- Academic Editor
†These authors contributed equally.
Background: Right ventricular
involvement in hypertrophic cardiomyopathy is uncommon. This study aimed to
evaluate clinical outcomes of the modified septal myectomy in patients diagnosed
with biventricular hypertrophic cardiomyopathy (BHCM), a subject seldom explored
in the literature. Methods:
We
conducted a retrospective cohort study from January 2019 to January 2023,
enrolling 12 patients with BHCM. Each patient underwent
a modified
septal myectomy and was followed
postoperatively. Clinical data and echocardiographic parameters, including the
ventricular outflow tract peak pressure gradient and maximum interventricular
septum thickness, were collected and analyzed. Results:
The study cohort had a median age of 43.0
(interquartile range 14.5–63.0) years at surgery, with four patients (33.3%)
being children. Two patients (16.7%) previously underwent percutaneous
transluminal septal myocardial ablation. Surgical relief of biventricular outflow
tract obstruction (BVOTO) was achieved in five patients (41.7%), aside from
those managed solely for left ventricular outflow tract obstruction. In five
instances, three-dimensional (3D) printing technology assisted in surgical
planning. The postoperative interventricular septum thickness was significantly
reduced (21.0 mm preoperative vs. 14.5 mm postoperative, p
Hypertrophic cardiomyopathy (HCM) is a genetic heart condition affecting 1:500 people and is primarily caused by mutations in genes encoding sarcomere proteins [1]. HCM has diverse phenotypic expressions resulting in clinical presentations ranging from no symptoms to sudden cardiac death (SCD). The vast majority of HCM cases are characterized by an abnormal thickness of the interventricular septum (IVS), while a few cases involve the apical and mid-segments of the left ventricle [2]. A rarer variant, biventricular hypertrophic cardiomyopathy (BHCM) may lead to the obstruction of both the left and right ventricular outflow tracts [3, 4]. Due to its rarity, surgical data on BHCM are scarce and experience in safely and effectively performing surgery for BHCM is limited. The objective of the present study was to evaluate the effectiveness of the modified septal myectomy in patients with BHCM at our center.
In this retrospective cohort study, we
enrolled twelve consecutive patients with BHCM who underwent surgical treatment
at our institution between January 2019 and January 2023. Eligible patients were
clinically diagnosed with primary BHCM, confirmed either pathologically or
through genetic testing. Patients with Noonan syndrome, Costello syndrome, aortic
valve stenosis, and other diseases causing hypertrophy and ventricular outflow
tract obstruction were excluded. HCM was defined as an end-diastolic left
ventricular wall thickness
Baseline characteristics, including age, gender, symptoms, personal history, and family history, were collected during the initial clinic visit. Surgical data, preoperative biological data, and electrocardiography results were retrieved from the electronic medical record system of our center. Follow-up data were acquired by outpatient review at 1, 3, 6, and 12 months postoperatively, or by telephone interviews.
Prior to surgery, all patients underwent two-dimensional echocardiographic assessments to confirm the HCM diagnosis, subtype, peak gradient at ventricular outflow tracts, and other intracardiac structural diseases, in accordance with the British Society of Echocardiography practical guidelines [10]. For complex cases requiring detailed preoperative planning, patient-specific three-dimensional (3D) reconstruction and printed models were utilized [11].
All surgeries were performed under general
anesthesia with cardiopulmonary bypass support.
Transesophageal echocardiography was used
to verify the BHCM diagnosis, evaluate hemodynamic parameters, and identify any
other structural anomalies. Surgical approaches and the extent of resection were
tailored for each patient based on preoperative imaging and intraoperative
exploration. Extended septal myectomy in the left ventricle was performed for an
LVOT peak gradient
In the first place, the extended septal myectomy was applied to relieve the LVOT obstruction. The incision started 3–5 mm to the right of the nadir of the right aortic sinus, extended leftward to the mitral anterior commissure, and downward to the apex of the left ventricle. Aberrant muscle bundles and papillary muscles were partially excised until the apex was visible through the incision. To relieve RVOT obstruction, a 3–4 cm longitudinal ventriculotomy incision was made 5–8 mm below the subpulmonary artery, away from the left anterior descending coronary artery. The IVS and right ventricle were exposed using two retractors (Fig. 1). Myocardial fibers and stiffness were detected by finger exploration. The cardiac hypertrophy located at the subpulmonary valve or conus arteriosus level was removed while avoiding damage to the subvalvular tricuspid valve apparatus. The infundibular muscle bundles were resected when they were hypertrophic, causing RVOT obstruction. If necessary, a pericardial patch with continuous 5–0 prolene sutures was used for RVOT enlargement. The resected myocardia were weighed, and the volumes were measured separately (Fig. 2).
