- Academic Editor
Background: Rheumatoid arthritis (RA) is a systemic and chronic autoimmune disease that is characterized by persistent joint inflammation. RA patients experience a considerably increased risk of cardiovascular-related morbidity and mortality. The current study investigated the association between triglyceride glucose (TyG) index and major adverse cardiovascular events (MACEs) in a predominantly male cohort of RA patients. Methods: A total of 1613 RA patients (81.53% male) were selected from the Kailuan study. The TyG index was calculated as the logarithmic product of fasting blood triglyceride and fasting blood glucose divided by two. MACEs were defined as the composite of non-fatal myocardial infarctions and non-fatal strokes. Cox proportional hazards analysis was performed to study the association between the TyG index and MACEs. Results: A total of 59 MACEs occurred during the median follow-up time of 5.32 years. Following adjustment for age and gender, analysis by multivariable Cox proportional hazards (model 1) showed that an elevated TyG index was associated with an increased risk of MACEs (quartile 2, hazard ratio (HR): 2.741, 95% confidence interval (CI): 1.220–6.157, p = 0.015; quartile 4, HR: 2.521, 95% CI: 1.074–5.917, p = 0.034). After adjustment for other variables, Cox proportional hazards analysis (model 2) showed that an elevated TyG index was independently associated with an increased risk of MACEs (quartile 2, HR: 2.348, 95% CI: 1.009–5.465, p = 0.048). In addition, subgroup analysis showed a higher TyG index was significantly linked to an increased risk of MACEs in patients aged more than 65 years (quartile 2, HR: 6.048, 95% CI: 1.311–27.908, p = 0.021; quartile 4, HR: 12.074, 95% CI: 1.438–101.358, p = 0.022). Conclusions: The TyG index was associated with an increased risk of MACEs in a predominantly male cohort of RA patients. This index may be helpful for the prediction of MACEs in male patients with RA. Clinical Trial Registration: Registration number in the Chinese clinical trial registry: ChiCTR-TNRC-11001489.
Rheumatoid arthritis (RA) is a chronic, inflammatory, and disabling disease that affects about 18 million people worldwide [1]. The major consequence of RA is work disability [2], with a rate being relatively high at 20% to 35% after 5 years of the disease course [3, 4, 5]. Furthermore, RA patients have a 1.5-fold higher mortality rate than the general population [6]. The most frequently reported cause of death in RA patients is cardiovascular disease (CVD) [7, 8]. Indeed, RA is closely associated with a substantially increased risk of CVD, largely due to the presence of atherosclerosis (AS) [9], which is the thickening or hardening of the arteries due to the plaque accumulation in the inner arterial lining. In addition to traditional cardiovascular risk factors, chronic inflammation may also be regarded as an independent risk factor for AS in RA patients [10].
Recently, a large body of evidence supports the notion that insulin resistance (IR) is critical in both RA and CVD [11, 12]. Indeed, the triglyceride glucose (TyG) index may be considered a surrogate marker for IR [13]. The systemic inflammation associated with RA contributes to the development of abnormal lipid and glucose metabolisms [14, 15]. Recent studies have also shown that the TyG index is a strong and useful predictor of cardiovascular outcomes in various disease settings [16, 17]. However, investigations into potential associations between the TyG index and cardiovascular outcomes in RA patients are still lacking, especially in males. Hence, the current study aimed to investigate whether the TyG index could be used to predict major adverse cardiovascular events (MACEs) in a predominantly male cohort of RA patients.
A total of 1702 patients diagnosed with RA during 2014–2016 were initially extracted from 118,500 participants in the Kailuan study database. Of these, 89 patients with histories of myocardial infarction (MI) (n = 21), stroke (n = 38), and cancer (n = 16), or with missing data (n = 14) were excluded, leaving 1613 RA patients that were included in the current study (Fig. 1).
 Fig. 1.
Fig. 1.Flowchart of this study. RA, rheumatoid arthritis; MI, myocardial infarction; TyG index, triglyceride glucose index.
Fasting blood tests were performed for all patients, with all samples being
processed in the central laboratory of the Kailuan General Hospital. The TyG
index was calculated: ln (fasting triglycerides (mg/dL)
All patients were followed until December 2020, with a median of 5.3 years. The study endpoint was defined as the occurrence of a MACE, including MI, ischemic stroke, and hemorrhagic stroke.
