- Academic Editor
†These authors contributed equally.
Background: Gender is a well-recognized risk factor in atrial
fibrillation (AF)-related ischemic stroke. The association of gender with the use
of oral anticoagulants (OACs) and prognosis remains unknown. Methods:
The National Health Insurance Research Database in Taiwan identified 203,775
patients with AF aged
Atrial fibrillation (AF) is the most prevalent sustained cardiac arrhythmia, and
it increases risks of ischemic stroke, intracranial hemorrhage (ICH), and
mortality [1, 2, 3]. Sex differences in terms of clinical presentations, risk
profile, response to treatment, and prognosis are observed in patients with AF
[4]. In particular, women with AF frequently experience more symptomatic AF
episodes, have worse quality of life, more drug-related arrhythmias, and are less
likely to take oral anticoagulants (OACs) [5, 6, 7, 8, 9]. Among patients undergoing
catheter ablation for AF, female sex was independently associated with a higher
risk of adverse events [10] and more frequent AF recurrences [11]. Further, a
higher mortality rate is observed in female patients with AF. Furthermore, the
female sex is considered as a disease modifier for AF-related ischemic stroke and
contributes one point in the CHA
The present study used the “National Health Insurance Research Database
(NHIRD)” released by the Taiwan National Health Research Institutes. The
National Health Insurance (NHI) system is a mandatory universal health insurance
program that provides comprehensive medical care coverage to all Taiwanese
residents. The NHIRD consists of detailed healthcare data from
The study protocol is similar to our previous studies which have been published
[19, 20]. Patients aged
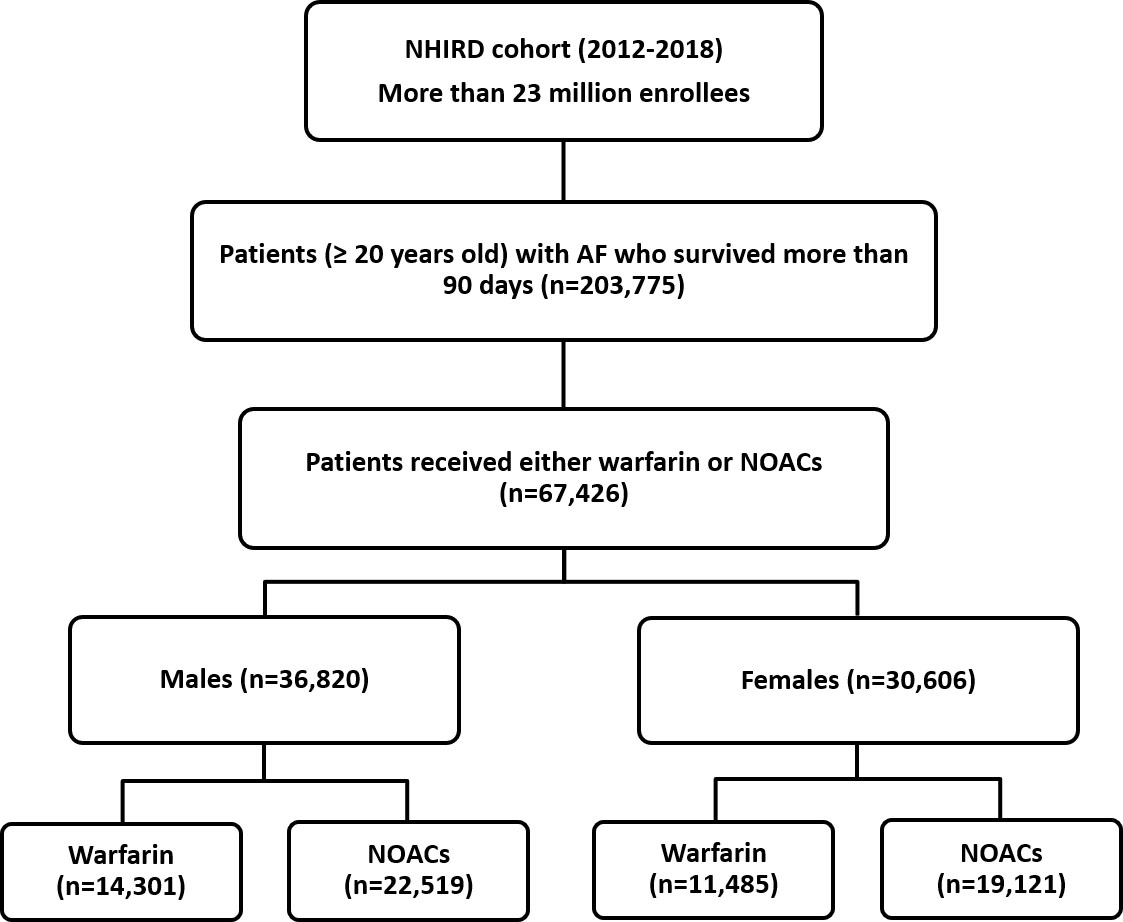 Fig. 1.