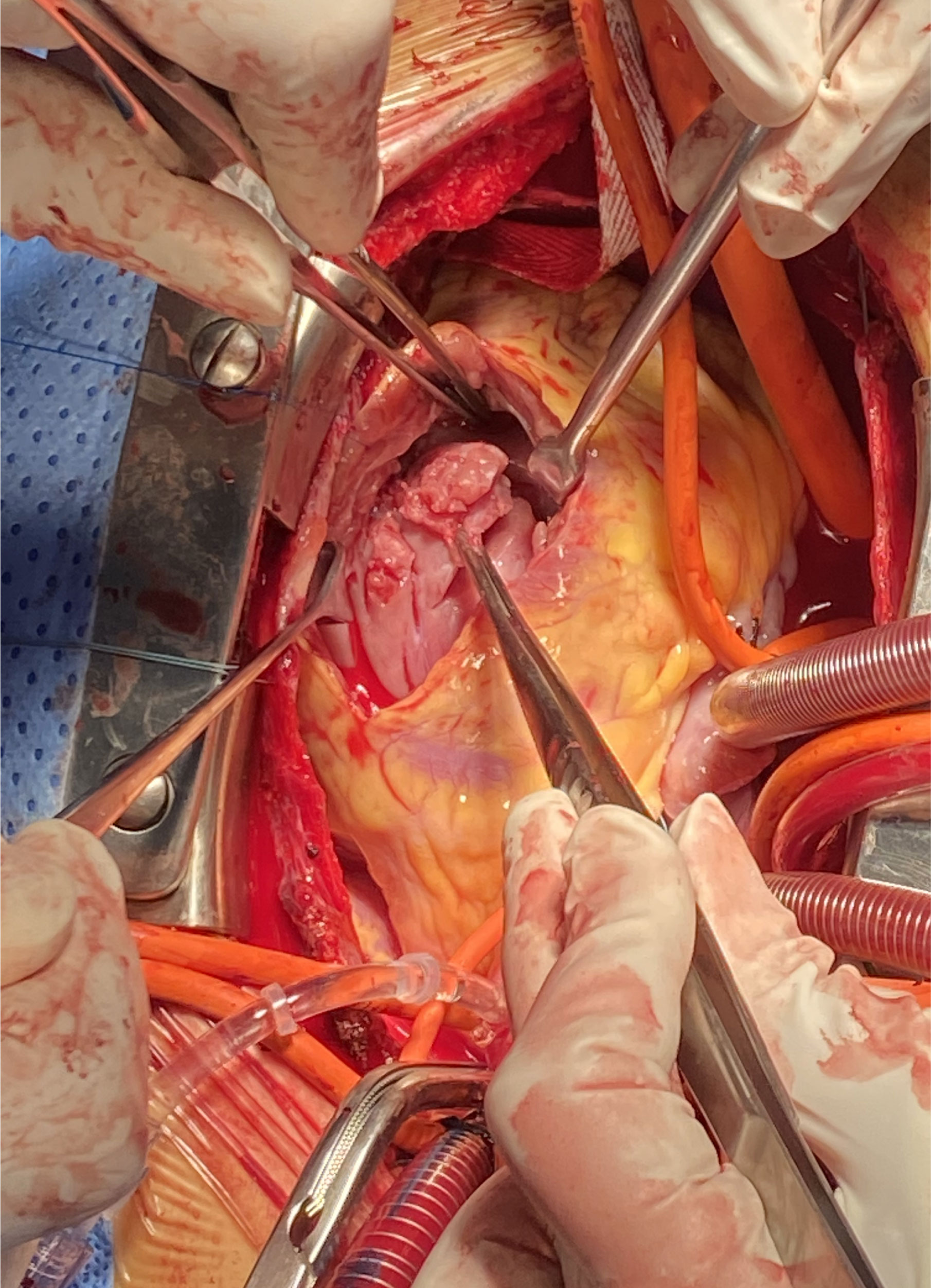 Fig. 1.
