- Academic Editors
Background: Calcified nodules (CN) have been linked to unfavorable
clinical outcomes. However, there is a lack of systematic studies on non-culprit
lesions with CN in patients with acute coronary syndromes (ACS). This study aims to investigate the frequency, distribution,
predictors, and outcomes of CN in non-culprit lesions among ACS patients.
Methods: We included 376 ACS patients who received successful stent
placement in their culprit lesions. Intravascular ultrasound (IVUS) was performed
to evaluate non-culprit lesions in left main arteries and all three coronary
arteries (CA). CN was defined as accumulations of small nodular calcium deposits
exhibiting a convex shape protruding into the lumen. Results: CNs was
identified in 16.9% (121 of 712) per artery and 26.9% (101 of 376) per patient.
They were predominantly located at the mid portion of the right coronary artery
(26.3%) and the bifurcation site (59.9%). Patients with CN were older (63.57
Pathologically, calcified nodules (CN) are characterized by a disruption of the fibrous cap, the presence of fibrin or platelet-rich thrombus, and the accumulation of eruptive, dense, CN that penetrate the luminal surface. Compared to plaque rupture (PR) and plaque erosion (PE), CN are considered to be a less common cause of acute coronary syndromes (ACS) [1, 2]. Intravascular ultrasound (IVUS) is a highly sensitive and specific imaging modality that provides detailed qualitative and quantitative information on underlying plaque morphology, including the detection of coronary calcium. Given the potential impact of lesion calcification with this unique morphology on clinical outcomes, recent studies have associated CN with adverse events following percutaneous coronary intervention (PCI), such as target lesion revascularization (TLR) [3, 4, 5] and refractory in-stent restenosis (IRS) [6]. However, the morphological features and clinical consequences of CN in non-culprit lesions in ACS patients are unknown. The goals of this in-vivo IVUS study are to establish the frequency, distribution, angiographic and intravascular ultrasound (IVUS) presentation, predictors, and outcomes of CN in ACS patients with non-culprit lesions.
The study group included 986 patients with ACS who had primary PCI between
October 2015 and May 2020. ST-segment elevation myocardial infarction (STEMI) and
non-ST-segment elevation myocardial infarction (NSTEMI) were both classified as
ACS [7]. In addition, cases of unstable angina pectoris (uAP) without cardiac
enzyme elevation were included. The presence of a culprit lesion was diagnosed
based on angiographic morphology, electrocardiogram (ECG) data, and anomalies in left ventricular
wall motion. A culprit lesion was more likely to be associated with more severe
stenoses and evidence of recent plaque disruption, with a filling defect on
angiography suggestive of thrombus. Lesions with a visible diameter stenosis of
more than 30% are considered to be non-culprit. Among the 986 patients,
exclusion criteria were as follows: (1) chronic total occlusion of coronary
arteries (CA); (2) tortuous vessels that would pose difficulties in advancing the
IVUS catheter; (3) vessel diameter
 Fig. 1.
Fig. 1.The study flow chart. Abbreviations: ACS, acute coronary syndrome; CN, calcified nodule; PCI, percutaneous coronary intervention; IVUS, intravascular ultrasound.
Following the successful stenting of all culprit lesions, IVUS was conducted to evaluate non-culprit lesions in the left main coronary arteries (LMCA) and all three coronary arteries. Procedural decisions were made based on the individual PCI operator’s judgment and discretion. Dual antiplatelet therapy was maintained for not less than one year after PCI. Physicians collected relevant data through new hospital admissions, telephone conversations, or clinic appointments subsequent to the PCI.
Quantitative coronary angiography (QCA) analysis was performed on the non-culprit lesion using QAngio software (v2.1.9, Medis, Leiden, the Netherlands) for offline analysis. The QCA study took into account numerous factors, including minimal lumen diameter, lesion length, reference vessel diameter, angiographic calcium (moderate or severe) and angiographic haziness [8, 9]. Based on the American Heart Association classification, the three epicardial arteries were divided into different segments, which included the left main (LM) artery segment (5), proximal segments (1, 6, 11), mid segments (2, 7, 13), and distal segments (3, 4, 8–10, 12, 14, 15) [10].
IVUS images were acquired using a 40-MHz OptiCross™ catheter (Boston
Scientific, Marlborough, MA, USA) within all three epicardial arteries. After
intracoronary administration of nitroglycerin (100–200 µg;
H44020569, Guangzhou Baiyun Mountain Mingxing pharmaceutical Company, Guangzhou,
China), IVUS was automatically pulled back at 0.5 mm/s from distal to proximal
references. CN was defined as a convex surface, protruding calcification from
luminal surface [11]. For each subject, CN were classified as either single
(occurring 1 solitary CN in one patient only) or multiple (occurring in a single
vessel with more than or equal to two nodules or in at least two vessels with one
nodule each). Following CN identification, the proximal and distal reference
segments representing the most normal-looking cross sections within 10 mm of the
nodule were chosen for additional research (Fig. 2). The slice with the narrowest
lumen and the highest plaque burden (PB) was chosen as the minimal lumenal area
(MLA) site among these reference segments. For quantitative investigation, the
cross-sectional areas (CSA) of the external elastic membrane (EEM), lumen, and
plaque plus media at the CN, MLA site, and proximal and distal reference segments
were assessed. PB was estimated by multiplying the plaque plus media CSA by 100
and then dividing by the EEM CSA. At the CN and MLA sites, the remodeling index
was calculated by dividing the EEM by the average EEM of the proximal and distal
reference segments. Lumen area stenosis was calculated by 1 minus MLA divided by
the average reference lumen CSA at the calcified nodule site and at the MLA site.