Fig. 1.Patient enrollment flowchart. A total of 203,775 patients aged
The International Classification of Diseases, Ninth Revision, Clinical
Modification (ICD-9-CM) codes were used to confirm the diagnosis. We defined
patients with a certain disease only when it was a discharge diagnosis or
confirmed more than twice in the outpatient department to ensure the accuracy of
diagnosis [19, 20, 21]. The CHA
The clinical endpoints included the occurrence of death, ischemic stroke, intracerebral hemorrhage (ICH), major bleeding, and composite adverse events (death or ischemic stroke or ICH or major bleeding). Ischemic stroke and ICH were diagnosed with concomitant brain imaging studies, including computed tomography or magnetic resonance imaging. Major bleeding was ICH or bleeding originating from the gastrointestinal, genitourinary, or respiratory tract that requires hospitalization. Each endpoint was independently analyzed of the others without being censored. The accuracy of diagnosis of ischemic stroke in Taiwan’s NHIRD was approximately 94% [27]. Another validation study demonstrated that the diagnostic accuracy of ischemic stroke in NHIRD was high, with a positive predictive value and sensitivity of 88.4% and 97.3%, respectively [28].
Data were presented as the mean value and standard deviation for normally
distributed continuous variables and proportions for categorical variables. The
unpaired two-tailed t-test was used to assess differences between
continuous values. Nominal variables were compared by chi-square test or Fisher’s
exact test. Incidence rates of events were calculated by dividing the number of
events by person-year at risk. The Kaplan-Meier method was used to plot the
cumulative incidence of clinical events with statistical significance examined by
the log-rank test. Multivariate Cox proportional hazards models were used for
risk prediction adjusting for significant baseline variables. All statistical
significances were set at a p-value of
Our study population consisted of 203,775 patients aged
| Variables | All | Males | Females | p value | |
| (n = 203,775) | (n =112,836) | (n = 90,939) | |||
| Age, years; mean value (SD) | 72.76 (13.52) | 70.7 (13.94) | 75.3 (12.53) | ||
| Age |
102,641 (50.37) | 49,041 (43.46) | 53,600 (58.94) | ||
| Age 65–74 years, n (%) | 48,290 (23.7) | 27,961 (24.78) | 20,329 (22.35) | ||
| CHADS |
2.49 (1.56) | 2.33 (1.54) | 2.69 (1.56) | ||
| CHA |
3.8 (2.00 | 3.15 (1.87) | 4.62 (1.84) | ||
| CHA |
3.36 (1.87) | 3.15 (1.87) | 3.62 (1.84) | ||
| HAS-BLED score | 2.91 (1.43) | 2.85 (1.47) | 2.98 (1.37) | ||
| Comorbidities, n (%) | |||||
| Congestive heart failure | 73,989 (36.31) | 37,600 (33.32) | 36,389 (40.01) | ||
