- Academic Editors
†These authors contributed equally.
Background: Left atrial appendages (LAAs) play an important role in
regulating left atrial function, and much evidence supports the possibility that
changes in left atrial structure may cause or worsen mitral regurgitation. This
study intended to investigate the outcomes of patients with mitral regurgitation
who underwent left atrial appendage closure (resection or endocardial closure)
during isolated surgical ablations. Methods: Patients with mild or
moderate mitral regurgitation who received isolated surgical ablations for atrial
fibrillation (AF) in our center from 2013 to 2022 were referred. During
follow-up, each clinical visit was composed of medical interrogation, a 24 h
Holter, and echocardiographic evaluation. Death, atrial fibrillation, worsening
of mitral regurgitation, and stroke were evaluated as outcomes. Freedom from
outcomes whose results were adjusted by inverse probability of treatment
weighting for causal effects after acquiring propensity scores. Results:
A total of 456 patients were enrolled in this study. During a median follow-up of
48 months, 30 deaths and 11 cases of stroke were observed. After adjustments, no
significant differences in terms of death or stroke were observed among the three
groups. Patients who underwent resection or endocardial closure during surgical
ablations had a higher risk of mitral regurgitation worsening during follow-up
(p
Left atrial appendage (LAA) is highly associated with the formation of left atrial thrombosis and atrial fibrillation (AF) [1, 2, 3]. Surgical LAA intervention, including LAA ligation and LAA excision, has been a common treatment for AF when patients undergo cardiac surgery [4, 5]. While some evidence supported that LAA resection or endocardial closure may cause the loss of left atrial physiological functions, like reservoir and contractile functions [6, 7, 8]. Some evidence also showed that the left atrial pressure and size increased after LAA was excised or excluded [9, 10], which revealed that a potential relationship may be present between LAA and LA remodeling. It is well known that atrial remodeling could contribute to functional mitral regurgitation (MR) [11, 12], and sometimes may lead to the worsening of MR. This study focused on patients with MR diseases who underwent surgical ablation, trying to illustrate whether LAA interventions could affect the outcomes of patients with fundamental MR diseases.
Patients with mild or moderate degenerative MR who received isolated surgical ablations for paroxysmal or persistent AF diagnosed on a 12-lead electrocardiogram in our center from 2013 to 2022 were referred. The exclusion criteria were as follows: (1) patients with organic valvular diseases who needed invasive interventions according to the guidelines; (2) patients with mechanical or biological prosthesis; (3) patients who received any other cardiac surgeries during ablations; (4) other etiologies of mitral valve diseases like rheumatic mitral valve diseases and secondary mitral valve regurgitation; (5) permanent pacemaker implantations. Patients were grouped according to the treatment methods for LAA (LAA resection, endocardial closure or preservation)
Two-dimensional echocardiography and doppler color flow imaging (IE33; Philips Medical Systems, Andover, MA, USA) were performed on all patients. Despite the routine geometric examination of left heart (left atrium and left ventricle). MR and tricuspid regurgitation (TR) grade were assessed by a multiparametric approach [13], including qualitative, semi-quantitative, and quantitative parameters, which was graded from 0 to 4, where 0, none or trivial; 1+, mild; 2+, moderate; 3+, moderate to severe; and 4+, severe. Mitral annular diameter (MAD) was acquired from a four-chamber apical view at the end of expiration. The measurement of the mitral tethering height (TH) was done by calculating the distance from the mitral annular plane to the leaflet coaptation point orthogonally.
Ablation was carried out with bipolar Cardioablate (Medtronic, Minneapolis, MN, USA) or an Atricure clamp (Atricure, West Chester, OH, USA). After the aorta clamping and cardioplegia perfusion, incising the LA and performing the LA ablation lines, which included bilateral pulmonary vein isolations (PVI), a roof connecting line between both islands of PVIs and a line from the left PVI ablation lesion to the posterior mitral annulus. Then, LAA closure ablation was performed by making a radiofrequency lesion around the base of LAA. Patients who underwent LAA interventions experienced either LAA resection or LAA endocardial closure. The LAA was excised with surgical staplers (Covidien, Medtronic, Minneapolis, MN, USA). Whether to perform LAA interventions or preservations depended on several factors, which included the anatomical features of LAA, the risk of atrial rupture evaluated by surgeons, the risk of stroke and surgeons’ preferences. All patients experienced the division of the Marshall ligament. The right atrial ablation lesions included the cavotricuspid isthmus ablation, superior vena cava to inferior vena cava, lateral free-wall to anterior-medial tricuspid valve annulus; and medial free-wall to the anterior-medial tricuspid valve annulus. More details about the above procedures can be found in our Supplementary Material.