Categorical variables were presented as proportions, and continuous variables as
mean
Table 1 shows the baseline characteristics for the 1613 RA patients included in
this study. The mean age of the patients was 55.41
| Variables | Total (n = 1613) | Quartile 1 (n = 403) | Quartile 2 (n = 403) | Quartile 3 (n = 404) | Quartile 4 (n = 403) | p value |
| Age, years | 55.41 |
54.17 |
55.59 |
56.54 |
55.33 |
0.125 |
| Male, n (%) | 1315 (81.53) | 336 (83.37) | 323 (80.15) | 321 (79.46) | 335 (83.13) | 0.516 |
| BMI, kg/m |
25.29 |
23.93 |
24.86 |
26.06 |
26.31 |
|
| SBP, mmHg | 137.40 |
130.40 |
136.36 |
140.58 |
142.23 |
|
| DBP, mmHg | 81.80 |
78.73 |
80.93 |
82.95 |
84.60 |
|
| FBG, mmol/L | 6.00 |
5.20 |
5.59 |
6.13 |
7.09 |
|
| TC, mmol/L | 5.01 |
4.80 |
5.12 |
5.29 |
4.82 |
|
| TG, mmol/L | 1.36 (0.90–2.09) | 0.72 (0.60–0.85) | 1.13 (1.00–1.29) | 1.65 (1.45–1.89) | 3.37 (2.50–4.56) | |
| HDL-C, mmol/L | 1.37 |
1.49 |
1.38 |
1.31 |
1.30 |
|
| LDL-C, mmol/L | 2.91 |
2.71 |
2.93 |
3.04 |
2.97 |
|
| hs-CRP, mg/L | 1.25 (0.40–2.70) | 0.91 (0.34–2.25) | 1.24 (0.40–2.30) | 1.45 (0.63–2.85) | 1.30 (0.40–2.90) | 0.011 |
| TyG index | 8.81 |
7.95 |
8.51 |
8.96 |
9.81 |
|
| Hypertension, n (%) | 1015 (62.93) | 194 (48.14) | 237 (58.81) | 284 (70.30) | 300 (74.44) | |
| Diabetes, n (%) | 294 (18.23) | 32 (7.94) | 42 (10.42) | 84 (20.79) | 136 (33.75) | |
| Snoring, n (%) | 615 (38.13) | 128 (31.76) | 162 (40.20) | 173 (42.82) | 152 (37.72) | 0.022 |
| Smoking, n (%) | 753 (46.68) | 187 (46.40) | 178 (44.17) | 182 (45.05) | 206 (51.12) | 0.325 |
| Drinking, n (%) | 482 (29.88) | 104 (25.81) | 129 (32.01) | 113 (27.97) | 136 (33.75) | 0.106 |
TyG index, triglyceride glucose index; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein.
Patients in quartile 4 were more likely to have higher body mass index, systolic blood pressure, and diastolic blood pressure, alongside higher levels of fasting blood glucose and triglyceride. Quartile 4 patients also had higher incidences of hypertension and diabetes.
A total of 59 MACEs were documented during the median follow-up period of 5.32 years. A total of 8 MACEs occurred in quartile 1, 22 occurred in quartile 2, 13 in quartile 3, and 16 in quartile 4. As shown in Fig. 2, patients in quartile 2 had the highest cumulative incidence of MACEs, although a significant difference was not reached (quartile 1: 2.48%, quartile 2: 6.63%, quartile 3: 3.96%, quartile 4: 4.05%, p = 0.060).
