- Academic Editor
†These authors contributed equally.
Background: Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are a class of widely used hypoglycemic agents for the treatment of type 2 diabetes mellitus (T2DM). In addition to lowering blood glucose, SGLT2i protects the heart and kidney, significantly reduces cardiovascular events, and delays the progression of heart failure and chronic kidney disease. However, previous studies have not exhaustively discussed the association between SGLT2i and the risk of developing cardiac arrhythmias. The purpose of this study is to assess the association of SGLT2i with cardiac arrhythmias in patients with T2DM and without T2DM in cardiovascular outcome trials (CVOTs). Methods: We performed a meta-analysis and systematic review of CVOTs that compared SGLT2i with placebo. MEDLINE, Web of Science, The Cochrane Library and Embase were systematically searched from inception to December 2022. We included CVOTs reporting cardiovascular or renal outcomes with a follow-up duration of at least 6 months. Results: A total of 12 CVOTs with 77,470 participants were included in this meta-analysis (42,016 SGLT2i vs 35,454 control), including patients with T2DM, heart failure (HF), or chronic kidney disease (CKD). Follow-up duration ranged from 9 months to 5.65 years. Medications included empagliflozin, canagliflozin, dapagliflozin and ertugliflozin. SGLT2i were associated with a lower risk of tachycardia (risk ratio (RR) 0.86; 95% confidence interval (CI) 0.79–0.95), supraventricular tachycardia (SVT; RR 0.84; 95% CI 0.75–0.94), atrial fibrillation (AF; RR 0.86; 95% CI 0.75–0.97) and atrial flutter (AFL; RR 0.75; 95% CI 0.57–0.99) in patients with T2DM, HF and CKD. SGLT2i could also reduce the risk of cardiac arrest in CKD patients (RR 0.50; 95% CI 0.26–0.95). Besides, SGLT2i therapy was not associated with a lower risk of ventricular arrhythmia and bradycardia. Conclusions: SGLT2i therapy is associated with significantly reduced the risk of tachycardia, SVT, AF, and AFL in patients with T2DM, HF, and CKD. In addition, SGLT2i could also reduce the risk of cardiac arrest in CKD patients. Further researches are needed to fully elucidate the antiarrhythmic mechanism of SGLT2i.
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are widely used to treat type 2 diabetes mellitus (T2DM) by inhibiting sodium and glucose reabsorption in the renal proximal convoluted tubules, resulting in natriuresis and glucosuria, promoting osmotic diuresis and lowering blood glucose [1]. SGLT2i were originally developed as novel hypoglycemic agents, but, with the deepening of research, these drugs have been discovered to have a wide range of additional effects, such as lowering body weight, metabolic shift, and reducing inflammatory responses [2].
In order to determine the association between new antidiabetic therapies and cardiovascular risk, the US Food and Drug Administration has set criteria for cardiovascular safety trials, which were also known as cardiovascular outcome trials (CVOTs) [3]. Recently, the advent of many large COVTs for SGLT2i has provided more information to optimize the treatment of T2DM, heart failure (HF) and chronic kidney disease (CKD), and to potentially reduce the risk of cardio-renal complications [4]. Compared with conventional randomized controlled trials (RCTs), COVTs are more helpful to identify the potential cardiovascular risk of drugs and their cardiovascular benefits. CVOTs are mostly RCTs with follow-up of more than 2 years. They included patients with confirmed cardiovascular disease (CVD) or at high risk of CVD. The primary endpoint of COVTs is major adverse cardiovascular events (MACE), and the degree of cardiovascular risk and follow-up duration of patients are important influencing factors.
Diabetes mellitus is a known risk factor for various cardiac arrhythmias, such as supraventricular tachycardia (SVT), ventricular arrhythmias (VA), and cardiac arrest, while bradyarrhythmias are also common in diabetic patients [5, 6, 7, 8]. Pathophysiological remodeling of cardiac function occurs at multiple levels in patients with HF, and abnormal changes in ion channels are likely to lead to various cardiac arrhythmias [9, 10]. CKD can also lead to increased cardiovascular risk such as cardiac arrhythmias and HF [11]. The present study identified potential antiarrhythmic benefits of SGLT2i, which can reduce cardiac arrhythmias by affecting cellular calcium ion current, calcium homeostasis, and reducing oxidative stress [12]. Therefore, the aim of this meta-analysis and systematic review was to gather the study results from all large CVOTs with SGLT2i to evaluate the effect of SGLT2i on common cardiac arrhythmia outcomes (tachycardia, SVT, VA, cardiac arrest, and bradyarrhythmia) in patients with T2DM, HF, and CKD.
This systematic review and meta-analysis were designed and conducted in accordance with the Cochrane Handbook of Systematic Reviews for interventions [13] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement [14]. The PRISMA checklist is shown in Supplementary Table 1. This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO), with the ID number CRD42023405574.
To identify all COVTs, we systematically explored MEDLINE (PubMed), Web of Science, The Cochrane Library, and Embase from database inception to December 10, 2022. Data from included clinical trials was obtained from ClinicalTrials.gov (https://clinicaltrials.gov/). In this study, medical subject headings (MeSH) strategy was used to search the literature using a combination of MeSH major topics and entry terms. The search terms were as follows: sodium-glucose transporter 2 inhibitors, SGLT2 inhibitor, SGLT2 inhibitors, SGLT 2 inhibitors, SGLT-2 inhibitors, sodium-glucose transporter 2 inhibitor, sodium glucose transporter 2 inhibitor, sodium glucose transporter 2 inhibitors, gliflozins, gliflozin, SGLT-2 inhibitor, SGLT 2 inhibitor, empagliflozin, dapagliflozin, canagliflozin, sotagliflozin, ertugliflozin, henagliflozin, randomized controlled trial, randomized, placebo, type 2 diabetes mellitus, chronic kidney disease and heart failure. Detailed search strategies are presented in Supplementary Table 2.
There was no restriction with respect to the language, date of publication, or publication status. To determine the antiarrhythmic effect of SGLT2i, we excluded trials where patients received combination therapy, and selected placebo as the comparator. We included RCTs comparing SGLT2i with placebo in adult patients with T2DM, HF or CKD and reported outcomes of interest as cardiac arrhythmias. Studies that met the inclusion criteria were included: (1) RCTs comparing any SGLT2i with placebo; (2) RCTs met FDA guidance for cardiovascular safety trials; (3) follow-up duration of at least six months; (4) participants aged 18 years or older with diagnosed T2DM and/or HF and/or CKD. Exclusion criteria: (1) studies without cardiac arrhythmia outcomes and those with duplicate data; (2) reviews, case reports, conference abstracts, and other non-RCT studies; (3) studies where data are incomplete or cannot be converted into usable data.