As we described in the prior study [14], oral anticoagulation was recommended to
everyone for the first three months after
procedures. For patients who had LAA
preserved, oral anticoagulation was discontinued if one had no AF recurrence and
a CHADS2 score
Clinical visits were arranged for all patients at 1, 3, and 12 months after procedures, and then annually thereafter. Each clinical visit was composed of medical interrogation, physical examination, X-ray, Holter examination, and echocardiographic evaluation. The primary outcome was defined as the worsening of MR (MR grade increased from mild to moderate, mild to severe or moderate to severe). Adverse clinical events including all-cause death, recurrent atrial fibrillation and stroke were recorded. Excluding the routine echocardiographic parameters, items related to the anatomy of the mitral valve including MAD and tethering height (TH) were also recorded at each visit.
Continuous variables which fit the normal distribution were presented as mean
(standard deviation), while other continuous variables were presented as median
(interquartile range). Categorical values were presented as percentages, and odds
ratios (OR) were presented with 95% confidence intervals (CIs). Unadjusted
comparisons in terms of categorical variables between different cohorts were done
by the chi-square test and Fisher’s test. Unadjusted comparisons in terms of
continuous variables between different cohorts were done by the two-sample
t test and Kruskal-Wallis H test. Propensity scores in this model were
acquired through the generalized boosted model. The absolute standardized mean
difference (ASMD) was used to measure the difference between two univariate
distributions of a single baseline variable [15]. Imbalance was presented when a
value was
A total of 456 patients were enrolled in this study. Of these, 278 underwent LAA
interventions (146 cases of resection, 132 cases of endocardial closure), and the
remaining 177 had LAA preserved. The average age was 62
| Characteristics | LAA excision | LAA closure | LAA preservation | Unadjusted maximal ASMD (%) | IPTW-Adjusted maximal ASMD (%) | |
| (n = 146) | (n = 132) | (n = 178) | ||||
| Male, no. (%) | 53 (36.3) | 65 (49.2) * | 69 (38.8) |
26.3 | 9.5 | |
| Age (years), mean (SD) | 62.1 (6.5) | 61.1 (7.4) | 62.1 (8.5) | 12.1 | 6.4 | |