Fig. 1.Surgical approach for relief of right ventricular outflow tract obstruction.
 Fig. 2.
Fig. 2.Septum myocardium and muscle bundles resection during the procedure. (a) From left ventricle. (b) From right ventricle.
Continuous variables are presented as
either medians with interquartile ranges or means
The baseline characteristics of the 12
enrolled patients are presented in Table 1. The overall sample had a median age
of 43.0 (interquartile range [IQR]: 14.5–63.0) years at surgery.
Pediatric patients made up a third of the
sample (4, 33.3%, exact ages: 14, 16, 14, and 12 years). Over half of the
patients (7, 58.3%) were female. The average IVS thickness was 21.0 mm (IQR:
20.0–26.3 mm), with 7.0 mm (IQR: 5.8–9.1 mm) of right ventricular free wall. All
patients exhibited systolic anterior motion (SAM), and showed mild-to-moderate
symptoms such as syncope, shortness of breath on exertion, or chest tightness
despite the
maximum
tolerated dose of either
 Fig. 3.
Fig. 3.In complex cases of biventricular hypertrophic obstructive cardiomyopathy, the septum myocardium (*) was resected under the guidance of three-dimensional (3D) printing techniques. (a) An individualized 3D printing model with the myocardium that was expected to be removed (green color). (b) Actual myocardium resection.
| Overall (n = 12) | BVOTO (n = 5) | LVOTO (n = 7) | p-value | ||
| Age, years | 43.0 (14.5, 63.0) | 42.0 |
38.4 |
0.808 | |
| Pediatric patients, n (%) | 4 (100%) | 3 (60.0%) | 1 (14.3%) | 0.222 | |
| Female, n (%) | 7 (58.3%) | 3 (60.0%) | 4 (57.1%) | 1 | |
| Family history of HCM, n (%) | 2 (16.7%) | 1 (20.0%) | 1 (14.3%) | 1 | |
| Previous PTSMA, n (%) | 2 (16.7%) | 1 (20.0%) | 1 (14.3%) | 1 | |
| SCD, % | 3.0 (2.4, 3.8) | 5.3 |
3.0 |
0.183 | |
| Pro-BNP, pg/mL | 2774.2 |
2007.2 |
3322.0 |
0.207 | |
| Cardiac function, n (%) | 1 | ||||
| II | 5 (41.7%) | 2 (40.0%) | 3 (42.9%) | ||
| III | 6 (50.0%) | 3 (60.0%) | 3 (42.9%) | ||
| IV | 1 (8.3%) | 0 | 1 (14.3%) | ||
| MR Grade |
8 (66.7%) | 4 (80.0%) | 4 (57.1%) | 0.576 | |
| IVS, mm | 21.0 (20.0, 26.3) | 20.0 (16.5, 25.5) | 22.0 (20.0, 31.5) | 0.321 | |
| LVPW thickness, mm | 15.1 |
12.0 |
17.3 |
0.008 | |
| RVW thickness, mm | 7.0 (5.8, 9.1) | 6.2 (5.9, 7.2) | 7.1 (5.6, 9.7) | 0.328 | |
| LVOT gradient, mmHg | 85.2 |
94.6 |
78.4 |
0.458 | |
| RVOT gradient, mmHg | - | 37.6 |
- | - | |
| SAM, n (%) | 12 (100%) | 5 (100%) | 7 (100%) | - | |
| LAD, mm | 41.3 |
43.4 |
39.7 |
0.387 | |
| LVEF, % | 67.0 (65.0, 69.5) | 66.8 |
64.9 |
0.495 | |
| RVEF |
63.6 |
65.6 |
61.3 |
0.332 | |
| RVED volume index |
72.3 |
68.8 |
75.2 |
0.606 | |
| LVED volume index |
95.9 |
83.9 |
106.0 |
0.020 | |
Abbreviations: HCM, hypertrophic cardiomyopathy; PTSMA, percutaneous transluminal septal myocardial ablation; SCD, sudden cardiac death; BNP, brain natriuretic peptide; MR, mitral regurgitation; IVS, interventricular septum; LVPW, left ventricular posterior wall; RVW, right ventricular wall; LVOT, left ventricular outflow tract; RVOT, right ventricular outflow tract; SAM, systolic anterior motion; LVEF, left ventricular ejection fraction; RVEF, right ventricular ejection fraction; LAD, left atrial dimension; RVED, right ventricular end-diastolic; LVED, left ventricular end-diastolic; BVOTO, biventricular outflow tract obstruction; LVOTO, left ventricular outflow tract obstruction.