| Hypertension | 158,518 (77.79) | 84,836 (75.19) | 73,682 (81.02) | ||
| Diabetes mellitus | 73,302 (35.97) | 38,349 (33.99) | 34,953 (38.44) | ||
| Previous stroke/TIA | 49,939 (24.51) | 26,779 (23.73) | 23,160 (25.47) | ||
| Vascular diseases | 24,759 (12.15) | 14,596 (12.94) | 10,163 (11.18) | ||
| COPD | 54,270 (26.63) | 34,114 (30.23) | 20,156 (22.16) | ||
| Hyperlipidemia | 94,780 (46.51) | 49,339 (43.73) | 45,441 (49.97) | ||
| Autoimmune diseases | 13,017 (6.39) | 4831 (4.28) | 8186 (9) | ||
| Cancer | 26,249 (12.88) | 15,366 (13.62) | 10,883 (11.97) | ||
| Abnormal renal function | 42,276 (20.75) | 23,574 (20.89) | 18,702 (20.57) | 0.0703 | |
| Abnormal liver function | 41,364 (20.3) | 23,964 (21.24) | 17,400 (19.13) | ||
| Anemia | 31,954 (15.68) | 14,207 (12.59) | 17,747 (19.52) | ||
| History of bleeding | 58,866 (28.89) | 32,331 (28.65) | 26,535 (29.18) | 0.0093 | |
| Alcohol excess/abuse, n (%) | 3677 (1.8) | 3271 (2.9) | 406 (0.45) | ||
| Use of NSAIDs, n (%) | 9316 (4.57) | 5109 (4.53) | 4207 (4.63) | 0.2911 | |
| Use of anti-platelet drugs, n (%) | 83,559 (41.01) | 49,168 (43.57) | 34,391 (37.82) | ||
| Aspirin | 64,326 (31.57) | 38,792 (34.38) | 25,534 (28.08) | ||
| Clopidogrel | 19,463 (9.55) | 11,834 (10.49) | 7629 (8.39) | ||
| Dipyridamole | 7830 (3.84) | 4250 (3.77) | 3580 (3.94) | 0.0475 | |
| Ticlopidine | 3368 (1.65) | 1746 (1.55) | 1622 (1.78) | ||
| Anticoagulant | |||||
| Warfarin | 27,971 (13.73) | 15,504 (13.74) | 12,467 (13.71) | 0.8392 | |
| NOACs | 43,825 (21.51) | 23,722 (21.02) | 20,103 (22.11) | ||
| Rate-control agents | |||||
| Beta-blockers | 88,936 (43.64) | 47,562 (42.15) | 12,014 (13.21) | ||
| CCBs | 25,487 (12.51) | 13,473 (11.94) | 12,017 (13.21) | ||
| Digoxin | 25,801 (12.66) | 13,784 (12.22) | 12,014 (13.21) | ||
| Rhythm-control agents | |||||
| Amiodarone | 42,467 (20.84) | 23,543 (20.86) | 18,924 (20.81) | 0.7602 | |
| Dronedarone | 4469 (2.19) | 2123 (1.88) | 2346 (2.58) | ||
| Propafenone | 18,088 (8.88) | 9757 (8.65) | 8331 (9.16) | ||
| Flecainide | 900 (0.44) | 501 (0.44) | 399 (0.44) | 0.8589 | |
| Sotalol | 368 (0.18) | 207 (0.18) | 161 (0.18) | 0.7343 | |
| ACEIs/ARBs | 88,257 (43.31) | 47,935 (42.48) | 40,322 (44.34) | ||
| Statins | 38,455 (18.87) | 21,131 (18.73) | 17,324 (19.05) | 0.0642 | |
ACEIs/ARBs, angiotensin converting enzyme inhibitors/angiotensin receptor blockers; CCBs, calcium channel blockers; COPD, chronic obstructive pulmonary disease; NOACs, non-vitamin K antagonist oral anticoagulants; AF, atrial fibrillation; NSAIDs, non-steroidal anti-inflammatory drugs; SD, standard deviation; TIA, transient ischemic attack.
