1 Department of Cardiology, First Affiliated Hospital of Xinjiang Medical University, 830054 Urumqi, Xinjiang, China
Abstract
Background: The correlation
between 5
Keywords
- 5′-nucleotidase (5′-NT)
- coronary artery disease (CAD)
- CD73
- prognosis
In recent years, the incidence rate and mortality of coronary artery disease (CAD) have risen significantly, posing a growing threat to human health and safety [1], as well as increasing the economic burden on patients and society [2]. While percutaneous coronary intervention (PCI) technology advancements have revolutionized CAD management [3], some patients still experience adverse clinical outcomes [4]. Therefore, recent studies have focused on prognosis prediction and finding new post PCI markers. Some of the emerging PCI markers include apolipoprotein E (ApoE) [5], plasminogen activator inhibitor-1 (PAI-1) [6], and anti-apolipoprotein B-100 autoantibody (anti-apoB-100 Ab) [7]. Additionally, metrics including the neutrophil/lymphocyte ratio, monocyte/lymphocyte ratio, systemic inflammation response index, systemic immune-inflammation index offer insights into a patient’s inflammatory and immune status [8, 9, 10, 11, 12, 13, 14, 15, 16].
The role of ecto-5
The data used in this study came from a single-center prospective cohort study (PRACTICE). We analyzed the clinical records of CAD patients who underwent PCI at the First Affiliated Hospital of Xinjiang Medical University. The dataset spans from 2016-October 2021, including sex, age, smoking history, chronic disease history, laboratory results, and image examination data.
The inclusion criteria consists of: (1) The results of severe
coronary angiography, with at least one main coronary artery having
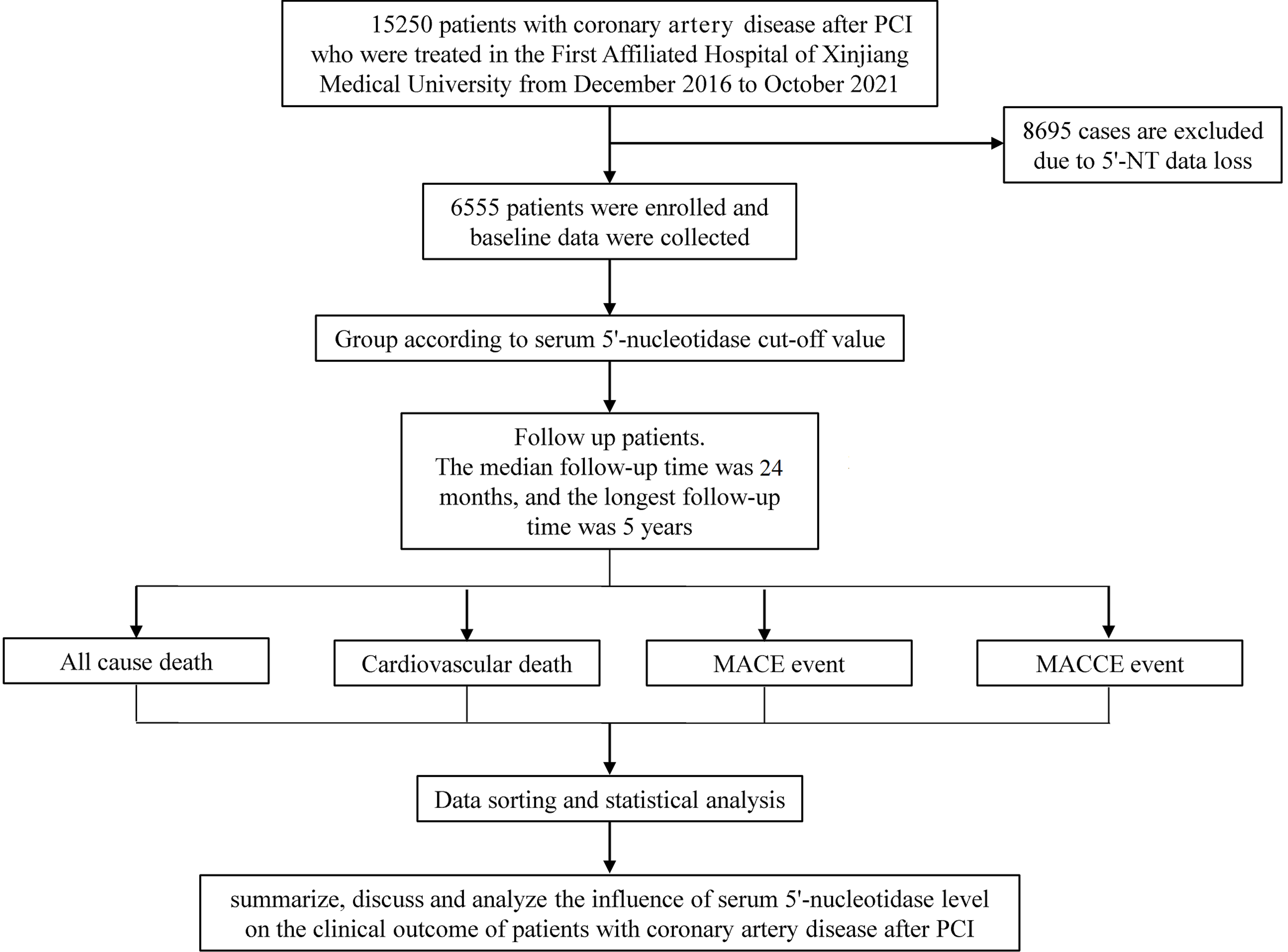