| BMI, mean (SD) | 23.6 (3.7) | 24.3 (3.3) | 23.7 (3.7) | 20.2 | 6.8 | |
| NYHA, no. (%) | ||||||
| II | 130 (89.0) | 118 (89.4) | 157 (88.2) | 2.7 | 3.5 | |
| III | 16 (11.0) | 14 (10.6) | 21 (11.8) | 2.7 | 3.5 | |
| Hypertension, no. (%) | 50 (34.2) | 52 (39.4) | 52 (29.2) | 21.5 | 9.1 | |
| Diabetes, no. (%) | 20 (13.7) | 18 (13.6) | 23 (12.9) | 2.3 | 4.8 | |
| HLP, no. (%) | 32 (21.9) | 27 (20.5) | 42 (23.6) | 7.6 | 8.2 | |
| Smoke, no. (%) | 21 (14.4) | 27 (20.5) | 37 (20.8) | 16.4 | 2.6 | |
| Alcohol, no. (%) | 22 (15.1) | 25 (18.9) | 37 (20.8) | 14.7 | 4.1 | |
| Stroke, no. (%) | 17 (11.6) | 17 (12.9) | 23 (12.9) | 3.9 | 8.8 | |
| Thyroid dysfunction, no. (%) | 5 (3.4) | 2 (1.5) | 4 (2.2) | 12.4 | 5.6 | |
| PMI, no. (%) | 7 (4.8) | 8 (6.1) | 14 (7.9) | 12.6 | 4.7 | |
| HF, no. (%) | 10 (6.8) | 6 (4.5) | 17 (9.6) | 19.4 | 1.8 | |
| COPD, no. (%) | 6 (4.1) | 5 (3.8) | 7 (3.9) | 1.7 | 0.7 | |
| CKD, no. (%) | 2 (1.4) | 4 (3.0) | 4 (2.2) | 11.3 | 4.9 | |
| CHA2DS2-VASC score | ||||||
| 0–1 | 47 (32.2) | 49 (37.1) | 100 (56.2) | 1.6 | 9.4 | |
| 99 (67.8) | 81 (61.4) | 78 (43.8) | 1.6 | 9.4 | ||
| Ablation history, no. (%) | 10 (6.8) | 13 (9.8) | 12 (6.7) | 11.7 | 9.2 | |
| LVEDD (mm), mean (SD) | 45.6 (4.6) | 45.9 (4.5) | 46.1 (4.6) | 10.6 | 1.5 | |
| LVESD (mm), mean (SD) | 31.8 (5.5) | 31.5 (5.7) | 31.6 (5.1) | 4.9 | 6.8 | |
| LVEF (%), mean (SD) | 61.4 (5.3) | 61.7 (6.0) | 61.8 (7.2) | 6.4 | 2.0 | |
| LAD (mm), mean (SD) | 46.9 (6.1) | 45.9 (6.5) * | 46.0 (6.8) | 24.6 | 3.1 | |
| MAD (mm), mean (SD) | 31.5 (3.4) | 31.4 (2.7) | 31.5 (2.6) | 1.6 | 6.2 | |
| MR grade, no. (%) | ||||||
| + |
129 (88.4) | 130 (98.5) * | 178 (100) * | 16.3 | 6.6 | |
| +++ | 17 (11.6) | 2 (1.5) * | 0 (0) * | 16.3 | 6.6 | |
| TR grade, no. (%) | ||||||
| Trivial or mild | 87 (59.6) | 82 (62.1) | 97 (50.5) |
7.7 | 5.1 | |
| Moderate | 57 (39.0) | 50 (37.9) | 77 (43.2) | 7.7 | 5.1 | |
| Severe | 2 (1.4) | 0 (0) | 4 (2.2) | 7.7 | 5.1 | |
SD, standard deviation; LAA, left atrial appendage; BMI, body mass index; NYHA,
New York Heart Association; HLP, hyperlipemia; PMI, post myocardial infarction;
HF, heart failure; COPD, chronic obstructive pulmonary disease; CKD, chronic
kidney disease; LVEDD, left ventricular end diastolic
diameter; LVESD, left ventricular end systolic diameter; LVEF, left ventricular
ejection fraction; LAD, left atrial diameter; MAD, mitral annular diameter;