In this study, outcomes of interest were reported as a serious adverse event according to Medical Dictionary for Regulatory Activities (MedDRA version 18.0) to reduce potential bias. Primary outcomes were incidence of tachycardia, supraventricular tachycardia, ventricular arrhythmia, cardiac arrest and bradyarrhythmia. Tachycardia in this meta-analysis included SVT and VA. SVT in this meta-analysis included sinus arrhythmia/tachycardia, atrial tachycardia, atrial fibrillation and atrial flutter. VA in this meta-analysis included ventricular tachycardia, torsade de pointes, ventricular extrasystoles, ventricular flutter and ventricular fibrillation. Bradyarrhythmia in this meta-analysis included sinus node dysfunction (SND), atrioventricular block (AVB) and conduction tissue disease (CTD).
Data search and extraction were performed independently by 2 investigators (XJW and XXZ). Any disagreements were resolved by author consensus or by consulting last author (QYL). The data extraction content included: study title, year of publication, ClinicalTrials.gov identifier, research type, study population, sample size, patients characteristics (age and follow-up duration), treatment information (type of SGLT2i and dose) and outcome data (number of events for each cardiac arrhythmia outcome).
According to the recommendations of the Cochrane Collaboration, Revised Cochrane risk-of-bias tool for randomized trials (ROB 2) [15] was used to assess the risk of bias of included studies. Risk of bias was assessed across five distinct domains, including: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome and bias in selection of the reported result. Judgments within the five domains mentioned above will lead to an overall risk of bias judgment, and these answers lead to judgments of low risk of bias, some concern, or high risk of bias.
Statistical analysis was performed using Cochrane ReviewManager (RevMan) 5.3
(The Cochrane Collaboration, Copenhagen, Denmark). The extracted data were
dichotomized data, so the risk ratio (RR) of the data was evaluated with a 95%
confidence interval (CI). Heterogeneity across studies was tested by using Q test
and I
I
Among the 1721 citations identified by literature search, 12 COVTs from 11 articles [16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26] were included in this study (Fig. 1). All trials were multinational, rigorously parallel-group, double-blind designed RCTs and sponsored by the industry. Publication of above studies ranged from 2015 to 2021, with 4 studies published in 2020. A total of 77,470 participants were included in this meta-analysis, including patients with T2DM, HF, or CKD. Follow-up duration ranged from 9 months to 5.65 years. The baseline characteristics of the trials included were summarized in Table 1.
 Fig. 1.
Fig. 1.Preferred reporting items for meta-analysis and systematic review flowchart of selection. CVOTs, cardiovascular outcome trials.
| Study (Clinical Trial) | Year | Study design (Study IDs) | Study population | Type of SGLT2i (dose, mg) | No. of patients | Age, mean (SD) or median, y | Follow-up, y | Primary outcome | |
| SGLT2i group | Placebo group | ||||||||
| EMPA-REG OUTCOME | 2015 | RCT (NCT01131676) | T2DM with high CV risk | Empagliflozin (10 and 25) | 4687 | 2333 | 63.1 (8.7) | 2.95 (mean) | MACE |
| CANVAS | 2017 | RCT (NCT01032629) | T2DM with history or high risk of CVE | Canagliflozin (100 and 300) | 2888 | 1442 | 62.4 (8.02) | 5.65 (mean) | MACE |
| CANVAS-R | 2017 | RCT (NCT01989754) | T2DM with history or high risk of CVE | Canagliflozin (100 and 300) |
2907 | 2905 | 64 (8.35) | 2.07 (meam) | MACE |
| DECLARE-TIMI 58 | 2018 | RCT (NCT01730534) | T2DM with history or high risk of CVD | Dapagliflozin (10) | 8582 | 8578 | 63.9 (6.8) | 4.2 (median) | MACE |
| CREDENCE | 2019 | RCT (NCT02065791) | T2DM and ACKD | Canagliflozin (100) | 2202 | 2199 | 63 (9.2) | 2.62 (median) | ESKD, doubling of serum creatinine level, Or death from renal or CV causes |
| DAPA-HF | 2019 | RCT (NCT03036124) | HFrEF with or without T2DM | Dapagliflozin (5 or 10) | 2373 | 2371 | 66.3 (10.9) | 1.52 (median) | worsening HF or CV death |
| DAPA-CKD | 2020 | RCT (NCT03036150) | CKD with or without T2DM | Dapagliflozin (10) | 2152 | 2152 | 61.8 (12.1) | 2.4 (median) | eGFR decline |
| VERTIS CV | 2020 | RCT (NCT01986881) | T2DM and CVD | Ertugliflozin (5 or 15) | 5499 | 2747 | 64.4 (8.1) | 3.5 (mean) | MACE |
| EMPEROR-Reduced | 2020 | RCT (NCT03057977) | HFrEF with or without T2DM | Empagliflozin (10) | 1863 | 1867 | 66.8 (11) | 1.33 (median) | CV death or hospitalization for HF |
| SCORED | 2020 | RCT (NCT03315143) | T2DM, CKD and risk for CVD | Sotagliflozin (200 or 400) | 5292 | 5292 | 69 | 1.33 (median) | MACE, CV death and hospitalization for HF |
| SOLOIST-WHF | 2021 | RCT (NCT03521934) | T2DM and HF | Sotagliflozin (200 or 400) | 608 | 614 | 70 | 0.75 (median) | CV death, urgent visit or hospitalization for HF |
| EMPEROR-Preserved | 2021 | RCT (NCT03057951) | HFpEF with or without T2DM | Empagliflozin (10) | 2997 | 2991 | 71.9 (9.4) | 2.18 (median) | CV death and hospitalization for HF |
CVOTs, cardiovascular outcome trials; T2DM, type 2 diabetes mellitus; CV, cardiovascular; CVD, cardiovascular disease; CVE, cardiovascular events; MACE, major adverse cardiovascular events; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; ACKD, albuminuric chronic kidney disease; ESKD, end-stage kidney disease; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; SGLT2i, sodium-glucose cotransporter 2 inhibitors. ※: 100 mg for first 13 weeks then 300 mg.
After quality assessment of all studies according to ROB 2’s evaluation process, we judged all 12 COVTs were low risk of bias (Supplementary Fig. 1). Due to the low heterogeneity across various studies, fixed effect model was used for all comparisons.