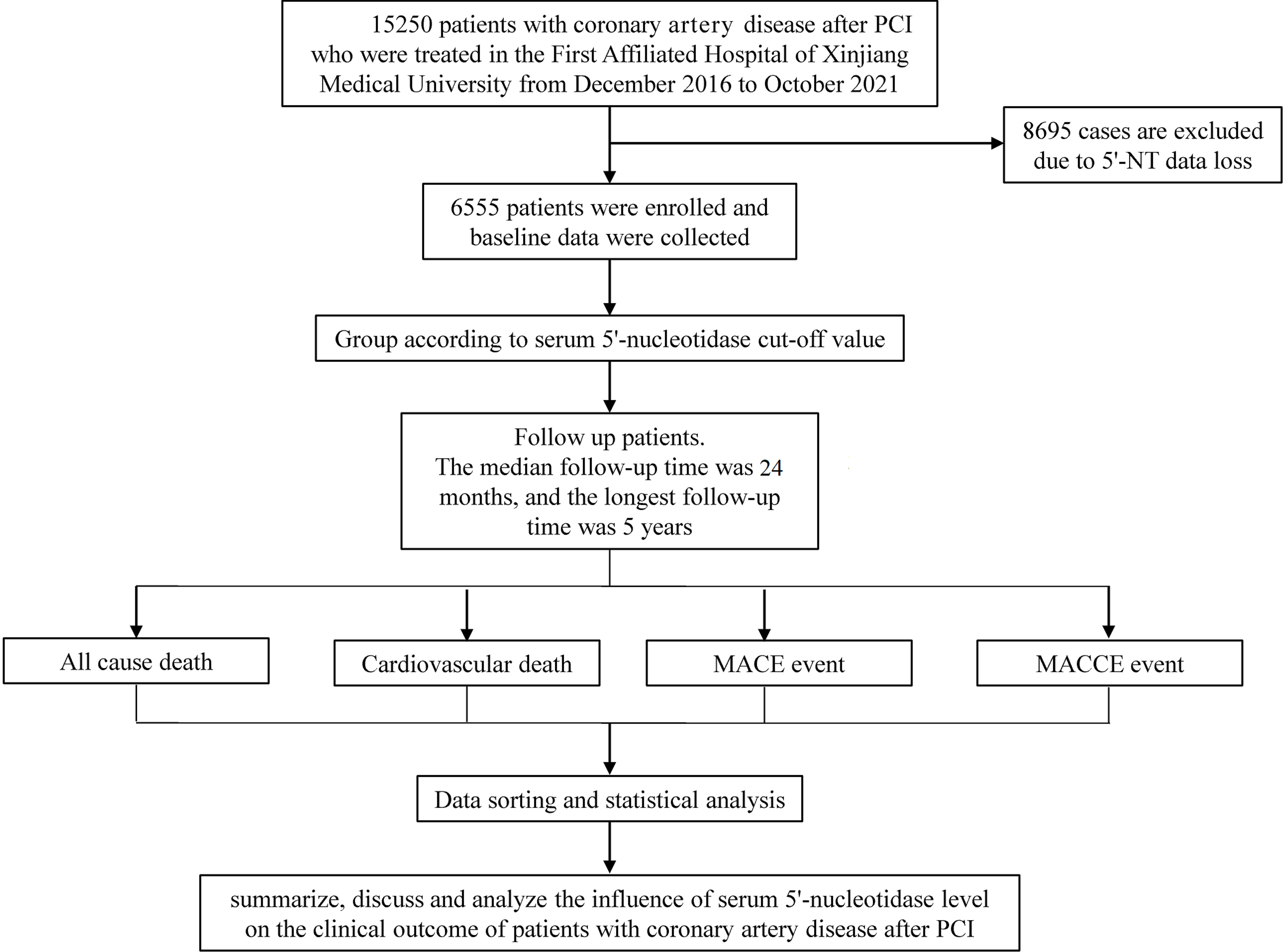 Fig. 1.
Fig. 1.Detailed inclusion and exclusion criteria. PCI, percutaneous
coronary intervention; 5
This study required a comprehensive collection of clinical, laboratory, and imaging data from all patients. Blood was collected in the morning following an overnight fast. All laboratory examinations included in the study were conducted at the Physical Examination Center of the Affiliated Hospital of Xinjiang Medical University.
The enzyme rate method was used to measure serum 5
(1) The patients were told to not eat excessively greasy or high-protein foods the day before the blood test and to avoid excessive alcohol consumption. (2) After 8 p.m. the day before the physical examination, patients fasted for 12 hours to avoid affecting the test results. (3) During the blood draw, the patient was told to relax to avoid the constriction of blood vessels caused by fear, which can increase the difficulty of blood collection.
The research conducted in this study was based on three kinds of follow-up data: the hospital’s inpatient system, outpatient electronic medical record system, and telephone interviews. Post-PCI patients were generally followed at 1 month, 3 months, 6 months, 1 year, 3 years and 5 years. The median follow-up time was 24 months, with the longest being 5 years. The primary endpoints we examined were death, all-cause death (ACD), and cardiogenic death (CD). The secondary endpoints included major adverse cardiovascular adverse events (MACE) and major adverse cardiovascular and cerebrovascular adverse events (MACCE). MACE was further subdivided into three types: cardiac death, recurrent myocardial infarction, and target vessel revascularization; MACCE additionally considered nonfatal stroke on top of MACE.
