little information on the discrimination and
best cutoff value of VCW for severe SMR. Our study indicated that
VCW had a fairly strong correlation with VCA and showed
adequately good discrimination of severe SMR. Notably, the best cutoff value of
VCW was 0.78 cm—which is close to the value of 0.80 cm according to
the current guidelines—with adequately high sensitivity and specificity for
severe SMR based on VCA [4]. Further, VCA had a strong
correlation with VCA and showed good discrimination of severe SMR.
Moreover, the best cutoff value of VCA was 0.42 cm, with high
sensitivity and specificity for severe SMR based on VCA.
The current study and previous studies have demonstrated that the regurgitant
orifice area in SMR may be elliptical [8, 12], indicating that SMR severity
based on VCW and EROA is underestimated [4, 5, 6]. Furthermore, there
was a weak correlation between the VCA shape index and difference between
VCA and EROA; this finding conforms to that reported by Goebel
et al. [11], suggesting that the ellipticity of the regurgitant orifice
area rather than the extent of ellipticity is related to the underestimation of
SMR severity based on EROA.
4.2 Assessment of SMR Severity to Avoid its Underestimation
Patients with SMR having EROA of 0.30 cm, corresponding to
moderate SMR according to the current guidelines, have a potential risk of
underestimation of SMR severity because of the elliptical regurgitant orifice
area [4]. Of the 88 patients with EROA of 0.30 cm in the
current study, 38 (43.2%) had severe MR based on VCA. In such cases,
VCW of 0.78 cm and/or VCA of 0.42
cm might be useful in identifying discordantly severe SMR based on
VCA. If EROA is 0.30 cm, SMR severity is expected
to be truly severe based on VCA; however, EROA of 0.30
cm does not necessarily indicate nonsevere SMR based on VCA. If
VCW of 0.78 cm and/or VCA of 0.42
cm are calculated using VCW and VCW, SMR severity might be
considered discordantly severe despite the EROA of 0.30 cm.
After the exclusion of severe SMR according to the abovementioned assessment,
symptomatic patients may be evaluated using exercise-stress echocardiography to
confirm significantly worsening SMR, if applicable.
4.3 Clinical Implications
Although severe SMR is associated with adverse clinical outcomes [1, 2, 3], it may
be underestimated using conventional echocardiographic parameters, including
VCW and EROA. Moreover, an inaccurate assessment of SMR severity
can lead to misleading indications for optimal MV interventions, including MV
transcatheter edge-to-edge repair, which is known to be effective and is
recommended in patients with SMR with reduced LVEF [5, 20, 21]. Karam et
al. [22] reported that MV transcatheter edge-to-edge repair for SMR is equally
effective in patients with EROA of 0.30 cm and those with
EROA of 0.30 cm in terms of clinical outcomes, suggesting
that patients with EROA of 0.30 cm may have a higher severity
of SMR than expected based on EROA. To obtain an accurate evaluation of
SMR severity, VCA is useful as a substantially reliable echocardiographic
parameter [11]. However, the assessment of VCA is relatively
time-consuming and requires good quality of 3D-echocardiographic data [4].
VCW and VCA, which were calculated via simple equations
using VCW and VCW, showed fairly strong correlations with
VCA and good discrimination of severe SMR based on VCA. Therefore,
instead of VCA, VCW and VCA, with best cutoff
values of 0.78 cm and 0.42 cm, respectively, might be helpful in
identifying true severe SMR.
5. Study Limitations
This study has several important limitations. First, this was a small-scale
retrospective analysis of patients with SMR who underwent TEE, with a
considerable bias in data accumulation (i.e., selection bias). Second, our study
defined severe SMR as VCA of 0.39 cm based on the findings
of a previous study [11]. However, our results may not be accurate when using
other definitions of severe SMR based on modalities other than echocardiography,
including cardiac magnetic resonance imaging. Third, TEE and TTE were not
performed on the same day. Hence, there might have been differences in the
hemodynamic status at the time of TEE and TTE. Finally, we measured VCW
and VCW using 3D-TEE data, which may not be similar to VCW and
VCW determined using 2D-TEE. However, there were no significant
differences between VCW and VCW measured using 3D-TEE and
2D-echocardiography according to a previous study [8].
6. Conclusions
VCW and VCA based on 3D-TEE were strongly associated
with VCA. Therefore, in general, the regurgitant orifice area of SMR may
be elliptical, and SMR severity might be underestimated if determined using only
VCW and EROA. Hence, VCW and VCA, with
best cutoff values of 0.78 cm and 0.42 cm, respectively, were useful in
identifying severe SMR.
Abbreviations
SMR, Secondary mitral regurgitation; EROA, Effective regurgitant orifice area;
EROA, Effective regurgitant orifice area by proximal isovelocity surface
area method; 3D-TEE, Three-dimensional transesophageal echocardiography; VC, Vena
contracta; VCW, Vena contracta width; VCA, Vena contracta area; VCA,
three-dimensional vena contracta area; VCA, Vena contracta area as an
ellipse; VCW, Anteroposterior vena contracta width; VCW,
Mediolateral vena contracta width; VCW, Average of anteroposterior
and mediolateral vena contracta widths.
Availability of Data and Materials
Data will be shared on request to the corresponding author with the permission
of New Tokyo Hospital and St. Marianna University Hospital.
Author Contributions
HO and MI designed the study. HO acquired and analyzed the data. HO, MI, TN, YJA and SA
interpreted the results. HO and MI prepared the manuscript. All authors
contributed to editorial changes in the manuscript. All authors read and approved
the final manuscript. All authors have participated sufficiently in the work and
agreed to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
The study protocol was approved by the Institutional Review Board of New Tokyo
Hospital (0267) and was in accordance with the guidelines of the Declaration of
Helsinki. The requirement for informed consent was waived because of the
retrospective nature of this study.
Acknowledgment
Not applicable.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3. Fig. 4.
Fig. 4. Fig. 5.
Fig. 5. Fig. 6.
Fig. 6.