MR, mitral regurgitation; TR, tricuspid regurgitation; IPTW, inverse probability of treatment weighting; ASMD, absolute standardized mean difference.
p-values may not be interpreted as confirmatory but rather descriptive.
*p
All patients in this study successfully
underwent the ablation procedures, and no in-hospital mortalities were observed.
After a median follow-up time of 4 years, 30 deaths were observed. The follow-up
rate of this study was 98.5%. The overall survival rates at 3 years and 5 years
were 94.2% and 89.1%, respectively. No differences were observed among the
three groups after IPTW adjustment (All p
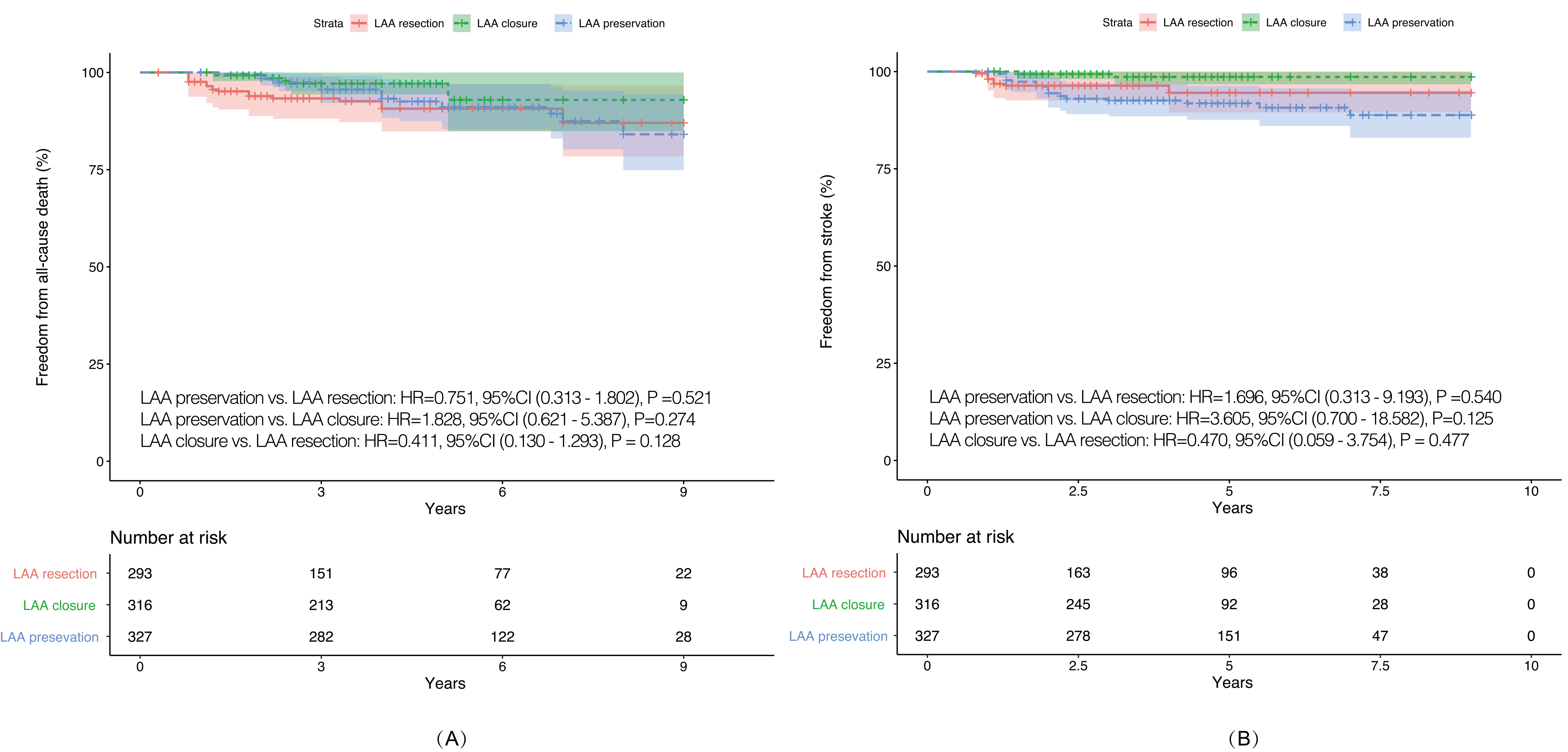 Fig. 1.
Fig. 1.IPTW adjusted Kaplan-Meier Curves in terms of Mortality and Stroke. (A) IPTW adjusted Kaplan-Meier curves in terms of all-cause mortality. (B) IPTW adjusted Kaplan-Meier curves in terms of stroke. IPTW, inverse probability of treatment weighting; LAA, left atrial appendage; HR, hazard ratio; CI, confidence interval.
As shown in Table 2, medications after discharges showed no differences among
the three groups. Worsening of MR occurred in 20.2% (92 cases) of participants
during follow-up. Of these, 52 cases of MR aggravated from mild to moderate, 10
cases aggravated from mild to severe, and 30 cases aggravated from moderate to
severe. Worsening of MR was more likely to occur among patients who underwent LAA
resected or closed (LAA preservation vs. LAA endocardial closure:
p = 0.004, 95% CI (0.232–0.762); LAA preservation vs. LAA
resection: p
| LAA excision | LAA closure | LAA preservation | p value | |
| (n = 146) | (n = 132) | (n = 178) | ||
| AADs, no. (%) | 72 (49.3) | 61 (46.2) | 87 (48.9) | 0.855 |
| 86 (58.9) | 83 (62.9) | 96 (53.9) | 0.280 | |
| Oral anticoagulant, no. (%) | 41 (28.1) | 38 (28.9) | 44 (24.7) | 0.680 |
| Loop-diuretic, no. (%) | 51 (34.9) | 50 (37.9) | 66 (37.1) | 0.867 |
| ARA, no. (%) | 32 (21.9) | 24 (18.2) | 35 (19.7) | 0.733 |
| ACEI, no. (%) | 28 (19.2) | 22 (16.7) | 33 (18.5) | 0.854 |
Any changes in medications for the worsening of MR were not included in this table. AADs, anti-arrhythmia drugs; ACEI, angiotensin-converting enzyme inhibitors; ARA, aldosterone receptor antagonist; LAA, left atrial appendage; MR, mitral regurgitation. p-values may not be interpreted as confirmatory but rather descriptive.