This study applied SPSS 26.0 statistical analysis software (IBM Corp., Armonk,
NY, USA) to process and analyze the obtained data. Continuous data (measurement
data) are presented as the mean
At baseline, there were no significant differences in smoking,
drinking, hypertension, urea or uric acid between the high- and low-5
| Parameters | Low serum 5′-NT (n = 4209) | High serum 5′-NT (n = 2346) | Chi-square or t | p value |
| Sex (male), n (%) | 3061 (72.7) | 1636 (69.7) | 6.628 | 0.01 |
| Smoking, n (%) | 1668 (39.6) | 916 (39.0) | 0.215 | 0.643 |
| Alcohol drinking, n (%) | 1011 (24.0) | 599 (25.5) | 1.861 | 0.173 |
| Hypertension, n (%) | 3047 (72.8) | 1655 (70.9) | 2.555 | 0.11 |
| Diabetes, n (%) | 1717 (40.8) | 1351 (57.6) | 170.637 | |
| Age, years | 60.7 |
60.1 |
2.207 | 0.027 |
| Urea, mmol/L | 10.77 |
10.45 |
0.342 | 0.733 |
| Uric acid, mmol/L | 335.6 (279.6, 404.1) | 358.0 (294.0, 439.0) | 1.018 | 0.309 |
| Total cholesterol, mmol/L | 3.70 |
3.95 |
–8.648 | |
| HDL-cholesterol, mmol/L | 1.10 |
1.07 |
4.344 | |
| LDL-cholesterol, mmol/L | 2.34 |
2.61 |
–11.014 | |
| CRP, mg/L | 9.60 |
19.12 |
–6.762 | |
| BNP, ng/L | 1897.64 |
1294.33 |
0.502 | 0.619 |
| AST, U/L | 25.13 |
51.84 |
–5.997 | |
| ALT, U/L | 25.28 |
47.48 |
–8.895 | |
| GGT, U/L | 27.35 |
63.55 |
–22.954 | |
| LVEF, % | 60.44 |
58.12 |
10.144 | |
| Single-vessel disease, n (%) | 749 (17.8) | 370 (15.8) | 4.358 | 0.037 |
| Multivessel disease, n (%) | 3460 (82.2) | 1976 (84.2) | 4.358 | 0.037 |
| LMCA, n (%) | 302 (7.2) | 213 (9.1) | 7.545 | 0.006 |
| RASi, n (%) | 1745 (41.5) | 1038 (44.2) | 4.788 | 0.029 |
| 2390 (58.4) | 1367 (60.6) | 2.737 | 0.098 | |
| Clopidogrel, n (%) | 2099 (49.9) | 1192 (50.8) | 0.533 | 0.465 |
| Ticagrelor, n (%) | 2110 (50.1) | 1154 (49.2) | 0.533 | 0.465 |
| Statins, n (%) | 3955 (94.0) | 2145 (91.4) | 14.964 | |