Among all patients with AF, 36,820 males and 30,606 females taking OACs were
further analyzed (Table 2). The distribution of baseline characteristics between
warfarin and NOAC users was very similar between the sexes. Both male and female
patients taking NOAC were older and had more underlying comorbidities except HF,
abnormal renal function, anemia, and a history of bleeding compared to those
using warfarin. NOAC users demonstrated higher CHA
| Variables | All (n = 67,426) | p value | Males (n = 36,820) | p value | Females (n = 30,606) | p value | ||||
| Warfarin | NOACs | Warfarin | NOACs | Warfarin | NOACs | |||||
| (n = 25,786) | (n = 41,640) | (n =14,301) | (n = 22,519) | (n = 11,485) | (n = 19,121) | |||||
| Age, years; mean value (SD) | 70.7 (13.94) | 75.86 (10.65) | 68.22 (12.92) | 74.00 (11.24) | 72.35 (12.15) | 78.05 (9.46) | ||||
| Age |
10,332 (40.07) | 24,673 (59.25) | 4832 (33.79) | 11,632 (51.65) | 5500 (47.89) | 13,041 (68.2) | ||||
| Age 65–74 years, n (%) | 6821 (26.45) | 11,449 (27.5) | 0.003 | 3837 (26.83) | 6788 (30.14) | 2984 (25.98) | 4661 (24.38) | 0.0018 | ||
| Sex (male), n (%) | 14,301 (55.46) | 22,519 (54.08) | 0.0005 | - | - | - | - | - | - | |
| CHADS |
2.33 (1.54) | 2.76 (1.43) | 2.34 (1.50) | 2.62 (1.42) | 2.61 (1.56) | 2.93 (1.42) | ||||
| CHA |
3.15 (1.87) | 4.21 (1.75) | 3.06 (1.83) | 3.56 (1.63) | 4.45 (1.85) | 4.96 (1.57) | ||||
| CHA |
3.15 (1.87) | 3.75 (1.62) | 3.06 (1.83) | 3.56 (1.63) | 3.45 (1.85) | 3.96 (1.57) | ||||
| HAS-BLED score | 2.85 (1.47) | 2.89 (1.27) | 2.58 (1.46) | 2.86 (1.33) | 2.66 (1.39) | 2.93 (1.19) | ||||
| Comorbidities, n (%) | ||||||||||
| Congestive heart failure | 11,309 (43.86) | 15,705 (37.72) | 5865 (41.01) | 7942 (35.27) | 5444 (47.4) | 7763 (40.6) | ||||
| Hypertension | 19,359 (75.08) | 34,814 (83.61) | 10,596 (74.09) | 18,256 (81.07) | 8763 (76.3) | 16,558 (86.6) | ||||
| Diabetes mellitus | 8827 (34.23) | 16,003 (38.43) | 4728 (33.06) | 8307 (36.89) | 4099 (35.69) | 7696 (40.25) | ||||
| Previous stroke/TIA | 6844 (26.54) | 11,906 (28.59) | 3743 (26.17) | 6415 (28.49) | 3101 (27) | 5491 (28.72) | 0.0011 | |||
| Vascular diseases | 2758 (10.7) | 4884 (11.73) | 1648 (11.52) | 2856 (12.68) | 0.0008 | 1110 (9.66) | 2028 (10.61) | 0.0079 | ||
| COPD | 5791 (22.46) | 10,693 (25.68) | 3565 (24.93) | 6647 (29.52) | 2226 (19.38) | 4046 (21.16) | 0.0002 | |||
| Hyperlipidemia | 11,761 (45.61) | 22,495 (54.02) | 6221 (43.5) | 11,562 (51.34) | 5540 (48.24) | 10,933 (57.18) | ||||
| Autoimmune diseases | 1492 (5.79) | 2833 (6.8) | 528 (3.69) | 1027 (4.56) | 964 (8.39) | 1806 (9.45) | 0.0017 | |||
| Cancer | 2635 (10.22) | 5387 (12.94) | 1494 (10.45) | 3131 (13.9) | 1141 (9.93) | 2256 (11.8) | ||||
| Abnormal renal function | 5237 (20.31) | 7543 (18.11) | 2971 (20.77) | 4312 (19.15) | 0.0001 | 2266 (19.73) | 3231 (16.9) | |||
| Abnormal liver function | 4785 (18.56) | 8743 (21) | 2807 (19.63) | 4850 (21.54) | 1978 (17.22) | 3893 (20.36) | ||||
| Anemia | 3692 (14.32) | 4825 (11.59) | 1584 (11.08) | 2172 (9.65) | 2108 (18.35) | 2653 (13.87) | ||||