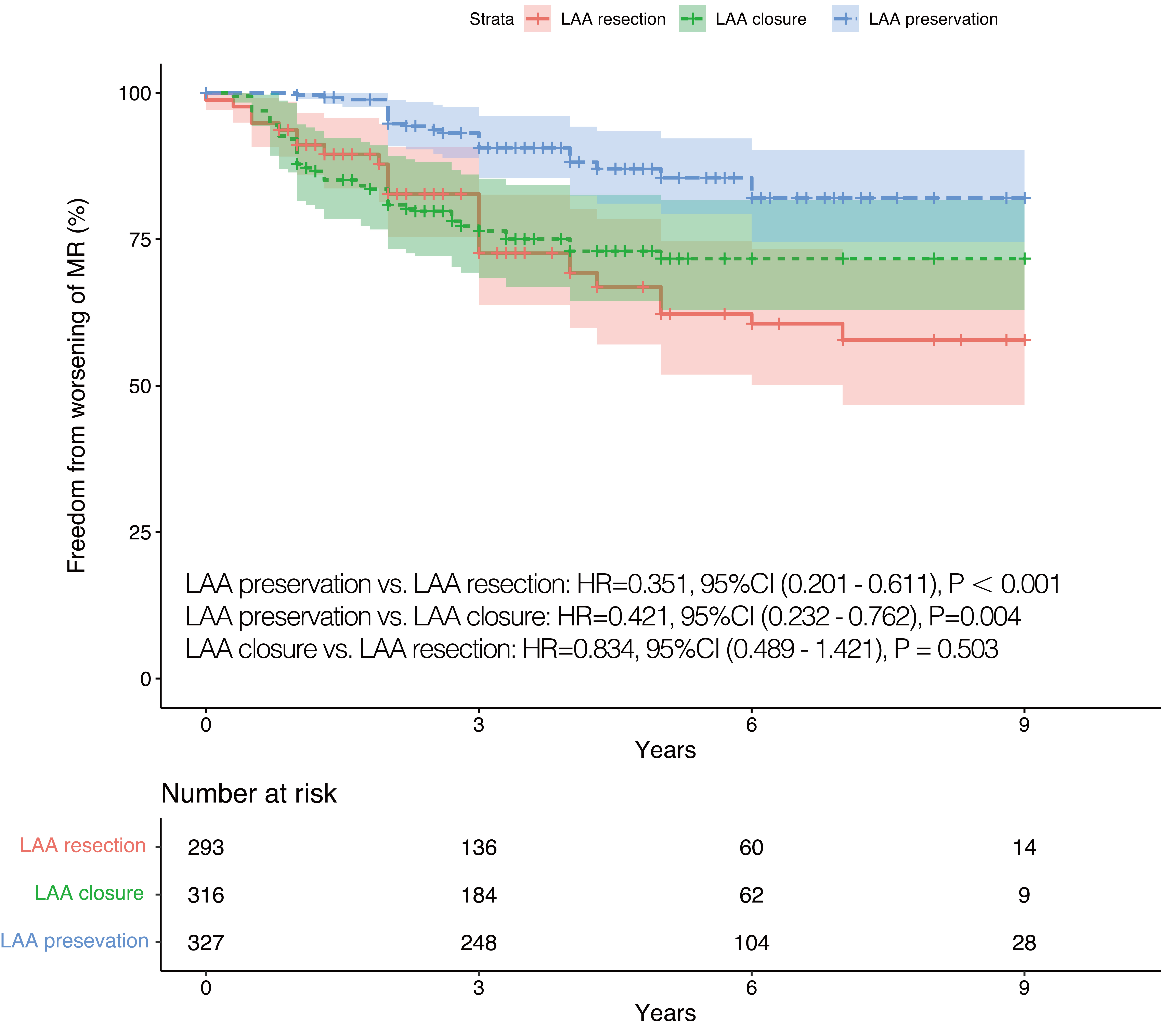 Fig. 2.