| Postoperative anticoagulation, n (%) | 330 (7.8) | 272 (11.6) | 25.451 | |
| SCAD, n (%) | 1844 (43.8) | 771 (32.9) | 75.276 | |
| ACS, n (%) | 2365 (56.2) | 1575 (67.1) | 75.276 | |
| Non-fatal myocardial infarction, n (%) | 153 (3.6) | 101 (4.3) | 1.816 | 0.178 |
| Stent thrombosis, n (%) | 5 (0.1) | 14 (0.6) | 11.907 | 0.001 |
Values are mean
Between the two 5
Through the receiver operating characteristic curve analysis, we calculated
Youden’s J statistic to identify the optimal cutoff point for 5
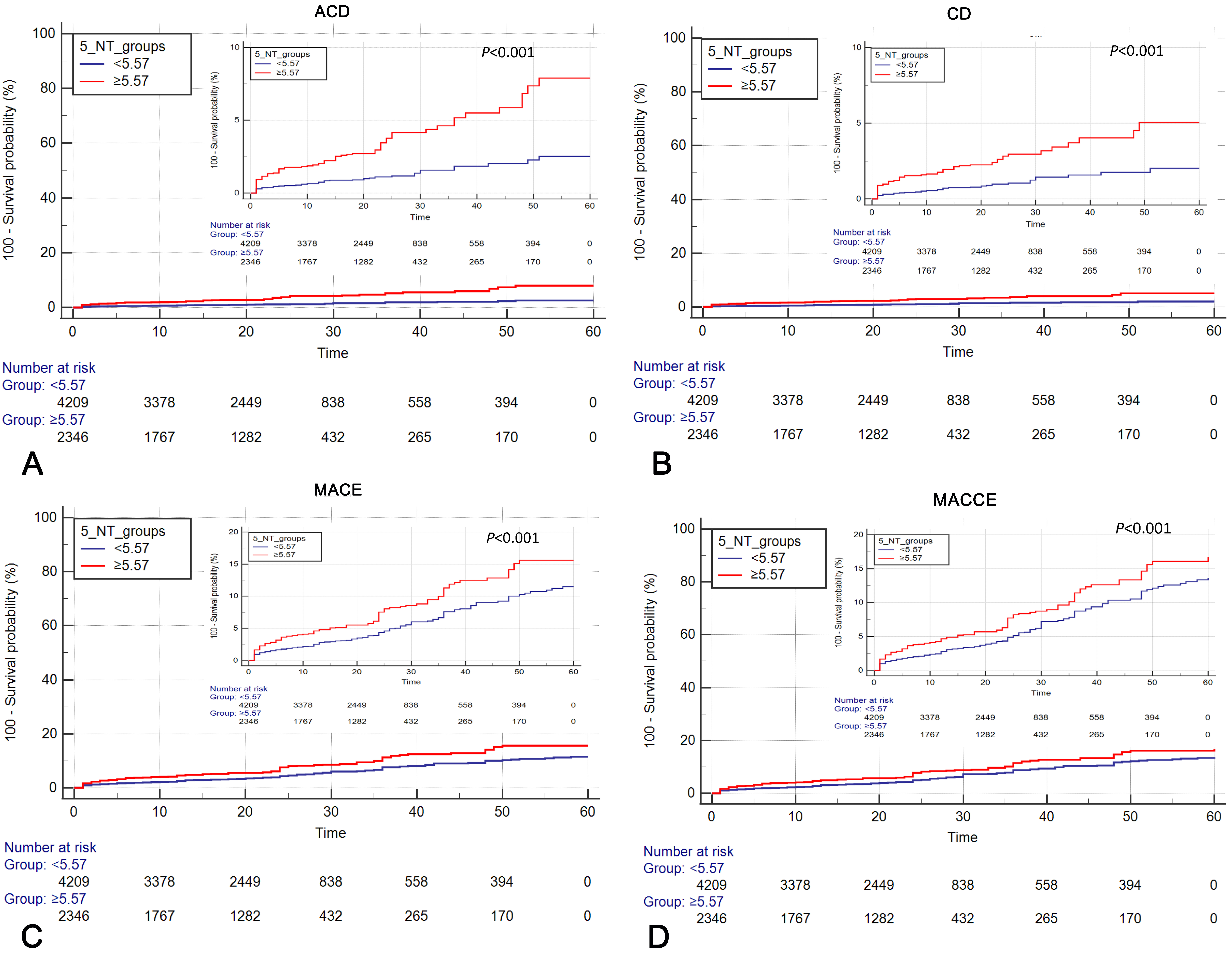
All 6555 patients were followed up for an average of 24 months and a maximum of
5 years. A total of 129 instances of ACD were reported during the follow-up
period, including 49 cases (1.2%) in the low-value group and 80 cases (3.4%) in
the high-value group. Similarly, 102 cardiovascular deaths (CDs) occurred,
including 42 cases (1.0%) in the low-value group and 60 cases (2.6%) in the
high-value group. MACE endpoints were noted in 363 patients, including 198
(4.7%) in the low-value group and 165 (7%) in the high-value group. A total of
397 patients reached the MACCE endpoint, including 227 (5.4%) in the low-value
group and 170 (7.2%) in the high-value group. Statistical analysis revealed a
significant increase in ACD, CD, MACE and MACCE incidences as serum 5
| Outcomes | Low serum 5′-NT (n = 4209) | High serum 5′-NT (n = 2346) | Chi-square | p value |
| ACD, n (%) | 49 (1.2) | 80 (3.4) | 39.384 | |
| CD, n (%) | 42 (1.0) | 60 (2.6) | 23.922 | |
| MACE, n (%) | 198 (4.7) | 165 (7.0) | 15.621 | |
| MACCE, n (%) | 227 (5.4) | 170 (7.2) | 9.092 | 0.003 |
The p value indicates p for trend. 5
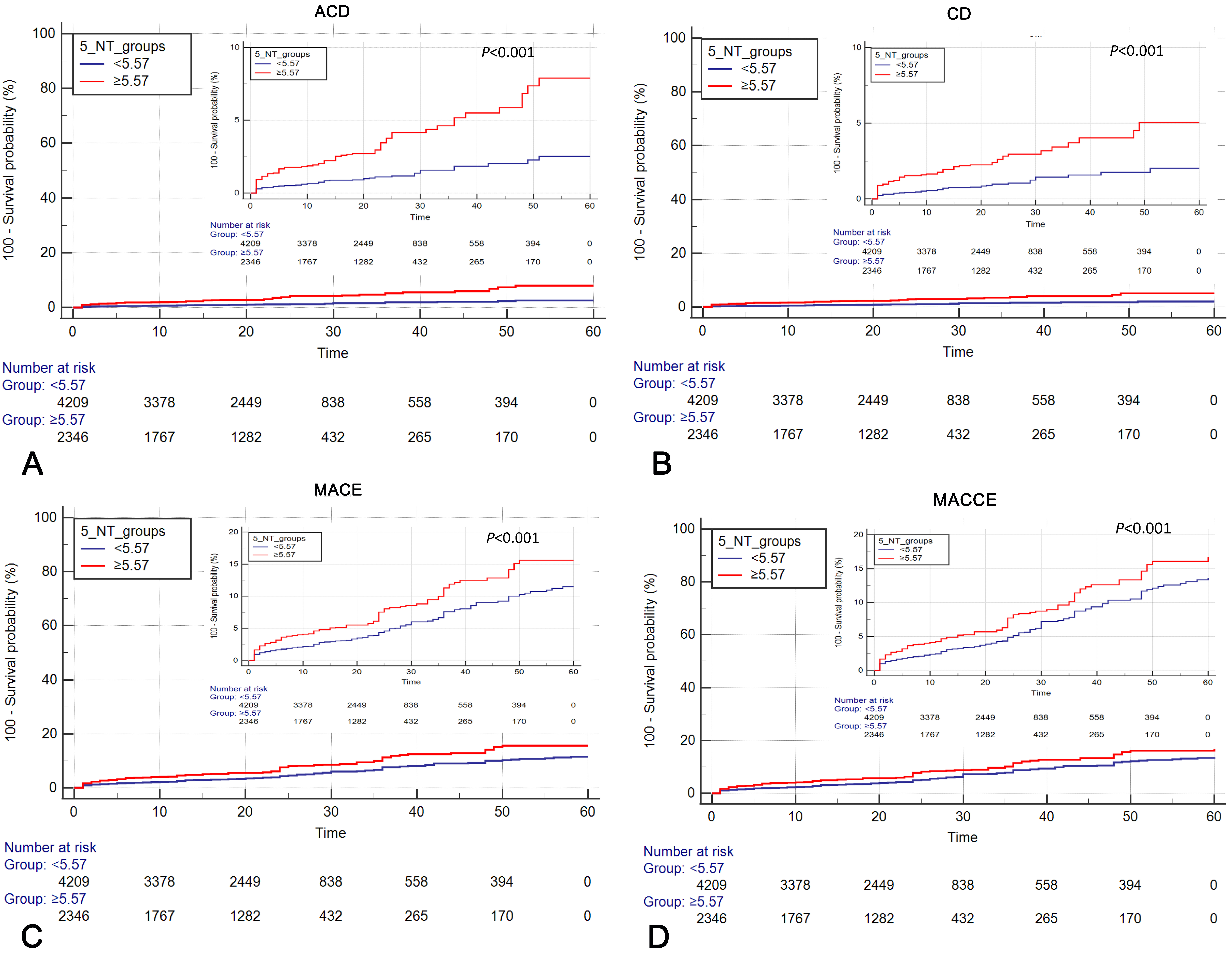 Fig. 2.
Fig. 2.Cumulative Kaplan‒Meier estimates of the time to first assessed
occurrence of results during the 60-month follow-up. (A) ACD. (B) CD. (C) MACE.