Tachycardia is a general term for tachyarrhythmia, including supraventricular
tachycardia and ventricular arrhythmia. All 12 COVTs reported the incidence of
tachycardia. The risk of tachycardia in the SGLT2i group was significantly lower
than that in the placebo group (Fig. 2; RR, 0.86; 95% CI: 0.79 to 0.95;
p = 0.002; I
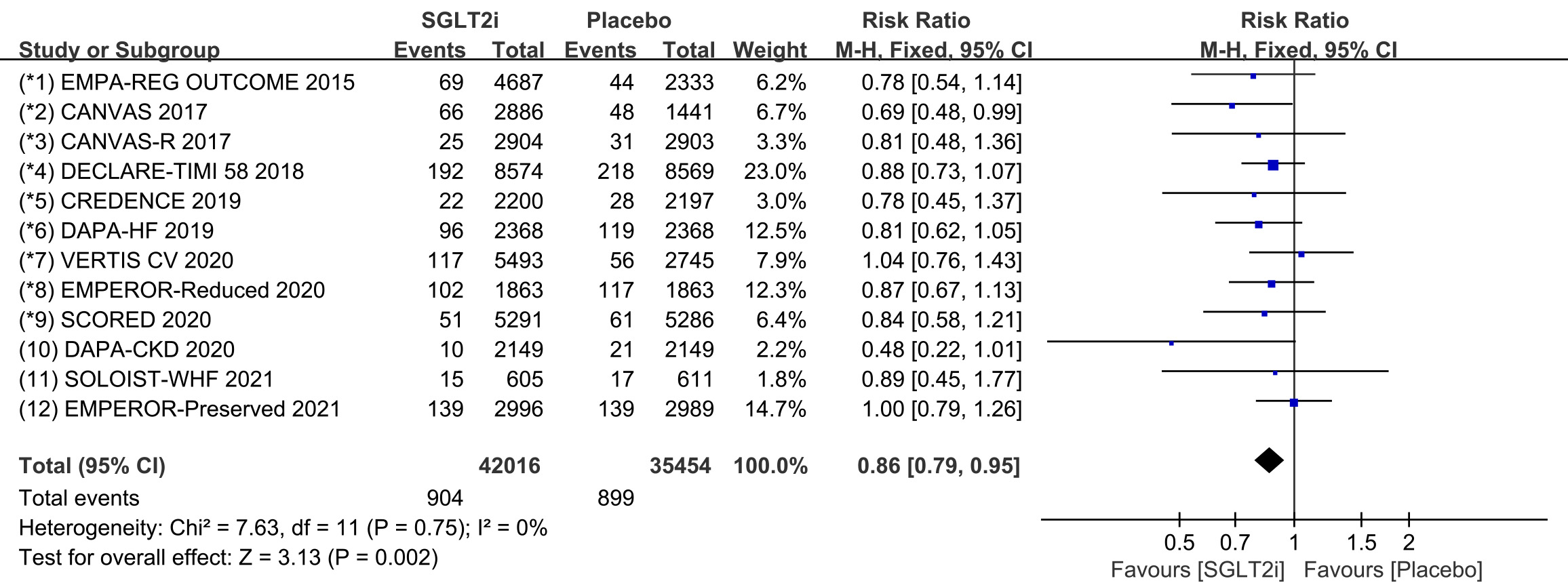 Fig. 2.
Fig. 2.The pooled effect of tachycardia incidence. SGLT2i, sodium-glucose cotransporter 2 inhibitors.
Raw data on supraventricular tachycardia, sinus arrhythmia, sinus tachycardia,
atrial tachycardia, atrial fibrillation and atrial flutter from clinical trials
were included in the meta-analysis of supraventricular tachycardia. All 12 COVTs
reported the incidence of supraventricular tachycardia. The risk of
supraventricular tachycardia in the SGLT2i group was significantly lower than
that in the placebo group (Fig. 3; RR, 0.84; 95% CI: 0.75 to 0.94; p =
0.002; I
 Fig. 3.
Fig. 3.SVT events with SGLT2i vs placebo in patients with T2DM, HF or CKD. SGLT2i, sodium-glucose cotransporter 2 inhibitors; SVT, supraventricular tachycardia; T2DM, type 2 diabetes mellitus; HF, heart failure; CKD, chronic kidney disease.
In addition, we performed subgroup analyses for the incidence of atrial
fibrillation and atrial flutter. All 12 COVTs reported the incidence of atrial
fibrillation and atrial flutter. The risk of atrial fibrillation in the SGLT2i
group was significantly lower than that in the placebo group (Fig. 4; RR, 0.86;
95% CI: 0.75 to 0.97; p = 0.02; I
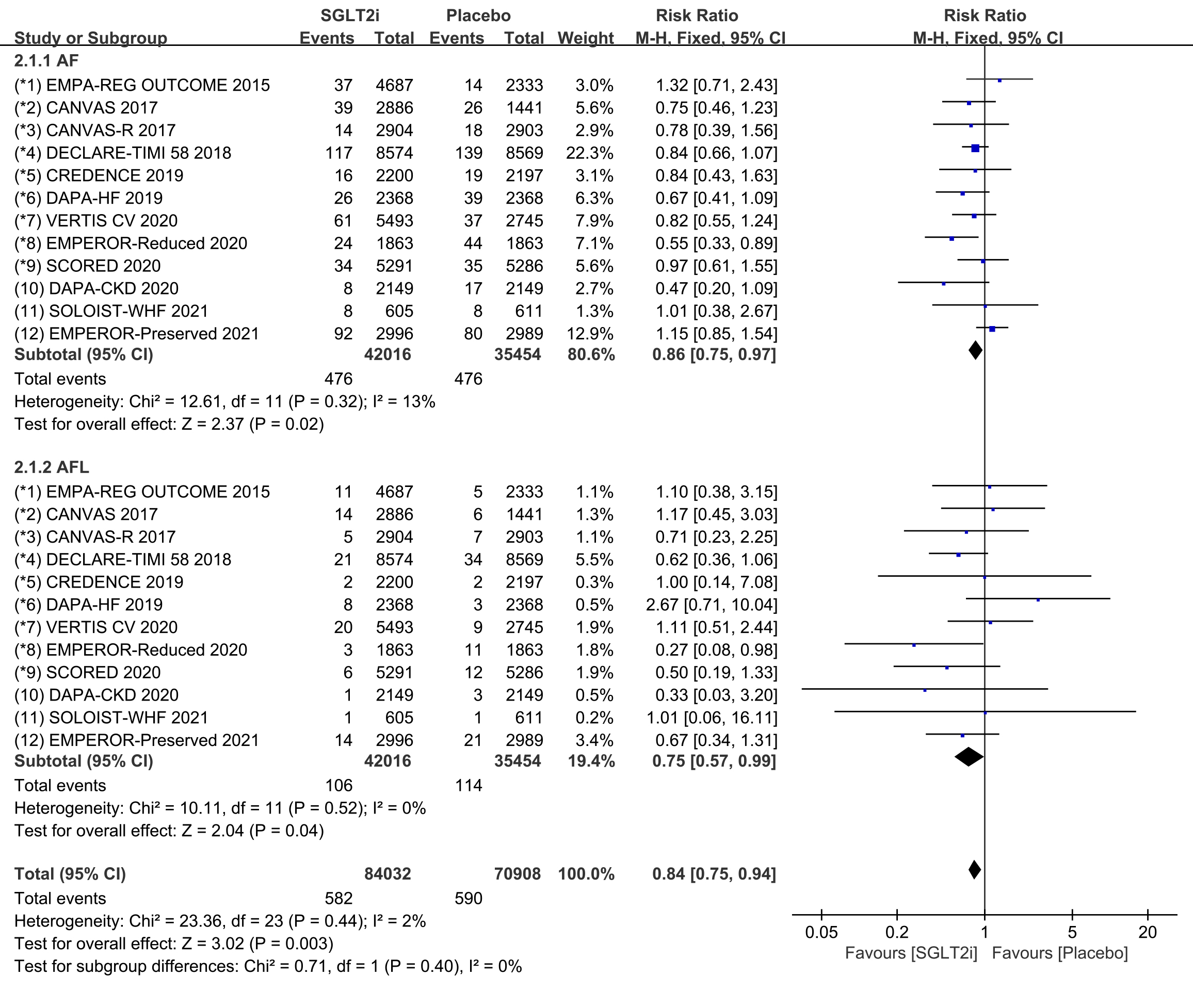 Fig. 4.