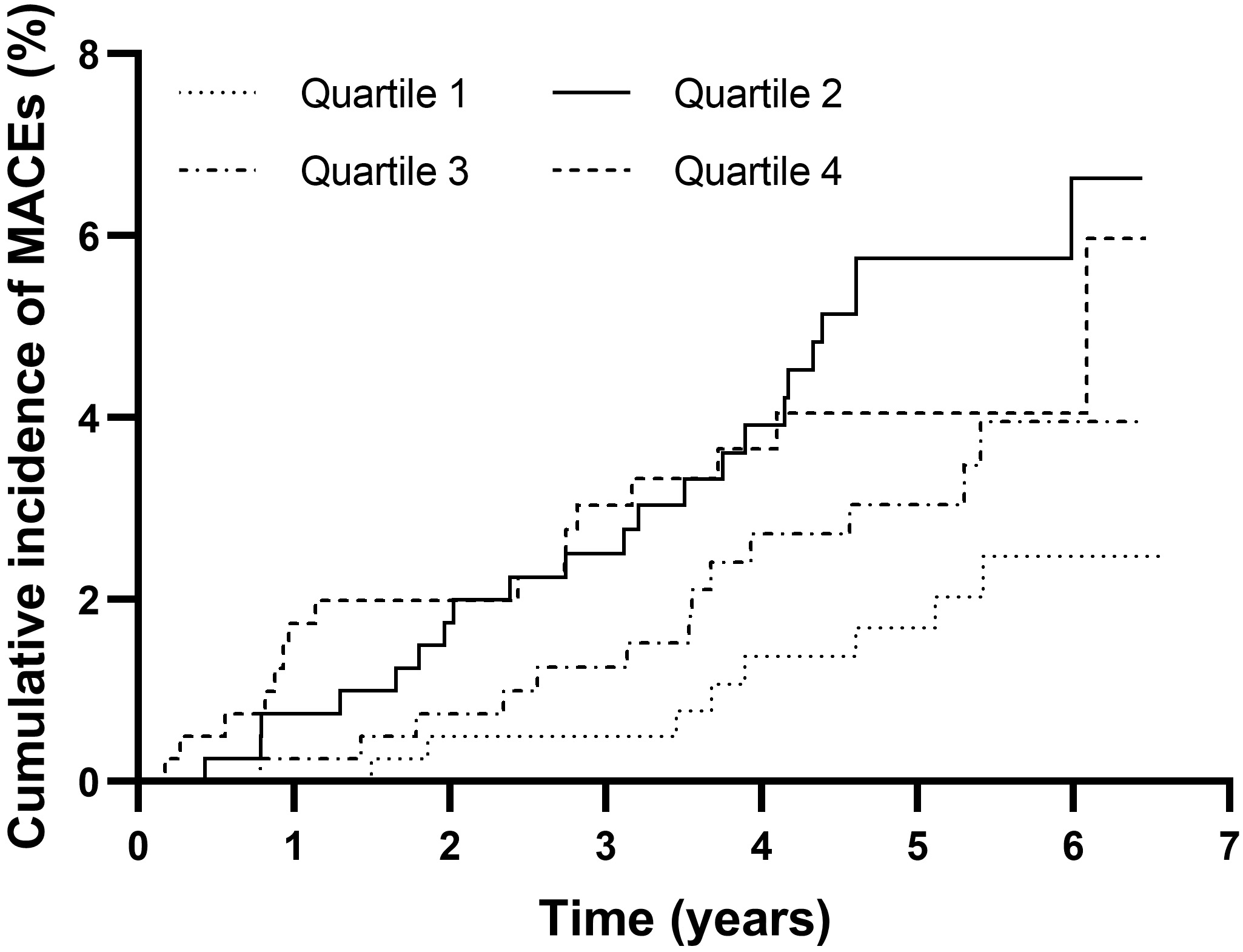 Fig. 2.
Fig. 2.Cumulative incidence of MACEs in the TyG index quartile groups. MACEs, major adverse cardiovascular events; TyG index, triglyceride glucose index.