(D) MACCE. 5
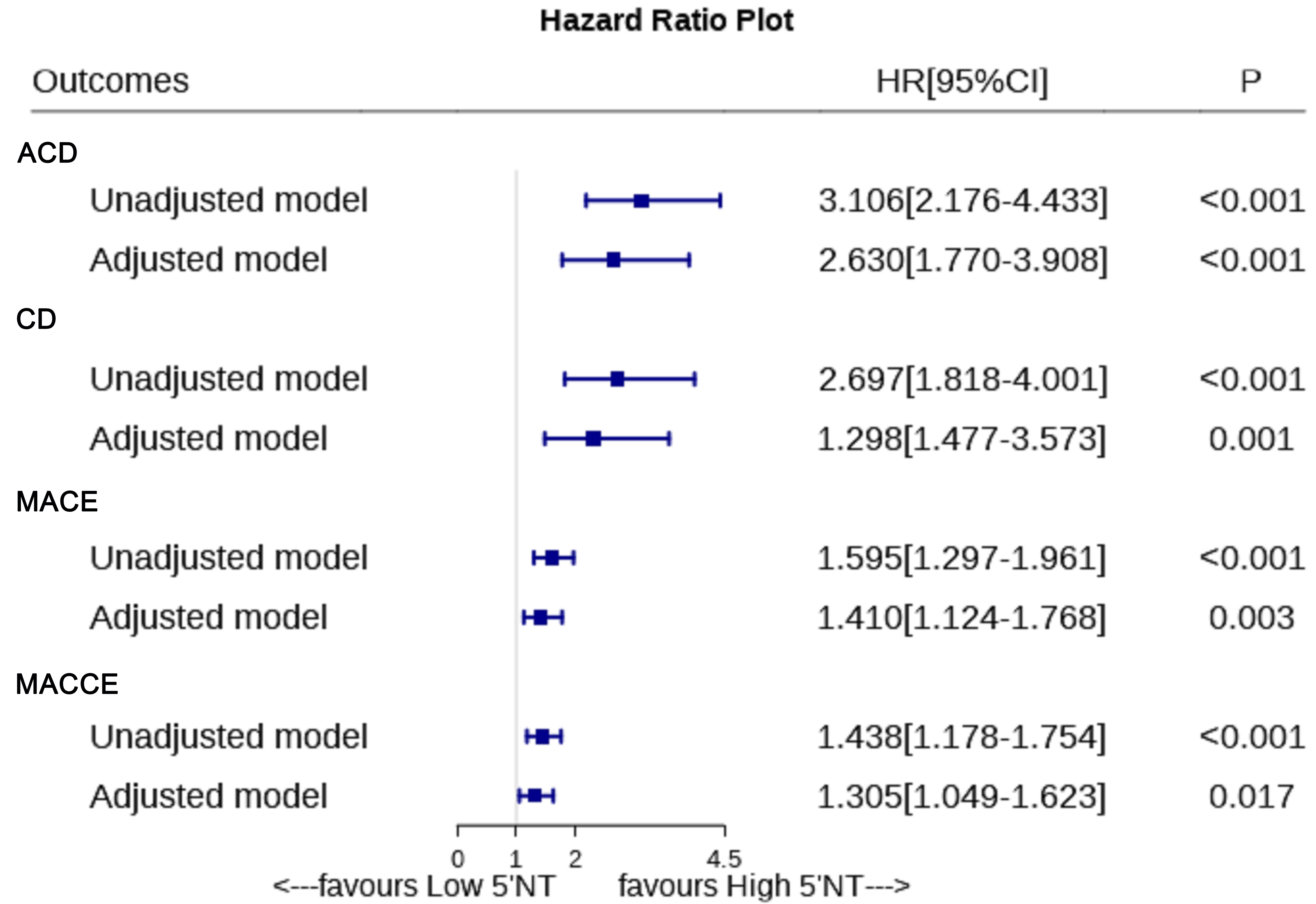
To mitigate potential biases, we employed a multivariate Cox
regression model. After adjusting for variables including sex, age, diabetes
history, HDL-C, LDL-C and other confounding factors, we assessed the impact of
serum 5
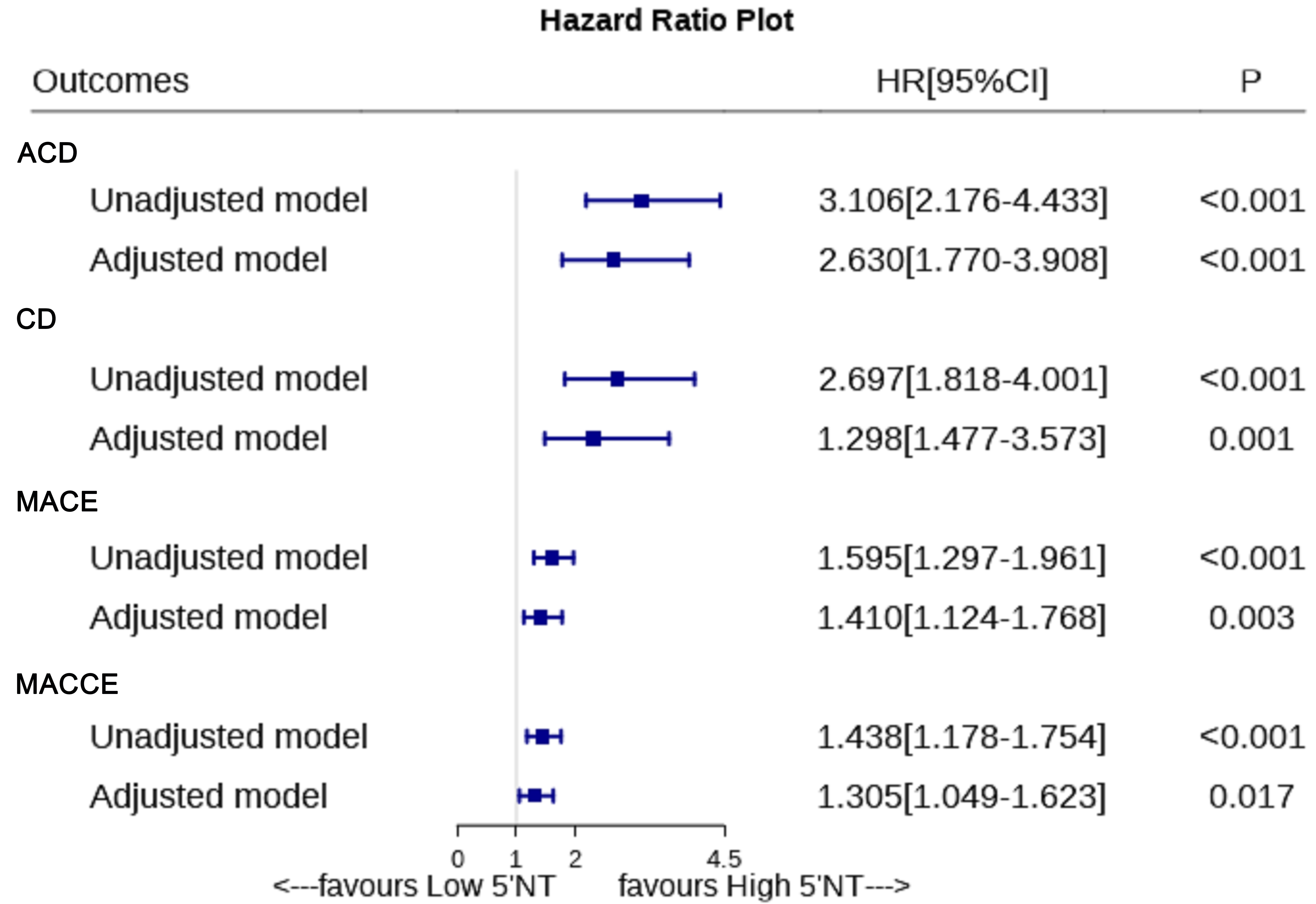 Fig. 3.