Fig. 2.IPTW adjusted Kaplan-Meier curves in terms of worsening of MR. IPTW, inverse probability of treatment weighting; LAA, left atrial appendage; HR, hazard ratio; CI, confidence interval; MR, mitral regurgitation.
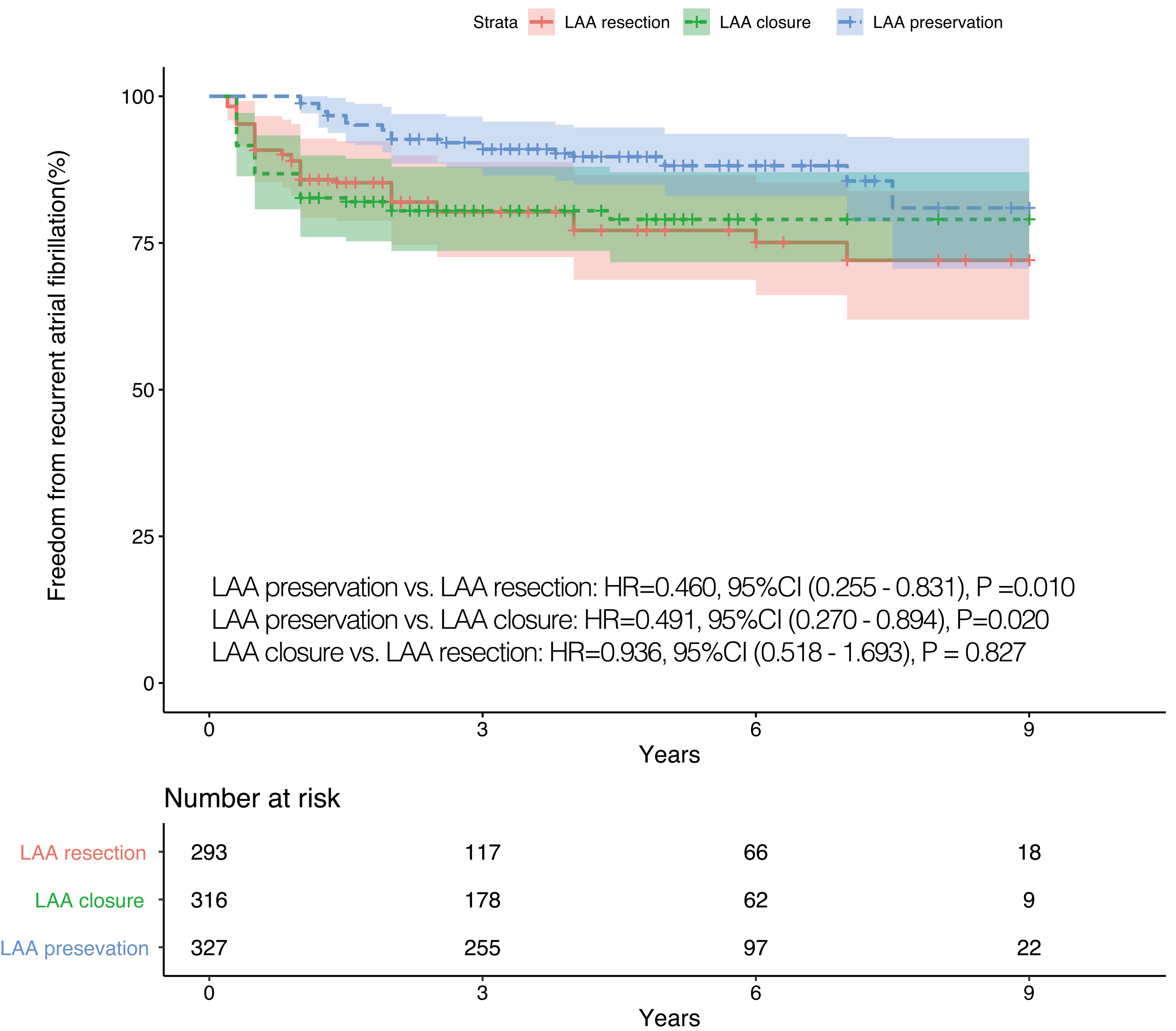 Fig. 3.