Fig. 4.AF and AFL events with SGLT2i vs placebo in patients with T2DM, HF or CKD. AF, atrial fibrillation; AFL, atrial flutter; SGLT2i, sodium-glucose cotransporter 2 inhibitors; T2DM, type 2 diabetes mellitus; HF, heart failure; CKD, chronic kidney disease.
Raw data on ventricular arrhythmia, ventricular tachycardia, torsade de pointes,
ventricular extrasystoles, ventricular flutter and ventricular fibrillation from
clinical trials were included in the meta-analysis of ventricular arrhythmia. All
12 COVTs reported the incidence of ventricular arrhythmia. The risk of
ventricular arrhythmia in the SGLT2i group was not significantly different from
that in the placebo group (Fig. 5; RR, 0.98; 95% CI: 0.82 to 1.16; p =
0.79; I
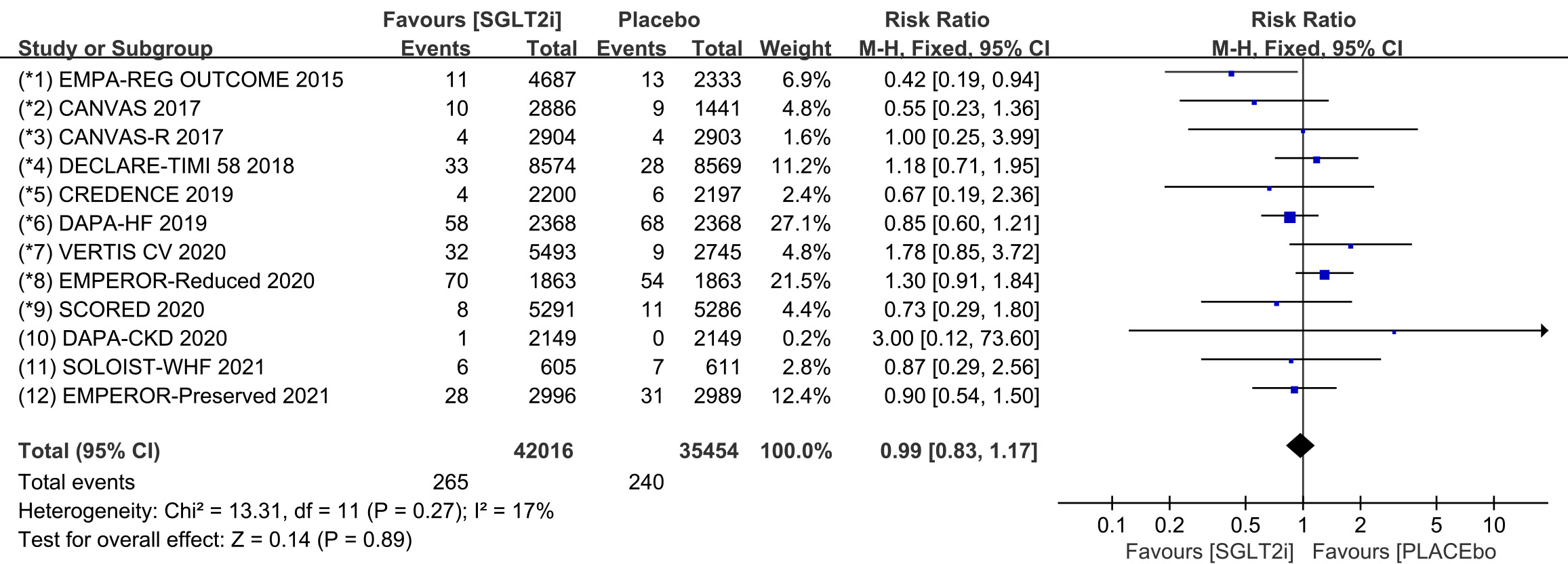 Fig. 5.
Fig. 5.VA events with SGLT2i vs placebo in patients with T2DM, HF or CKD. VA, ventricular arrhythmias; SGLT2i, sodium-glucose cotransporter 2 inhibitors; T2DM, type 2 diabetes mellitus; HF, heart failure; CKD, chronic kidney disease.
Subgroup analyses assessed the incidence of ventricular tachycardia (including
ventricular tachyarrhythmia and torsade de pointes) and ventricular fibrillation,
respectively. The incidence of ventricular tachycardia was reported in all 12
COVTs, and the incidence of ventricular fibrillation was reported in 11 COVTs.