As shown in Table 2, multivariate Cox proportional hazards regression analysis
(model 1) revealed that a higher TyG index was significantly associated with an
increased risk of MACEs (quartile 2, hazard ratio (HR): 2.741, 95% confidence
interval (CI): 1.220–6.157, p = 0.015; quartile 4, HR: 2.521, 95% CI:
1.074–5.917, p = 0.034). Quartile 3 in the TyG index was also
positively associated with an increased risk of MACEs, although this did not
reach statistical significance (HR: 1.645, 95% CI: 0.681–3.972, p =
0.268). Age was significantly associated with MACEs (HR: 1.066, 95% CI:
1.043–1.090, p
| SE | p value | HR | 95% CI | ||||
| Adjusted model 1 | |||||||
| Quartile 2 | 1.008 | 0.413 | 5.961 | 0.015 | 2.741 | 1.220–6.157 | |
| Quartile 3 | 0.498 | 0.450 | 1.226 | 0.268 | 1.645 | 0.681–3.972 | |
| Quartile 4 | 0.925 | 0.435 | 4.515 | 0.034 | 2.521 | 1.074–5.917 | |
| Age | 0.064 | 0.011 | 32.809 | 1.066 | 1.043–1.090 | ||
| Gender (male) | 0.431 | 0.363 | 1.414 | 0.234 | 1.539 | 0.756–3.132 | |
| Adjusted model 2 | |||||||
| Quartile 2 | 0.854 | 0.431 | 3.922 | 0.048 | 2.348 | 1.009–5.465 | |
| Quartile 3 | 0.285 | 0.510 | 0.313 | 0.576 | 1.330 | 0.489–3.617 | |
| Quartile 4 | 0.961 | 0.677 | 2.017 | 0.156 | 2.614 | 0.694–9.843 | |
| Age | 0.070 | 0.015 | 23.191 | 1.073 | 1.043–1.104 | ||
| Gender (male) | 0.102 | 0.417 | 0.060 | 0.807 | 1.107 | 0.489–2.506 | |
| BMI | 0.032 | 0.044 | 0.527 | 0.468 | 1.033 | 0.947–1.126 | |
| SBP | 0.000 | 0.008 | 0.000 | 0.991 | 1.000 | 0.984–1.016 | |
| DBP | 0.012 | 0.015 | 0.660 | 0.417 | 1.012 | 0.983–1.041 | |
| FBG | 0.118 | 0.075 | 2.454 | 0.117 | 1.126 | 0.971–1.305 | |
| TC | 0.092 | 0.128 | 0.524 | 0.469 | 1.097 | 0.854–1.408 | |
| TG | –0.201 | 0.176 | 1.306 | 0.253 | 0.818 | 0.579–1.155 | |
| HDL-C | –0.456 | 0.470 | 0.942 | 0.332 | 0.634 | 0.252–1.592 | |
| LDL-C | 0.058 | 0.218 | 0.072 | 0.789 | 1.060 | 0.692–1.624 | |
| hs-CRP | 0.012 | 0.019 | 0.401 | 0.527 | 1.012 | 0.975–1.050 | |
| Hypertension | 0.328 | 0.373 | 0.775 | 0.379 | 1.388 | 0.669–2.883 | |
| Diabetes | –0.413 | 0.408 | 1.023 | 0.312 | 0.662 | 0.298–1.472 | |
| Snoring | 0.128 | 0.274 | 0.218 | 0.640 | 1.137 | 0.664–1.947 | |
| Smoking | 0.378 | 0.291 | 1.693 | 0.193 | 1.459 | 0.826–2.579 | |
| Drinking | 0.442 | 0.306 | 2.094 | 0.148 | 1.557 | 0.855–2.834 | |
Note: Model 1 was adjusted for age and gender (male). Model 2 was adjusted for age and gender (male), body mass index, systolic blood pressure, diastolic blood pressure, fasting blood glucose, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, high-sensitivity C-reactive protein, hypertension, diabetes, snoring, smoking, and drinking. MACEs, major adverse cardiovascular events; TyG index, triglyceride glucose index; SE, standard error; HR; hazard ratio; CI, confidence interval; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein.
| Subgroup | Total (event) | p value | HR | 95% CI | p for interaction | ||
| Age | 0.227 | ||||||
| Quartile 1 | 91 (6) | - | - | - | |||
| Quartile 2 | 96 (9) | 0.021 | 6.048 | 1.311–27.908 | |||
| Quartile 3 | 89 (4) | 0.076 | 4.530 | 0.853–24.066 | |||
| Quartile 4 | 86 (6) | 0.022 | 12.074 | 1.438–101.358 | |||
| Quartile 1 | 312 (2) | - | - | - | |||
| Quartile 2 | 307 (13) | 0.848 | 0.891 | 0.273–2.911 | |||
| Quartile 3 | 315 (9) | 0.316 | 0.420 | 0.077–2.288 | |||
| Quartile 4 | 317 (10) | 0.763 | 0.672 | 0.051–8.874 | |||
TyG index, triglyceride glucose index; HR; hazard ratio; CI, confidence interval.