Fig. 3.Association of 5
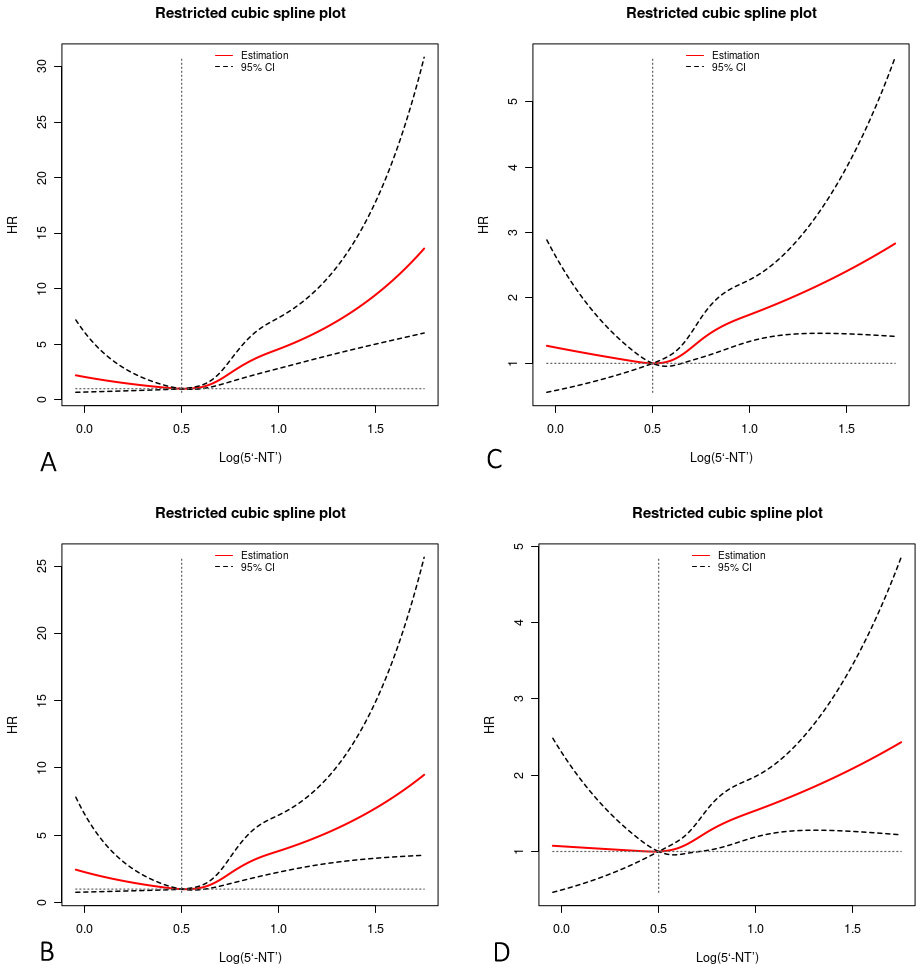
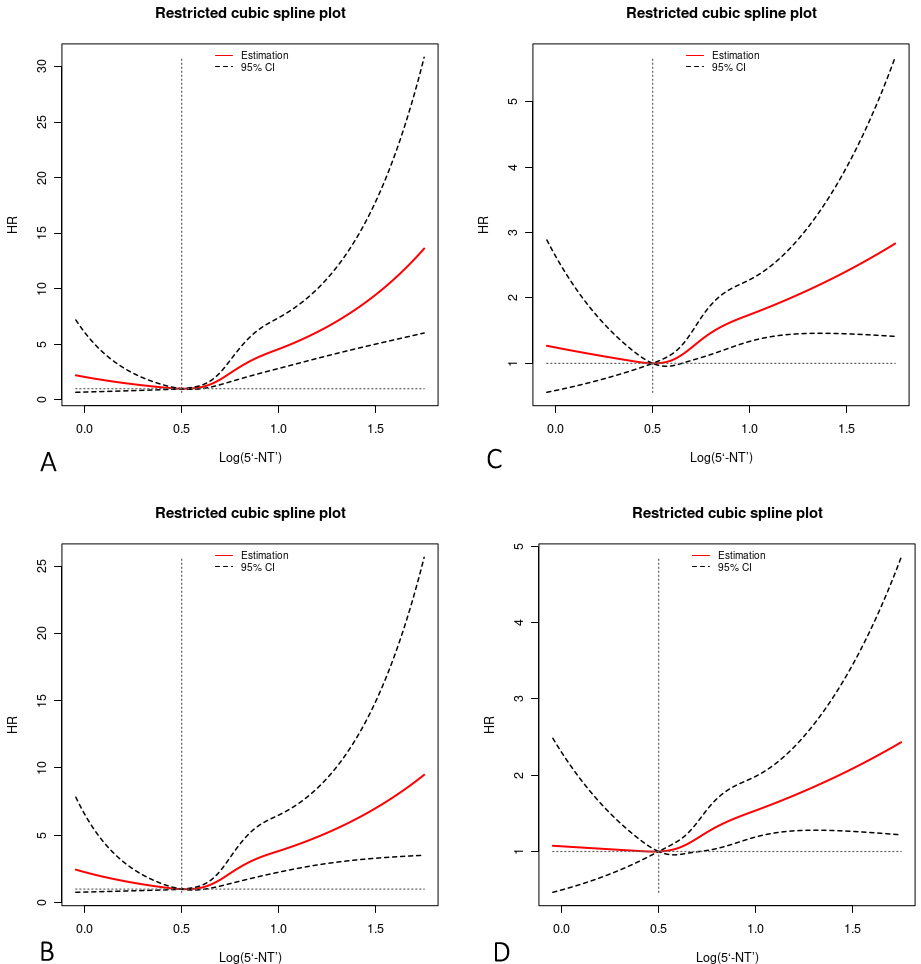 Fig. 4.