| History of bleeding | 6713 (26.03) | 11,353 (27.26) | 0.0004 | 3583 (25.05) | 6285 (27.91) | 3130 (27.25) | 5068 (26.5) | 0.1534 | ||
| Alcohol excess/abuse, n (%) | 414 (1.61) | 557 (1.34) | 0.0055 | 378 (2.64) | 491 (2.18) | 0.0052 | 36 (0.31) | 66 (0.35) | 0.6371 | |
| Use of NSAIDs, n (%) | 1047 (4.06) | 1647 (3.96) | 0.4999 | 603 (4.22) | 878 (3.9) | 0.1339 | 444 (3.87) | 769 (4.02) | 0.4966 | |
| Use of anti-platelet drugs, n (%) | 6480 (25.13) | 7719 (18.54) | 4003 (27.99) | 4733 (21.02) | 2477 (21.57) | 2986 (15.62) | ||||
| Aspirin | 4726 (18.33) | 5045 (12.12) | 2999 (20.97) | 3094 (13.74) | 1727 (15.04) | 1951 (10.2) | ||||
| Clopidogrel | 1521 (5.9) | 2462 (5.91) | 0.9401 | 957 (6.69) | 1629 (7.23) | 0.0455 | 564 (4.91) | 833 (4.36) | 0.0266 | |
| Dipyridamole | 795 (3.08) | 977 (2.35) | 437 (3.06) | 567 (2.52) | 0.0025 | 358 (3.12) | 410 (2.14) | |||
| Ticlopidine | 285 (1.11) | 193 (0.46) | 166 (1.16) | 111 (0.49) | 119 (1.04) | 82 (0.43) | ||||
| Rate-control agents | ||||||||||
| Beta-blockers | 13,041 (50.57) | 21,695 (52.1) | 0.0001 | 7106 (49.69) | 11,268 (50.04) | 0.514 | 5935 (51.68) | 10,427 (54.53) | ||
| CCBs | 3255 (12.62) | 5688 (13.66) | 0.0001 | 1708 (11.94) | 2846 (12.64) | 0.0471 | 1547 (13.47) | 2842 (14.86) | 0.0007 | |
| Digoxin | 5721 (22.19) | 4828 (11.59) | 2995 (20.94) | 2519 (11.19) | 2726 (23.74) | 2309 (12.08) | ||||
| Rhythm-control agents | ||||||||||
| Amiodarone | 7054 (27.36) | 8970 (21.54) | 3879 (27.12) | 4726 (20.99) | 3175 (27.64) | 4244 (22.2) | ||||
| Dronedarone | 529 (2.05) | 1458 (3.5) | 248 (1.73) | 704 (3.13) | 281 (2.45) | 754 (3.94) | ||||
| Propafenone | 1909 (7.4) | 3959 (9.51) | 1080 (7.55) | 1945 (8.64) | 0.0002 | 829 (7.22) | 2014 (10.53) | |||
| Flecainide | 94 (0.36) | 294 (0.71) | 64 (0.45) | 155 (0.69) | 0.0021 | 30 (0.26) | 139 (0.73) | |||
| Sotalol | 105 (0.41) | 89 (0.21) | 63 (0.44) | 51 (0.23) | 0.0008 | 42 (0.37) | 38 (0.2) | 0.0101 | ||
| ACEIs/ARBs | 12,161 (47.16) | 22,666 (54.43) | 6896 (48.22) | 12,029 (53.42) | 5265 (45.84) | 10,637 (55.63) | ||||
| Statins | 5136 (19.92) | 10,775 (25.88) | 2809 (19.64) | 5963 (26.48) | 2327 (20.26) | 4812 (25.17) | ||||
ACEIs/ARBs, angiotensin converting enzyme inhibitors/angiotensin receptor blockers; CCBs, calcium channel blockers; COPD, chronic obstructive pulmonary disease; NOACs, non-vitamin K antagonist oral anticoagulants; AF, atrial fibrillation; NSAIDs, non-steroidal anti-inflammatory drugs; SD, standard deviation; TIA, transient ischemic attack.
The mean follow-up of 2.89 years reported 12,850 deaths, 3033 ischemic strokes, 874 ICHs, 4125 major bleeding, and 16,750 composite adverse events. Compared to warfarin use, the NOAC group had lower incidence rates of death (6.99% versus 7.32%), ischemic stroke (1.47% versus 2.07%), ICH (0.40% versus 0.58%), major bleeding (2.17% versus 2.65%), and composite adverse events (9.80% versus 10.65%). The Kaplan-Meier analysis demonstrated higher rates of clinical events in the NOAC group compared to the warfarin group for both sexes, with female patients exhibiting a more prominent decrease in cumulative incidence of ischemic stroke with NOAC use compared to warfarin (Fig. 2).