All patients underwent surgical management
to address left ventricular outflow tract obstruction (LVOTO). Among these, five
(41.7%) patients who also achieved relief from
RVOT obstruction were included in the
biventricular outflow tract obstruction (BVOTO) group. In the specific case of a
14-year-old with a previous ablation procedure and RVOT gradient
In the LVOTO group, the mean weight of the excised myocardium was 9.6
In the BVOTO group, two patients required patch enlargement, and one exhibited
SAM after myectomy, necessitating a resumption of CPB for mitral valve
replacement. The excised myocardial tissue weighed 7.5
During the follow-up period, which
averaged 21.2
While most HCM cases involve hypertrophy of the septum and left ventricle, right ventricular involvement defines a unique HCM phenotype. Previous studies [12, 13] have reported that 15%–30% of HCM patients display BHCM, which includes both left/right ventricular and biventricular obstructions. Similar to its left ventricular counterpart, right ventricular HCM features four obstruction subtypes: outflow tract (most common), inflow tract, mid-ventricle, and apex [14, 15]. Despite its clinical significance, isolated RVOT obstruction is exceedingly rare, and the epidemiology of biventricular obstruction is largely unknown, mostly documented through case reports [16, 17]. In a 10-year study by Quintana E et al. [18] biventricular obstruction was reported in only 0.5% (11/2283) of patients treated surgically. Biventricular obstruction appears more frequently in children and accounts for 18.8% of childhood hypertrophic obstructive cardiomyopathy [19].
While BHCM can be easily detected through echocardiography, it’s crucial to differentiate it from other hypertrophy-causing diseases or phenotypes, such as storage diseases and RASopathies [20]. It requires a complete diagnostic workup, including family history, clinical presentation, laboratory tests, detailed imaging examination, and genetic testing [21]. Establishing a diagnosis of primary BHCM is essential to ensure that medical treatment and myectomy result in a good prognosis.
Right ventricular HCM usually leads to increased ventricular stiffness and decreased ventricular wall compliance, resulting in diastolic dysfunction and impaired right heart function, therefore, BHCM patients are more likely to present with symptoms such as palpitations, fatigue, dyspnea [22]. The histological underpinnings for complications like atrial fibrillation in HCM are atrial enlargement and myocardial fibrosis [23, 24, 25]. RVOT obstruction in BHCM is associated with a higher risk of cardiovascular events and progressive heart failure [26, 27]. Long-term right heart dysfunction can exacerbate the already compromised left heart function in these patients. Therefore, to improve surgical outcomes and patient safety, it is advisable to undertake surgical intervention before the onset of severe heart failure.
The current guidelines have limited recommendations for BHCM management [2, 5].
Treatment is typically individualized,
focusing on symptom relief, reducing complication risk, and preventing SCD [2, 5]. Conventional drug treatment often include
Emerging therapies including mavacamten (MYK461) and aficamten (CK-274) have demonstrated efficacy in reducing outflow tract gradients while enhancing cardiac function and alleviating symptoms [29, 30]. Mavacamten, in particular, acts by inhibiting myosin ATPase activity and inducing a compact myosin head configuration, and has been shown to be safe and well-tolerated for various HCM phenotypes [31]. Although these developments are promising, it’s important to note that there have yet to be studies specifically evaluating the impact of these novel treatments on BHCM.