Fig. 4.Restricted cubic spline plots for ACD, CD, MACE, and MACCE by
serum 5
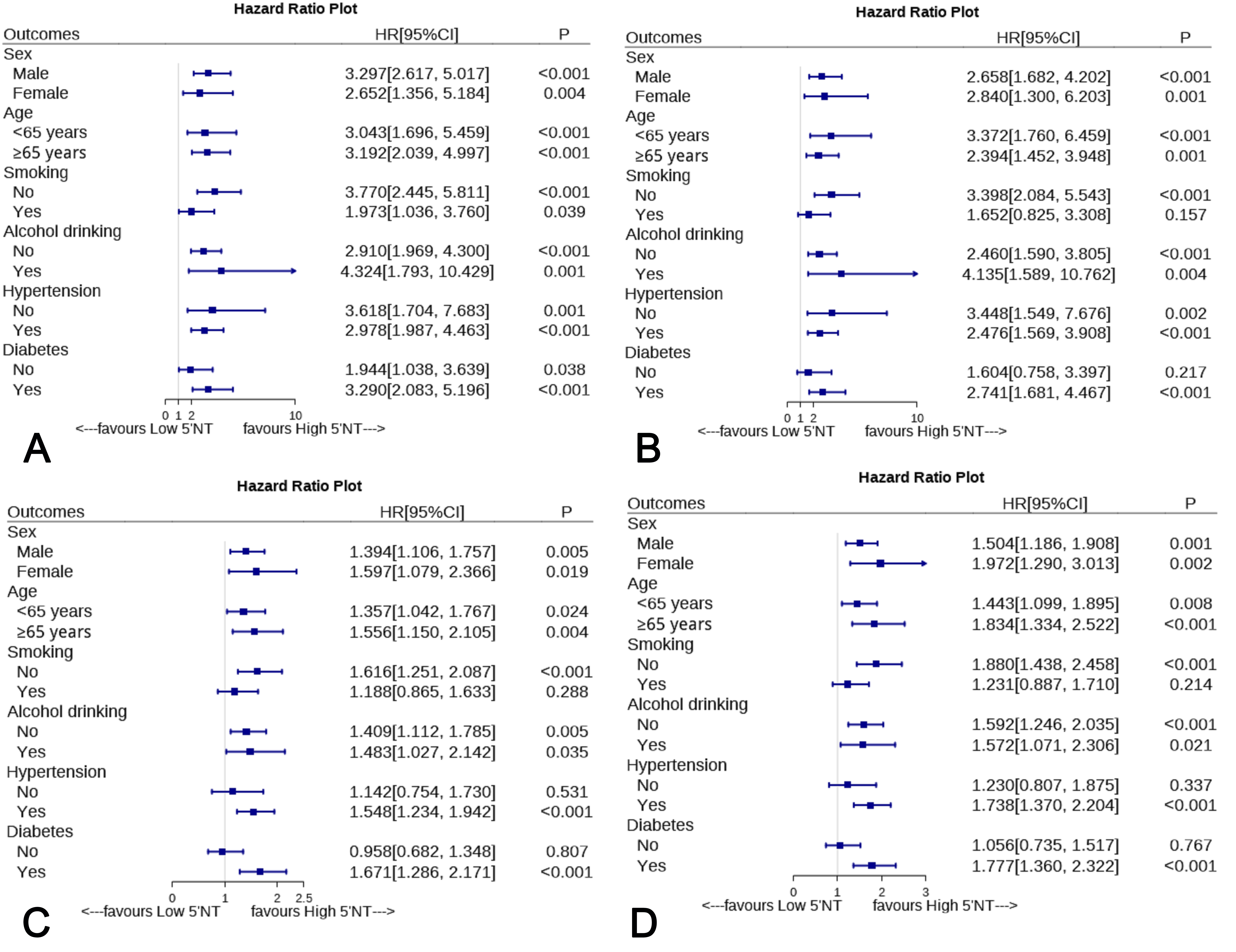
Fig. 5A–D reveal that age, sex, smoking, alcohol consumption, hypertension, or
diabetes did significantly impact the relationship between elevated 5
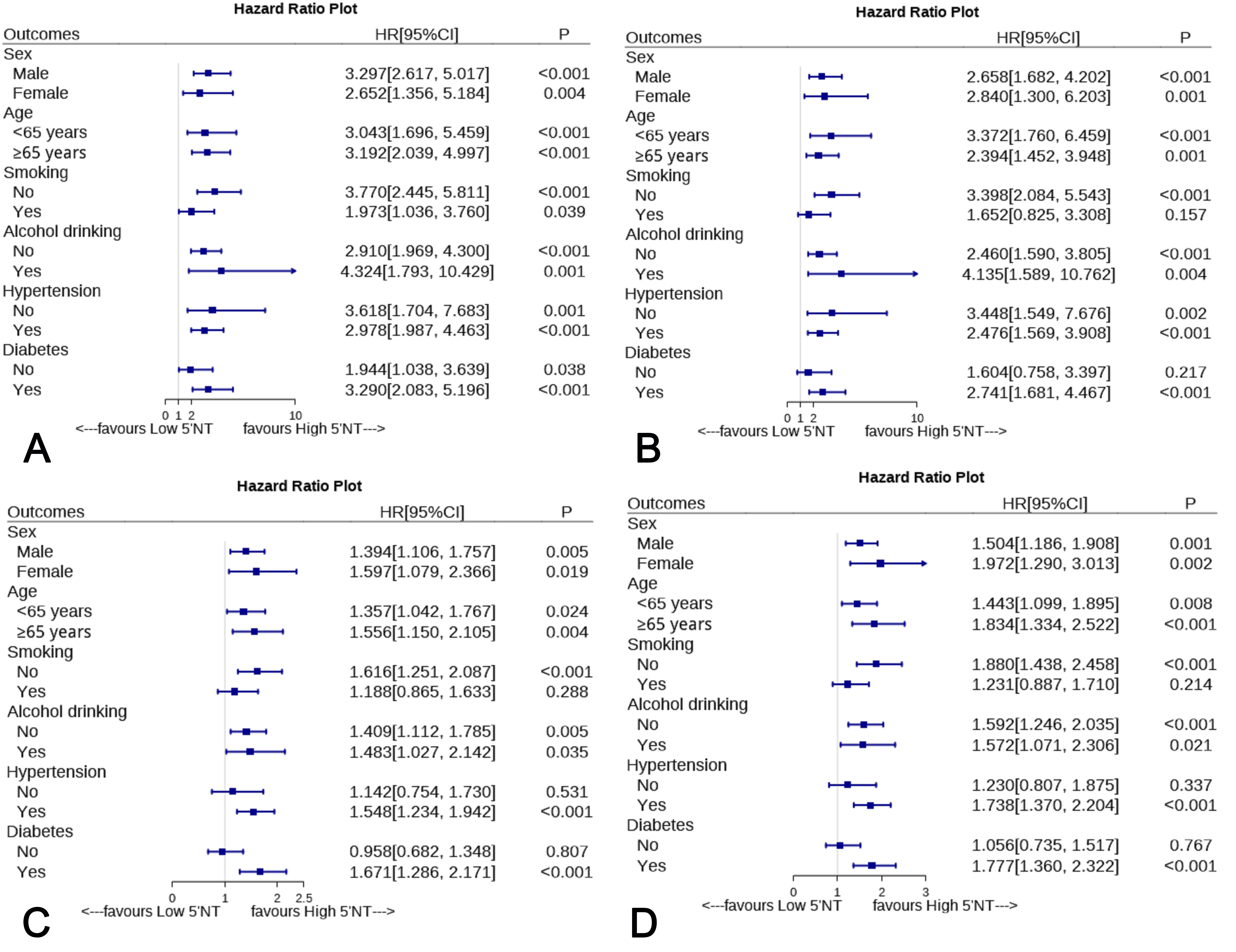 Fig. 5.