However, the risk of ventricular tachycardia (Fig. 6; RR, 0.97; 95% CI: 0.78 to
1.20; p = 0.78; I
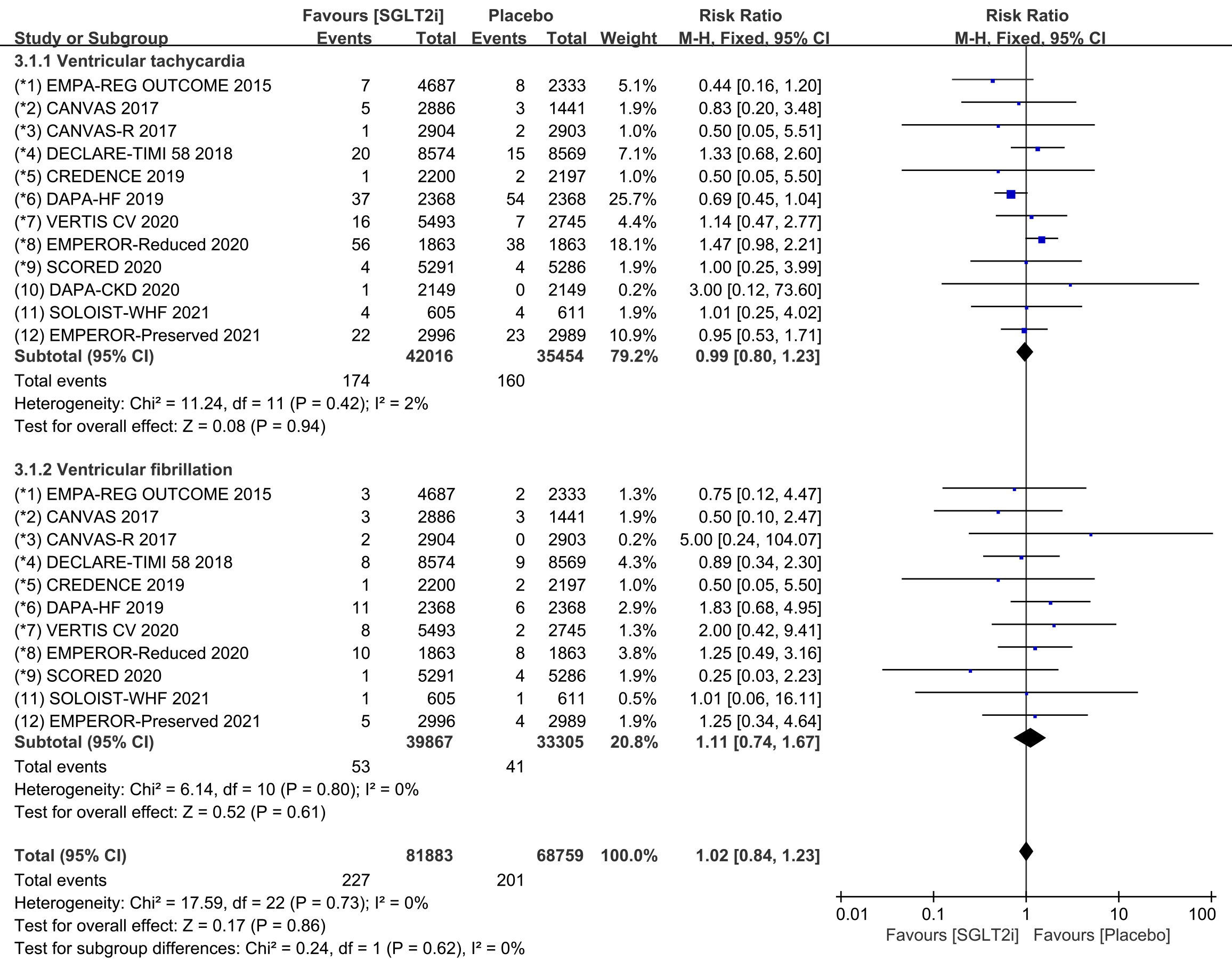 Fig. 6.
Fig. 6.Ventricular tachycardia and ventricular fibrillation events with SGLT2i vs placebo in patients with T2DM, HF or CKD. SGLT2i, sodium-glucose cotransporter 2 inhibitors; T2DM, type 2 diabetes mellitus; HF, heart failure; CKD, chronic kidney disease.
All 12 COVTs reported the incidence of cardiac arrest. The risk of cardiac
arrest in the SGLT2i group was not significantly different from that in the
placebo group (Fig. 7; RR, 0.83; 95% CI: 0.65 to 1.06; p = 0.13;
I
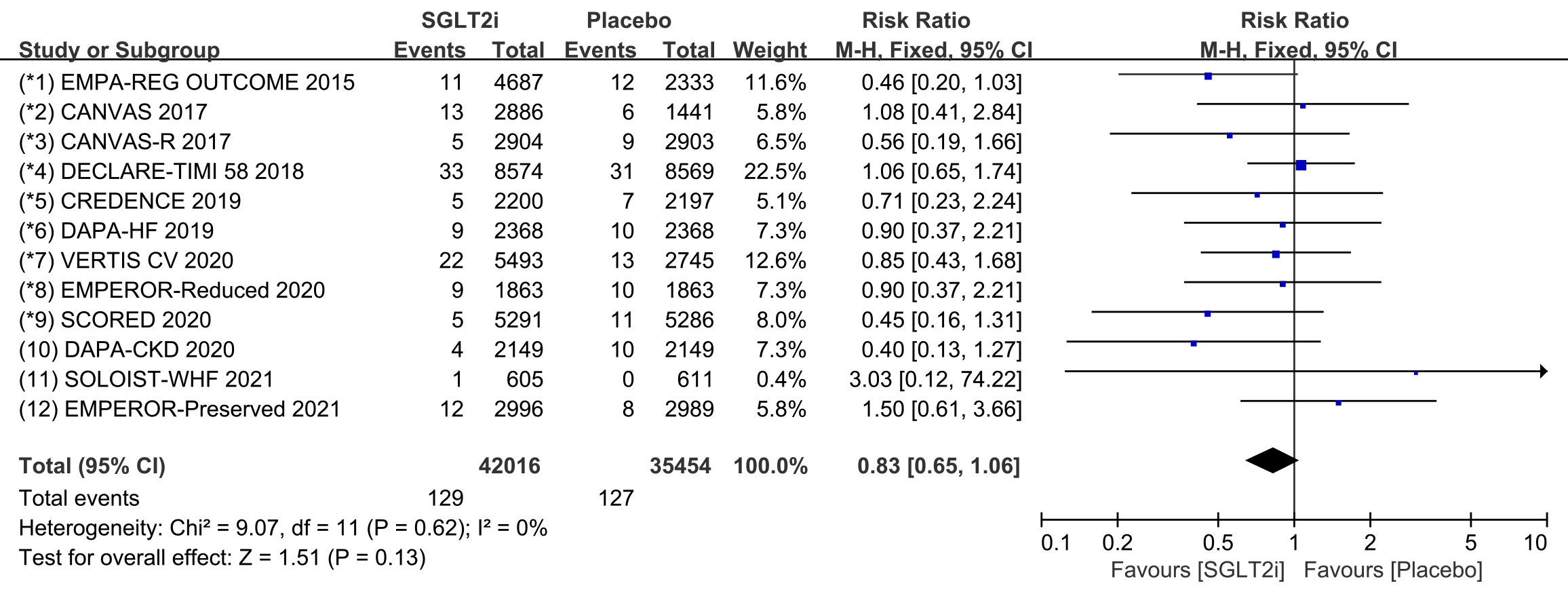 Fig. 7.
Fig. 7.The pooled effect of cardiac arrest incidence. SGLT2i, sodium-glucose cotransporter 2 inhibitors.