In our study, all BHCM patients exhibited an LVOTO gradient
While our study underscores the positive outcomes of septal myectomy in selected patients with BHCM, long-term results need to be established. It’s important to note that myectomy did not completely eliminate the risk of SCD. Postoperative complications, such as non-sustained ventricular tachycardia, as observed in our study, carry a less favorable prognosis for both adult and pediatric populations [36, 37]. Consequently, ongoing risk stratification is essential during the follow-up period for BHCM patients.
When surgically addressing RVOT obstruction, the conventional “see the apex” guideline is not applicable. Given that nearly 38.8% of patients experience a left bundle branch block following left ventricle septum myectomy [38], extra caution is warranted during extensive myectomy in the right ventricle’s IVS. Combination of both left and right bundle branch block will contribute to the requirement of permanent pacemaker implantation. Borisov KV [39] suggests that myectomy should start from the right ventricle’s conal part, and avoid the moderator band. This method has shown promise in minimizing changes in ventricular penetration and lowering the risk of complete conduction block.
In our study, we found a strong correlation between RVOT obstruction and IVS hypertrophy, which contributed to the narrowing of the outflow tract. To optimize surgical planning in complex cases, we used 3D printing techniques targeting the bulging part of the hypertrophied septum in the right ventricle (Fig. 4). This preoperative tool allowed surgeons to identify and precisely measure the myocardial tissue slated for resection. Additionally, we utilized Mimics software to estimate the right ventricular cavity size, ensuring adequate myectomy in BHCM cases with significantly narrowed cavities, and providing data functionally similar to magnetic resonance imaging.
 Fig. 4.
Fig. 4.Visualization of the hypertrophic myocardium (*) in right ventricle. (a) In the three-dimensional model. (b) In the actual myocardium resection.
The sterilized 3D printed model served as an intraoperative guide for the myectomy procedure. Post-surgery, we compared the size and volume of the excised myocardium with the predicted measurements from the model. Leveraging the printed model enabled greater precision, minimizing the risk of severe complications such as septal perforation. Additionally, the model helped to identify other anatomical irregularities such as hypertrophied trabeculae, anomalous papillary muscles, or other subvalvular anomalies. This information proves invaluable for clinicians in deciding whether additional procedures are necessary, thereby contributing to more accurate surgical planning and potentially improved patient outcomes.
This study comes with specific limitations. First, due to the rarity of BHCM, this single-center study had a limited sample size. Second, the retrospective nature of this research introduces potential biases, especially as some data—like cardiac magnetic resonance parameters—were not uniformly documented across all patients, potentially leading to a statistical bias. Finally, while our follow-up shows promising early outcomes, long-term adverse events, including late mortality, remain unknown. Future research with a larger sample size and extended follow-up would provide more robust conclusions.
In our cohort study, we successfully and safely alleviated BVOTO by employing septal myectomy through transaortic and pulmonary valve approaches in selected patients. These positive results reaffirm the modified septal myectomy as the gold standard in BHCM treatment, leading to symptom relief and enhanced quality of life. For complex cases, we recommend the use of 3D printing technology to guide surgical decisions and enhance surgical safety.
The original contributions presented in this study are included in this article, further inquiries can be directed to the corresponding authors with appropriate reasons.
TT, WZ, JRM, and JMC contributed to study concept and design; BQF, XDZ, RBW, and XYL were contributed to the acquisition of data; JL, JZ, WZ, and HMG were responsible for clinical diagnosis, surgery, patient follow-up and data interpretation; the first draft of the manuscript was finished by TT, WZ, and JRM; JL, BQF, XDZ, RBW, XYL, JMC, JZ, and HMG were involved in critical revision of the content. All authors have read and agreed to the published version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (KY-N-2021-035). The written informed consent to participate in this study were obtained from patients/participants.
Not applicable.
This study was funded by the Science and Technology Planning Project of Guangdong Province (2020B1111170011), Science and Technology Program of Guangzhou (202201010768), Cardiovascular Special Project of Guangdong Provincial People’s Hospital (2020XXG010), National Natural Science Foundation of Guangdong (2022A1515010157), Clinical High-Tech and Major Technologies of Guangzhou (2023P-ZD09), and Guangdong special funds for Science and Technology Innovation Strategy, China (Stability support for scientific research institutions affiliated to Guangdong Province-GDCI 2021).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