As shown in Table 3, further subgroup analysis showed that a higher TyG index
was significantly associated with the risk of MACEs in elderly patients
(
Numerous studies have shown that RA patients are at greater risk of CVD compared with the general population [18, 19]. The excess risk is due to RA-specific risk-factor profiles, such as systemic inflammation, rather than traditional CVD risk factors [20]. Furthermore, RA can be regarded as an independent risk factor for CVD [21]. Together with risk-based CVD management, the use of anti-rheumatic medication is fundamental procedure for reducing the incidence of CVD [22]. Still, it has been found that RA is associated with an increased risk of CVD [23]. Solomon et al. [24] reported that female RA patients had a significantly higher risk of MI compared to those without RA. Specifically, for women with a medical history of RA of at least 10 years, the adjusted relative risk (RR) was 3.10, and for those with a medical history of RA less than 10 years, the RR was 1.16 [24]. Fischer et al. [25] also reported an increased risk of acute MI in patients with RA, with an adjusted odds ratio (OR) of 1.47. Södergren et al. [26] found that RA patients had higher morbidities from acute MI than the general population, with all-cause mortality showing an HR of 1.67. Holmqvist et al. [27] reported a rapid increase in the risk of MI within 1–4 years after RA diagnosis.
Similarly, both the morbidity and mortality from stroke were found to be higher in RA patients than in the general population [28]. Trömmer et al. [29] reported that RA was associated with both stroke (HR = 1.42) and transient ischemic attack (TIA) (HR = 1.69). Furthermore, patients aged 18–40 years showed the highest risk group for stroke (HR = 3.45) [29]. However, Holmqvist et al. [27, 30] reported that the progress of ischemic stroke in RA patients was slower than for ischemic heart disease. Moreover, Tiosano et al. [31] reported that RA was independently associated with stroke (OR = 1.18), particularly among young adults aged less than 65 years (OR = 1.79). Meissner et al. [32] reported that adverse events, particularly serious infections, and inadequate CVD treatment increased the risk of stroke in RA patients.
Insulin resistance can be defined as a pathological condition in which normal insulin concentrations induce subnormal biological effects [33]. Thus, the TyG index is more cost-effective and readily available for the direct measurement of IR compared to the gold standard, which is the hyperinsulinemic-euglycemic clamp. Therefore, the TyG index can be considered a surrogate marker for IR [34]. Furthermore, an increasing body of evidence suggests that the TyG index can be used to predict MACEs in different disease settings [35, 36, 37, 38].
As far as we are aware, this is the first study to investigate the TyG index in
relation to MACEs in a predominantly male cohort of RA patients. We found that a
higher TyG quartile (quartile 2: 8.28
Since the Kailuan group is a company in the coal industry, considerably more males than females were employed, meaning that over 80% of the RA patients in this study were males. Our results are in contrast with the 3-fold higher incidence of RA found in women in the general population compared to men. Therefore, it is unclear whether the current findings are applicable to a more general RA population. On the one hand, CVD has long been regarded as a “male” disease [39], since males have an inherently greater risk for MACEs occurring than females. Research on gender differences in CVD indicates that males may not cope with stress as well as females, either physiologically, behaviorally, or emotionally, thereby increasing their risk of MACEs occurring. Stress-related factors can cause direct effects by influencing physiological parameters associated with the risk of CVD, as well as asserting indirect effects by increasing unhealthy behavioral and emotional responses associated with CVD (e.g., smoking, alcohol consumption, and depression) [40]. Alternatively, RA is known to affect about two to three times more women than men. Sex hormones play a critical role in regulating the immune response and are therefore strongly associated with the etiology of RA [41]. Presently, there has been a lack of research regarding cardiovascular risk factors for RA in men. Indeed, as far as we are aware, this is the first study to investigate the TyG index in relation to MACEs in a predominantly male cohort of RA patients. However, further research is needed to confirm whether the TyG index is a predictor of MACEs in RA patients.
In summary, our study found that the TyG index was associated with an increased risk of MACEs in a predominantly male cohort of RA patients. These results suggest that the TyG index is a potentially useful predictor of MACEs in predominantly male patients with RA. The pathological mechanism of IR and its effect on cardiovascular outcomes in RA patients warrants further investigation.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
QLH, SLW, and KBL designed the research study. JWZ, QQH, QLH, XP, HXC, SLW, and KBL participated in the acquisition, analysis, and interpretation of data for the work. JWZ, QQH, QLH, XP, HXC, SLW, and KBL contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
All procedures performed in the Kailuan study involving human participants were in accordance with the standards of the ethics committee of Kailuan Hospital (Approval number: [2006] yilunzi 5). A written informed consent was obtained from each patient.
Not applicable.
This work was supported by the Key Scientific Research Project (No. 20231775), Health Commission of Hebei Province, P.R. China.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