Subgroup analyses were conducted to evaluate the prevalence of cardiac arrest in
patients with T2DM, HF, and CKD. 8 COVTs reported the incidence of cardiac arrest
in T2DM patients. The results showed that the risk of cardiac arrest in the
SGLT2i group was not significantly different from that in the placebo group (Fig. 8; RR, 0.80; 95% CI: 0.60 to 1.07; p = 0.14; I
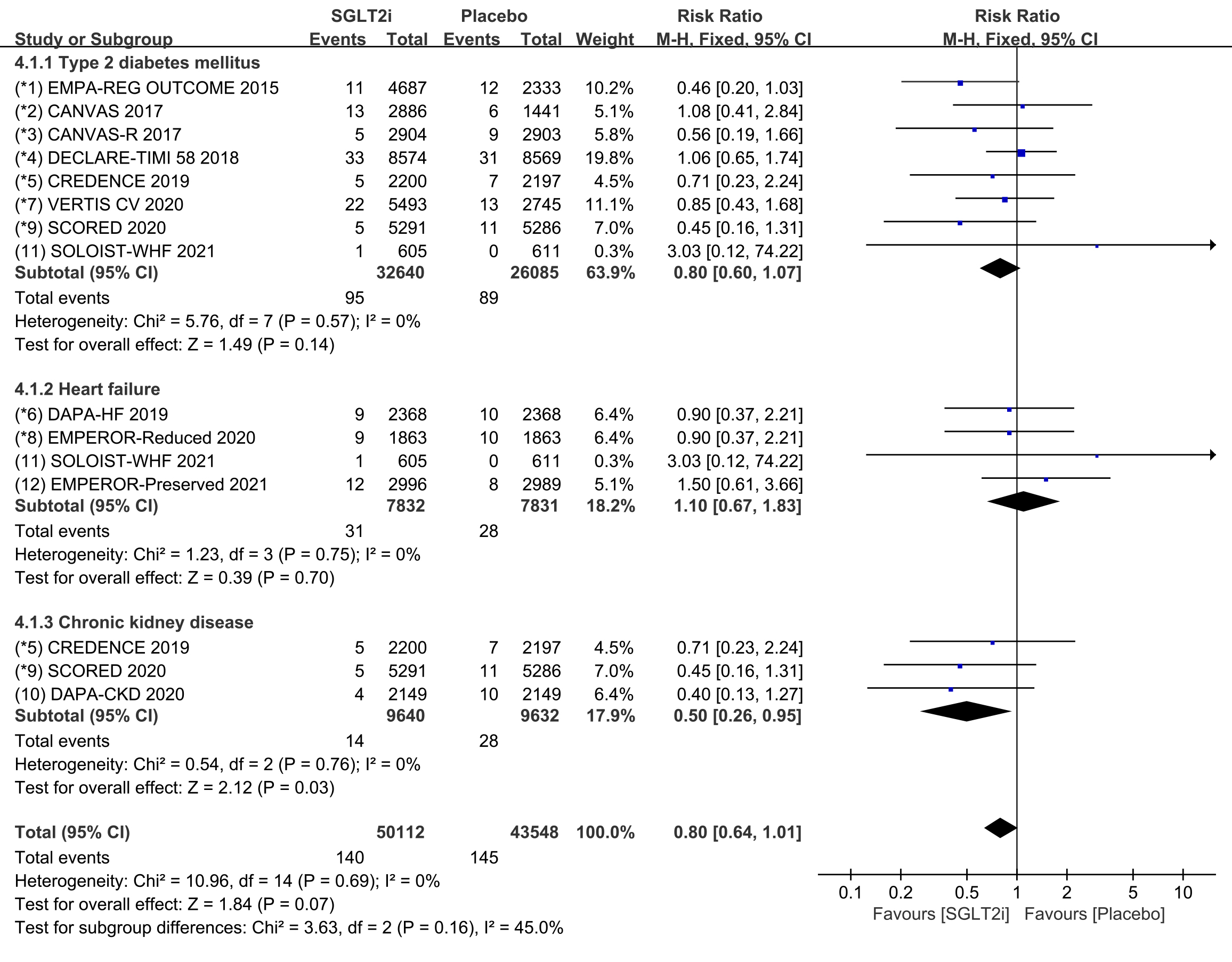 Fig. 8.
Fig. 8.Cardiac arrest events with SGLT2i vs placebo in patients with T2DM, HF or CKD. SGLT2i, sodium-glucose cotransporter 2 inhibitors; T2DM, type 2 diabetes mellitus; HF, heart failure; CKD, chronic kidney disease.
Raw data on SND, AVB and CTD from clinical trials were included in the
meta-analysis of bradycardia. All 12 COVTs reported the incidence of bradycardia.
The risk of bradycardia in the SGLT2i group was not significantly different from
that in the placebo group (Fig. 9; RR, 0.92; 95% CI: 0.77 to 1.09; p =
0.34; I
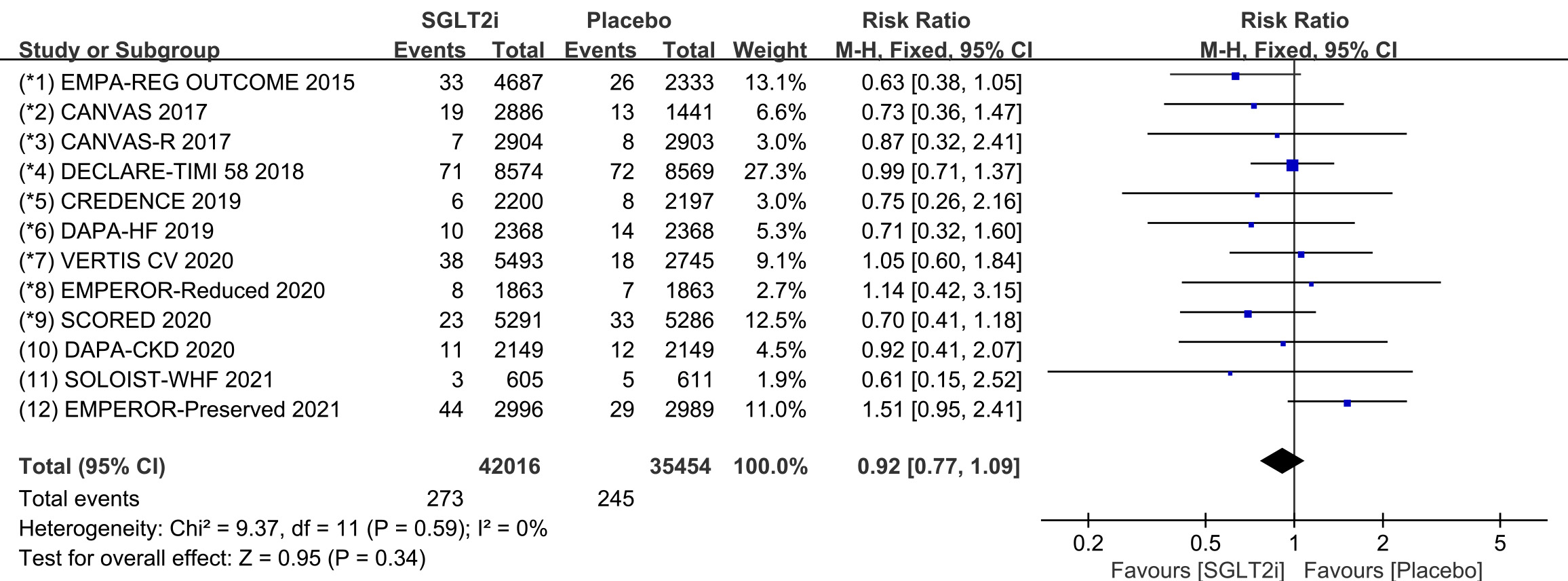 Fig. 9.