Fig. 5.Subgroup analyses of the relationship between serum 5
In this study, we propose that elevated 5
While the role and mechanism of 5
Some studies challenge the purported cardioprotective role of 5
It has been reported [33] that CD73 knockout led to arterial
calcification, a ubiquitous pathological process of atherosclerosis [34]. This
underscores the influence of CD73 on the occurrence and progression of
atherosclerosis. In a study using ApoE-/- mice, CD73
inactivation inhibited the migration, proliferation and foam cell transformation
of vascular smooth muscle cells (SMCs), thereby attenuating both AS and
hyperlipidemia [35]. This led to the proposal that
CD73 is an important regulator in the development of atherosclerosis
(AS). In their experiment, CD73 siRNA was used to downregulate
CD73 expression [35]. They mainly explain the role of CD73 in
the rupture of atherosclerotic plaques through the following mechanisms: First,
deactivating CD73 led to carotid artery ligation injuries, which
affected parameters such as the neointimal area, the neointimal/medial thickness
ratio, and the number of proliferative SMCs [35]. Second, the downregulation of
CD73 (at both the mRNA and protein levels) through siRNA significantly
inhibited both the migration and growth of human umbilical artery smooth muscle
cells (HUASMCs) [35]. Furthermore, the knockdown of CD73 significantly
reduced cyclin D1 levels, impacting the cell cycle [35]. This indicates that
CD73 can inhibit the release and migration of inflammatory factors in
HUASMCs, promoting their proliferation ability [35]. Third, administration
of CD73 siRNA significantly reduced lipid accumulation, implying a role
for CD73 in lipid metabolism [35]. Fourth, CD73 appears to
promote plaque formation by increasing blood lipid levels, specifically
triglycerides, TC, and plasma LDL-C [35]. The mechanism for these increases may
be related to CD73’s regulatory impact on hepatic mRNA expression genes
coding for hepatic lipase, peroxisome proliferator-activated
receptor
Another study suggested impaired CD73-derived adenosine production contributes to the development of atherosclerosis in mice and humans, leading to calcification of human lower limb arteries [37]. When compared to the age-matched healthy control group, peripheral artery disease (PAD) patients had significantly higher CD73 activity in the blood [37]. They suggest that the high CD73 activity observed in the circulation of PAD patients appears to be a result of the shedding and loss of CD73 expression in mature occlusive plaques [37]. Müller et al. [38, 39] proposed that signal-induced glycosylphosphatidylinositol-anchored CD73 was upregulated by lysosomal degradation (LD)-mediated lipid synthesis in adipose tissue via diacylglycerol (DIG) transfer from the adipose body to adipocytes. They found that adipocytes release microbubbles containing CD73, which enter immature or small adipocytes through gaps or blood circulation and adhere to the surface of lipid droplets [38, 39]. Once attached, the enzymes facilitate the breakdown cAMP (cyclic adenosine monophosphate) on the lipid droplet surface [38, 39]. The decrease in cAMP levels impact lipid metabolism enzymes dependent on cAMP phosphorylation, such as hormone-sensitive lipase (HSL) and glycerol-3-phosphate acyltransferase (GPAT), thereby enhancing esterification, inhibiting lipolysis and promoting lipid synthesis [38, 39]. Furthermore, they described the release of specific transcripts and microRNAs induced by stimulation [40]. These molecules control lipid synthesis and lipid droplet biogenesis from primary and differentiated rat adipocytes to microbubbles containing Gce1 and CD73 [40]. During their transfer and expression in small adipocytes, lipid synthesis is upregulated [40]. This suggests a potential mechanism for regulating lipid metabolism and adipocytes size, facilitated by microcapsules containing a specific set of GPI (glycolphosphatidylinositol)-anchored proteins and RNA [40]. In a separate study, Müller et al. [38] found that the esterification effects triggered by audiogenic stimulants could be nullified by depleting CD73-containing microvesicles secreted from adipocytes.
In our study, after adjusting for potential confounding variables, we found that
the serum 5
This study has limitations, both subjective and objective in nature. First, it
does not account for the influence of dietary habits, nutritional status, and
other related factors in patients with CAD. Second, we did not measure serum
5
This was the first study to investigate the relationship between elevated serum
5
Reasonable requests to access the data used in these analyses can be made to the first authors.
XX and YZ designed this study. TW and XX conducted research. XH, HY and YY provided assistance and suggestions for ELISA experiments. MA, XX and TW analyzed the data and wrote the paper. All authors have contributed to the editing and revision of the manuscript. All authors have read and approved the final manuscript. All authors have fully participated in this work and agree to be responsible for all aspects of the work.
This study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Ethical approval number: K201909-02). It is based on the standards of the Helsinki Declaration. All patients provided written informed consent and were explicitly allowed to collect relevant clinical data.
Not applicable.
This research was funded by the National Natural Science Foundation of China (82000238 and 82170345).
The authors declare no conflict of interest. Xiang Xie and Ying-Ying Zheng are serving as Guest Editors of this journal. We declare that Xiang Xie and Ying-Ying Zheng had no involvement in the peer review of this article and have no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Ezra Abraham Amsterdam.
References
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.