Fig. 3.IPTW adjusted Kaplan-Meier curves in terms of recurrent AF. IPTW, inverse probability of treatment weighting; LAA, left atrial appendage; HR, hazard ratio; CI, confidence interval; AF, atrial fibrillation.
Before ablations, the three groups of patients showed similar left atrial
diameter (LAD) and MAD (all p
 Fig. 4.
Fig. 4.Longitudinal echocardiographic features during follow-up among
different LAA treatment methods. (A) LAD among different LAA treatment methods;
(B) MAD among different LAA treatment methods; (C) TH among different LAA
treatment methods; *p
This article is the first study to investigate the impacts of different LAA treatments on patients with mitral diseases. We found that patients who underwent LAA resection or endocardial closure during surgical ablations had a higher risk of MR worsening than those who had LAA preserved. A worse coaptation of the mitral valve may be present among those who lost LAA, which may contribute to the worsening of MR.
The LAA derives from the primordial LA, which is a finger-like projection from the main body of LA [2]. The mechanical and endocrinological functions of LAA are hard to ignore [3]. LAA plays an important role in modulating the LA pressure through its distensibility. In addition, the concentration of atrial natriuretic peptide (ANP) is the largest in LAA, which could also help to modulate the LA pressure [1]. In 1990, Davis et al. [16] first reported that the slope of the LA pressure vs. normalized volume data increased significantly when the LAA was excluded. Recently, more evidence showed left atrial enlargement or left atrial remodeling was present after LAA interventions [9, 10, 17], which may be related to the postoperative decreases of ANP [18]. In the study of Kim et al. [8], they found postoperative LA transport functions were more favorable with LAA preservation than with LAA interventions among patients who underwent surgical ablations. The loss of left atrial physiological function may explain why patients who underwent LAA interventions showed larger LAD and MAD during follow-up in our study.
Patients with mild or moderate MR were commonly not considered as candidates for invasive MV interventions [19, 20]. In our study, the enlargements of LA and MV annulus were more frequently observed among patients who underwent LAA interventions, which was in accordance with the results from the above studies. Despite both enlargements of LA and MV annulus, one of the most symbolic characteristics of MR among patients who underwent LAA interventions is the shortening of MV tenting height [11]. During follow-up, the mean TH of patients who underwent LAA resections and endocardial closure were 4.63 mm and 5.04 mm, respectively, which were significantly lower than the value of the normal population in the study Ring et al. [21]. All the above evidence showed that LAA interventions could affect patients’ clinical outcomes by modulating LA functions.
Additionally, some relationships may be present between LAA interventions and recurrent AF. In the study of Melduni et al. [6], surgical LAA closure during routine non-AF-related cardiac surgery was independently associated with an increased risk of early postoperative AF. Similarly, patients who underwent LAA interventions in our study were at a higher risk of recurrent AF during follow-up, which may be caused by the increased pressure and decreased distensibility of LA.
This is a single-center, retrospective study, all baseline clinical features, rhythm status and MR status were retrospectively collected. Our study has the typical limitations of retrospective analysis. Additionally, the baseline tenting heights of different groups were lacking, because tenting height itself was not a common examination item in our center, we only acquired it in the follow-up echo.
Our findings further confirmed the regulating function of LAA, which could affect LA remodeling. Mitral regurgitation was more likely to get worse when patients with fundamental mitral diseases underwent LAA closure during isolated surgical AF ablations.
In the absence of LAA, dilation of the left atrium and the mitral annulus may lead to a reduction of the coaptation area, ultimately causing increased regurgitation.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
YZ, JD and JW made substantial contributions to conception and design; ChZ and QY contributed to the acquisition of data; CaZ contributed to the analysis and interpretation of data. CaZ and YZ were involved in drafting the manuscript; JD, JW, ChZ and QY reviewed this manuscript. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
The institutional review board at Beijing Anzhen Hospital, Capital Medical University has approved the study (IRB.20221201). All patients have given their written informed consent.
Not applicable.
This study was supported by the National Natural Science Foundation of China (Grant No.81770320).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