Fig. 9.The pooled effect of bradycardia incidence. SGLT2i, sodium-glucose cotransporter 2 inhibitors.
Subgroup analyses were conducted to assess the incidence of SND, AVB and CTD,
respectively. Raw data on SND, sinus bradycardia and sinus arrest from clinical
trials were included in the meta-analysis of SND. The incidence of SND was
reported in 11 COVTs, and the risk of SND in the SGLT2i group was not
significantly different from that in the placebo group (Fig. 10; RR, 0.94; 95%
CI: 0.66 to 1.33; p = 0.71; I
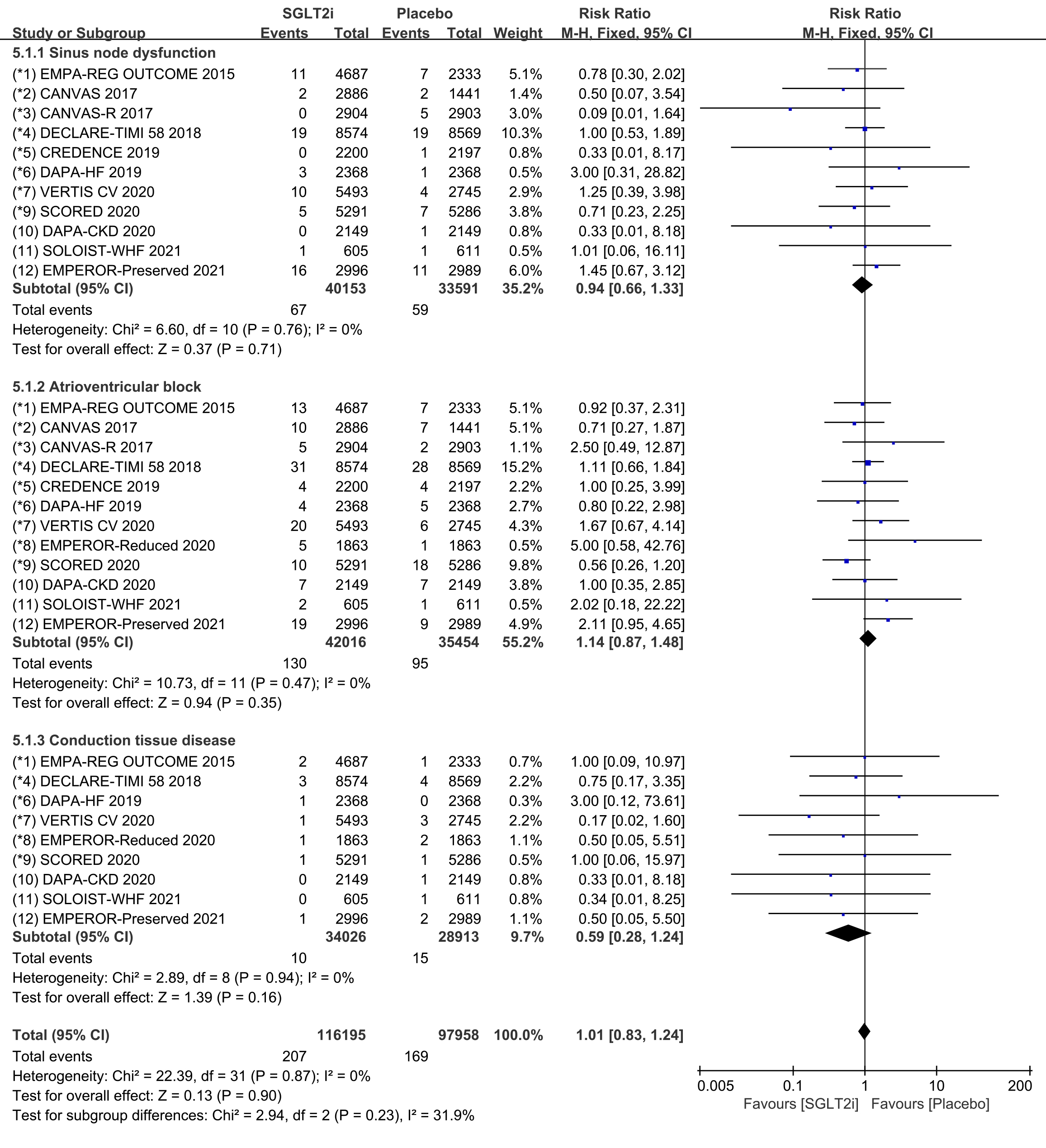 Fig. 10.
Fig. 10.SND, AVB and CTD events with SGLT2i vs placebo in patients with T2DM, HF or CKD. SND, sinus node dysfunction; AVB, atrioventricular block; CTD, conduction tissue disease; SGLT2i, sodium-glucose cotransporter 2 inhibitors; T2DM, type 2 diabetes mellitus; HF, heart failure; CKD, chronic kidney disease.
Raw data on AVB, AVB first degree, AVB second degree and AVB complete from
clinical trials were included in the meta-analysis of AVB. The incidence of AVB
was reported in 12 COVTs, and the risk of AVB in the SGLT2i group was not
significantly different from that in the placebo group (Fig. 10; RR, 1.14; 95%
CI: 0.87 to 1.48; p = 0.35; I
Raw data on bundle branch block right, bundle branch block left, bundle branch
block bilatera, bifascicular block and trifascicular block from clinical trials
were included in the meta-analysis of CTD. The incidence of CTD was reported in 9
COVTs, and the risk of CTD in the SGLT2i group was not significantly different
from that in the placebo group (Fig. 10; RR, 0.59; 95% CI: 0.28 to 1.24;
p = 0.16; I
In this meta-analysis and systematic review of 12 COVTs involving 77,470 patients with T2DM, HF, or CKD at risk of developing cardiac arrhythmias, we found that SGLT2i therapy was associated with a significant reduction in the risk of tachycardia, SVT, AF and AFL in patients with T2DM, HF and CKD. Besides, SGLT2i therapy could also reduce the risk of cardiac arrest in CKD patients. Therefore, SGLT2i may have important therapeutic effects against above types of cardiac arrhythmias, and can effectively prevent these serious cardiovascular adverse events in patients with T2DM, HF, and CKD. However, there was no significant difference in the risks of VA (ventricular tachycardia and fibrillation), bradycardia (SND, AVB, and CTD), and cardiac arrest in patients with T2DM and HF in the SGLT2i group compared to the placebo group. Nevertheless, we cannot rule out the potential therapeutic effect of SGLT2i on these cardiac arrhythmias, and more COVTs need to be included in the future to validate these findings.