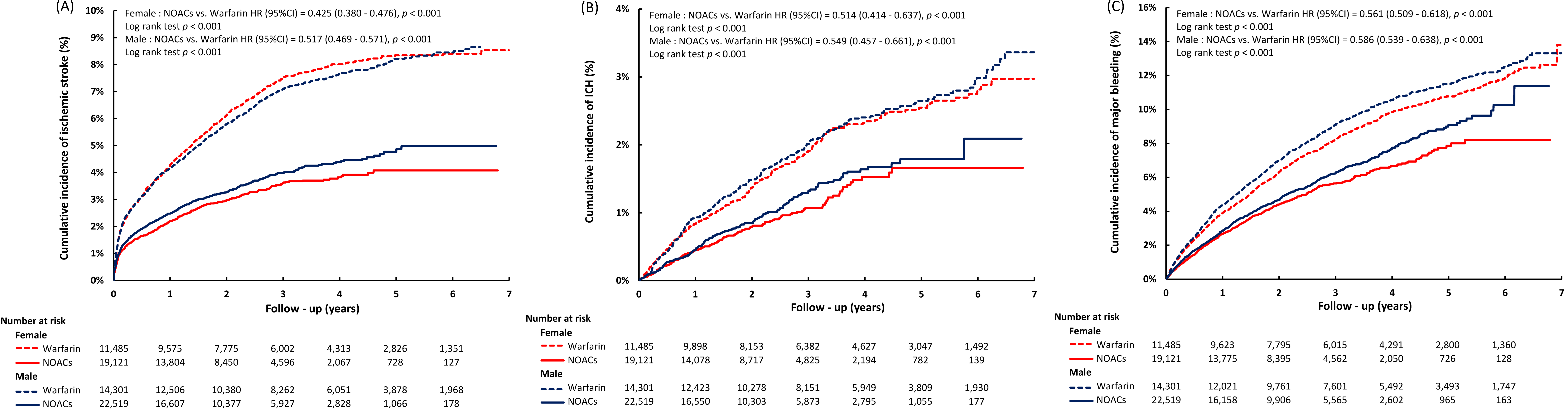 Fig. 2.
Fig. 2.Cumulative incidence curves of ischemic stroke (A), ICH (B), and major bleeding (C) in male and female patients in relation to OAC use. The c Kaplan-Meier analysis revealed higher cumulative incidence rates in the NOAC group compared to the warfarin group for both sexes. Additionally, the reduction in the cumulative incidence of ischemic stroke with NOAC use, as opposed to warfarin, was more pronounced in female patients than in male patients. ICH, intracranial hemorrhage; NOACs, non-vitamin K antagonist oral anticoagulants; OAC, oral anticoagulants; CI, confidence interval; HR, hazard ratio.
Multivariate Cox regression analysis revealed that NOAC was associated with
lower risk of death (adjusted hazard rate [aHR]: 0.726, 95% confidence interval
[CI]: 0.700–0.752, p
 Fig. 3.
Fig. 3.Incidence and risk of clinical endpoints between warfarin and NOAC use in both males and females. The whole study cohort demonstrated that NOAC was associated with lower risks of clinical endpoints compared to warfarin. Subgroup analysis revealed the consistently better outcomes associated with NOAC compared to warfarin in both sexes, whereas a greater risk reduction of ischemic stroke was observed in female patients with AF. AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; ICH, intracranial hemorrhage; NOACs, non-vitamin K antagonist oral anticoagulants.
This nationwide cohort analyzed the characteristics and long-term prognosis of
male and female patients with AF in terms of OAC types and presented the
following main results: (1) gender differences in baseline characteristics and
medication use in patients with AF, where female patients with AF demonstrated
higher CHA
Data in terms of OAC use in both sexes differ in previous reports. The CARMEN-AF registry [29] and the Global Anticoagulant Registry in the Field (GARFIELD-AF) [30] revealed no gender differences in OAC use. Conversely, the United States PINNACLE National Cardiovascular Data Registry from 2008 to 2014 reported that women with AF were more likely to receive aspirin but not OACs [31]. The present study revealed slight but significant differences in OAC prescription because NOAC use was more common in females and the percentage of warfarin use was similar between sexes. We hypothesized that a constellation of multiple factors, such as different periods, geographic factors, and underlying demographics caused gender differences in OAC use. For example, vascular diseases were more common in males and thus more males received aspirin or clopidogrel than women did. The need for multiple blood thinners might be a crucial factor for doctors while selecting medications.