SGLT2 inhibitors have a direct effect on cardiomyocyte metabolism and can improve cardiac function by reducing JunD expression [27, 28]. SGLT2i can balance autonomic system activity, induce an ameliorative regulation of sympathetic systemic tone, and reduce the recurrence of vaso-vagal syncope in T2DM patients [29]. In addition, SGLT2i also has anti-inflammatory and antiarrhythmic properties in patients with acute coronary syndrome, stable ischemic heart disease, multi-vessel coronary stenosis, and can significantly reduce in-hospital arrhythmic burden in treated patients [30, 31, 32, 33]. For cardiac arrhythmias, SGLT2i have multiple antiarrhythmic mechanisms, which include: (1) osmotic diuresis to lower blood glucose and reduce cardiac load: both the osmotic effect of glucose and natriuresis contribute to the diuretic effect of SGLT2i, resulting in plasma volume contraction, which hemodynamically unload the left ventricle, decreases myocardial oxygen consumption, filling pressure, and ventricular wall tension [34]; (2) regulation of cardiac ion balance: SGLT2i can affect a variety of cardiac ion currents to attenuate action potential duration prolongation and reduce the development of calcium-related cardiac arrhythmias by affecting calcium homeostasis and calcium ion current [12]; (3) regulation of mitochondrial function and improvement of myocardial remodeling: SGLT2i can increases mitochondrial calcium uptake and mitigate mitochondrial swelling in cardiomyocytes, thereby restoring the antioxidant capacity of mitochondria and exerting antiarrhythmic effects [35]; and (4) inhibition of sympathetic activity: SGLT2i may reduce the risk of arrhythmias by inhibiting levels of markers of the sympathetic nervous system (norepinephrine and tyrosine hydroxylase), attenuating the stimulation of afferent sympathetic activity, and reducing the activity of sympathetic nervous system [36]. Additionally, SGLT2i can reduce cardiac inflammation, myocardial oxygen consumption, and oxidative stress, all of which may contribute to reducing the risk of cardiac arrhythmias [12].
Sinus tachycardia, atrial tachycardia, atrioventricular junctional tachycardia, atrioventricular re-entrant tachycardia, AF and AFL are common SVT in clinical practice [37]. The pathogenesis of SVT is due to abnormalities or enhanced automaticity of non-pacemaker cells and may also involve oscillations in membrane potential because of abnormal pulse initiation [38]. A meta-analysis of 32 RCTs found that SGLT2i significantly reduced the risk of atrial arrhythmias, which partially supports the findings of this study [39]. However, since this study only included results from patients with AF and AFL, its rigor is limited compared to other meta-analysis. As a more severe SVT, AF is listed as a separate clinical guideline by the European Society of Cardiology (ESC) and the American heart association (AHA) [40, 41]. The results of several studies also support the findings of this meta-analysis that SGLT2i significantly reduces the risk of AF and AFL [42, 43, 44].
Compared to SVT, VA belongs to a more serious arrhythmia, and severe VA can be life-threatening. Ventricular tachycardia, ventricular extrasystoles, ventricular flutter, and ventricular fibrillation are common VAs [45]. Abnormal automaticity or enhanced automaticity of subordinate pacemaker cells originating in the His-Purkinje system or ventricular myocardium can cause the development of VA. Changes in transporter function and/or expression or ion channel and intercellular coupling secondary to underlying pathology, are also mechanisms leading to changes in myocardial action potential [46]. Similar to the results of this meta-analysis, several previous studies have found that SGLT2i treatment was not associated with a reduced risk of VA [39, 42, 47]. However, one study [41] found that SGLT2i reduced the risk of ventricular tachycardia, so more CVOTs should be included in the future to verify the association between SGLT2i and the risk of VA.
Cardiac arrest is a severe clinical emergency that is defined by the ESC as cessation of normal cardiac activity with hemodynamic collapse [45]. Despite few studies on cardiac arrest, the results of a meta-analysis [44] have been consistent with the findings of this study, and no association has been found between SGLT2i and the risk of developing cardiac arrest. Interestingly, our subgroup analysis of different disease populations showed that SGLT2i therapy significantly reduced the risk of cardiac arrest in CKD patients. CKD patients often experience adverse cardiomyopathic and vasculopathic conditions, resulting in left ventricular pressure and volume overload, which increases the risk of cardiac arrest in CKD patients [48]. Therefore, the findings of this study could provide new research clues for preventing cardiac arrest in patients with CKD.
In contrast to tachycardia, bradycardia is a type of arrhythmia that causes heart rate to slow down. Degenerative fibrosis of the sinoatrial node, atria, atrioventricular node, or other conduction tissues can lead to bradyarrhythmias [49]. Although drug therapy for bradyarrhythmia is mostly used for acute management, complementary therapies like traditional Chinese medicine, provide a new treatment method for bradyarrhythmias [50, 51, 52], so it is necessary to continuously expand the drug therapy for bradyarrhythmias. Unfortunately, this meta-analysis found that SGLT2i therapy did not reduce the risk of bradycardia, such as sinoatrial dysfunction, atrioventricular block, and conduction tissue disease in patients with T2DM, HF and CKD. While a meta-analysis has reported that SGLT2i was associated with a lower risk of bradycardia [43]. But we browsed the full text and found that the results of this study were not rigorous, because they only included data with the term “Bradycardia” and numerous other original data were ignored. Therefore, more CVOTs should be conducted to verify the association between SGLT2i therapy and the risk of bradycardia in the future.
This meta-analysis has several limitations. First, none of the included COVTs described a systematic method to evaluate cardiac arrhythmias, and arrhythmic events were reported as serious adverse events rather than outcomes. Besides, the arrhythmia terms in COVTs were coded according to MedDRA, but the descriptions of each type of arrhythmia were mixed and not uniform, which may have biased the results of the study. Finally, the lack of standardized definitions for arrhythmia endpoints in individual studies may also contribute to reporting bias.
This meta-analysis and systematic review demonstrated that SGLT2i therapy is effective in reducing the risk of tachycardia, SVT, AF, and AFL in patients with T2DM, HF, and CKD. In addition, SGLT2i may also reduce the risk of cardiac arrest in patients with CKD. These findings provide robust evidence to support the use of SGLT2i in reducing the risk of cardiovascular disease in patients with T2DM, HF, and CKD. More prospective trials are needed to confirm the antiarrhythmic effect of SGLT2i and to further elucidate their underlying mechanisms.
QYL and WLW designed this study. XJW and XXZ performed the research. WTZ and JXL provided help and advice on meta-analysis. XJW analyzed the data. XJW and XXZ wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
Not applicable.
This research was funded by the Fundamental Research Funds for the Central public welfare research institutes (Grant Nos. ZZ15-XY-PT-08), National Medicine Master’s Inheritance Studio Construction Project of the National Administration of Traditional Chinese Medicine (Weiliang Weng Academic Succession Studio) and National Natural Science Foundation of China (Grant Nos. 82004352).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