Generally, female patients with AF have a higher risk of stroke and systemic
thromboembolism, and AF-related embolic stroke in women is more severe and
disabling [32, 33, 34]. Warfarin was the mainstream of stroke prevention in patients
with AF before the introduction of NOAC, but the Medicare administrative claims
data revealed that warfarin reduced stroke less well in females. Further, female
patients with AF had a slightly higher risk of hospitalizations despite warfarin
use [35]. One possible explanation underlying this observation is the higher
chance of poor INR control in females [36]. Until now, no RCTs have compared
gender differences with OAC use. The DIRECT registry, a single-center prospective
observational registry of 806 patients with AF treated with NOACs, demonstrated
comparable bleeding events between men and women whereas the thromboembolic event
rate was higher in women [37]. One meta-analysis, including major RCTs of NOACs
versus warfarin in patients with AF (RE-LY, ROCKET-AF, ARISTOTLE, and AVERROES),
revealed a higher risk of systemic thromboembolism in females compared to males
when treated with warfarin, which did not occur with NOAC treatment [38]. One
review article revealed that the sex disparity in stroke is no longer seen after
introducing NOAC [38, 39]. Furthermore, one meta-analysis reported differential
benefits of NOACs between sexes in which male patients were more protected from
stroke/systemic thromboembolism and female patients from major bleeding events
[40]. Our present study was partly congruent with previous studies that both
sexes benefited from NOAC despite different background characteristics, and the
unfavorable prognosis in females no longer existed with NOAC. However, we
revealed a greater risk reduction of ischemic stroke with NOAC use in female
patients despite higher CHA
There are some limitations in the present study. First, males and females might possess different biochemistry data and demographic information, which were lacking in the database, but this was a common limitation in the registry database. Second, the diagnosis and occurrence of events were based on the diagnostic codes registered by the physicians responsible for patient treatments, and under-diagnosis could be excluded. However, the accuracy of diagnosis in Taiwan’s NHIRD has been previously validated [24, 25, 27, 28]. Third, INR levels and time in the therapeutic range of warfarin use were not available in the database. Fourth, because this is a retrospective observational study, the reasons underlying more risk reduction of ischemic stroke with NOAC in female patients is unknown. We postulated the benefit of NOAC over warfarin might be more prominent in females because female patients with AF were more likely to have poor INR control than male patients in previous study [25]. However, this is solely an assumption because INR data is not available in the present study. Finally, the doses and types of NOACs were not analyzed in our study, thus whether or not these factors would interfere with the results remains unknown.
This large-scale nationwide cohort revealed that the use of NOAC was associated with better long-term outcomes compared with warfarin in patients with AF in both sexes. Female patients with AF benefited more from NOAC in reducing ischemic stroke, regardless of a higher risk. More studies are required for solid results about gender differences in the era of NOAC and for possible mechanisms.
All data generated or analyzed during this study are included in this published article.
The specific contributions for this article by the listed authors are as follows: JNL and TFC drafted the manuscript and were responsible for the study idea, acquisition of database and critical review. YSH and CTT have involved in data analysis, plotting the figures, and drafting the manuscript. LK and SJC collected the data and have been involved in drafting the manuscript. TCT performed data analysis and reviewed the study critically for important intellectual content. TJC and SAC designed the study and reviewed the study critically for important intellectual content. All authors have reviewed the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
This study was conducted in accordance with the Declaration of Helsinki and the protocol was approved by the Medical Ethics Committee of Taipei Veterans General Hospital (Approval No. 2016-10-003BC). Informed consent was waived due to the nature of the study.
Not applicable.
This work was supported in part by grants from the National Science Council (MOST 110-2314-B-075-059, MOST 111-2314-B-075-004-MY2), and Taipei Veterans General Hospital (V111C-020, V112C-019, V111B-035, and V112B-002).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