Calcium analysis entailed detecting the location of calcium and measuring the
maximum arc of calcium. Volumes were calculated using Simpson’s rule. According
to the published research, the quantitative IVUS analysis included calcium
surface (smooth or irregular), visible tissue between the lumen and calcium
(absent or present), eccentricity of plaque (concentric or eccentric) and
calcified nodule surface echogenicity (isoechoic, or hyperechoic) [11].
Hyperechoic tissue measured as echogenicity is brighter than the reference vessel
adventitia with shadowing [12]. The CN identification and quantitative analyses
were carried out independently by two cardiologists who were blinded to the
clinical presentation. For the diagnosis of CN and IVUS image quantitative
analyses, the intra-observer and inter-observer variability demonstrated good
agreement by 2 independent cardiologists (XW and HH) who blinded to the clinical
presentation, coronary angiographic, and laboratory data (
 Fig. 2.
Fig. 2.Intravascular ultrasound images of a calcified nodule (CN). (A) White line indicate CN at the mid of right coronary artery. White dotted line indicate stent at culprit lesion. (B) Calcified nodule had a convex and irregular surface (white arrow), between intima and external elastic membrane. (C) White line indicate CN at the proximal of left anterior descending artery. (D) CN was superficial of the intima (white arrow). (E) White arrowhead indicate CN at the bifurcation site and the distal site of the branch. (F) CN was superficial of the intima (white arrow). White asterisk indicate the diagonal branch.
The study’s primary end point was the appearance of major adverse cardiovascular events (MACE) in non-culprit lesions. MACEs included cardiac-related mortality, the development of recurrent ACS attributable to progression of non-culprit lesions, or hospital readmission due to unstable or worsening angina. Secondary endpoints included the existence of each MACE component.
Statistical analyses were conducted using SPSS 22.0 (IBM Corporation, Armonk,
NY, USA). Continuous variables were presented as the mean
From October 2015 to May 2020, among 986 ACS patients undergoing PCI in our hospital, a total of 376 ACS patients (101 in the CN group and 275 in the without CN group) were included in the study population. Patients with CN were older and had a higher prevalence of diabetes mellitus (DM). There was no significant difference in the incidence of male gender, past myocardial infarction, hypertension, smoking, prior PCI, familial CA disease, chronic kidney disease (CKD), hemodialysis, and the presentation of ACS, between the two groups. The laboratory data and medical therapies at discharge were also similar between the two groups (Table 1). After PSM, the baseline characteristics of the two groups were well-balanced in the analysis (Table 2).
| Variables | with CN (n = 101) | without CN (n = 275) | p value | |
| Age (years) | 63.57 |
57.98 |
||
| Female, n (%) | 62 (61.4) | 178 (64.7) | 0.550 | |
| Hypertension, n (%) | 73 (72.3) | 206 (74.9) | 0.605 | |
| Diabetes, n (%) | 56 (55.4) | 116 (42.2) | 0.022 | |
| Current smokers, n (%) | 30 (29.7) | 74 (26.9) | 0.591 | |
| Prior myocardial infarction, n (%) | 19 (18.8) | 55 (20.0) | 0.797 | |
| Family history of CAD, n (%) | 21 (20.8) | 54 (19.6) | 0.804 | |
| History of dyslipidemia, n (%) | 38 (37.6) | 104 (37.8) | 0.973 | |
| LVEF (%) | 61.94 |
63.01 |
0.213 | |
| BMI, kg/m |
24.27 (22.83–26.38) | 24.33 (22.84–25.72) | 0.482 | |
| CKD (eGFR |
26 (25.7) | 72 (26.2) | 0.931 | |
| Hemodialysis, n (%) | 8 (7.9) | 22 (8.0) | 0.980 | |
| Multivessel disease, n (%) | 45 (44.6) | 118 (42.9) | 0.775 | |
| A history of PCI, n (%) | 15 (14.9) | 44 (16.0) | 0.786 | |
| ACS presentation, n (%) | 0.659 | |||
| STEMI | 12 (11.9) | 43 (15.6) | ||
| NSTEMI | 59 (58.4) | 154 (56.0) | ||
| UAP | 30 (29.7) | 78 (28.4) | ||
| Calcium (mg/dL) | 9.18 |
9.11 |
0.420 | |
| Phosphorus (mg/dL) | 3.61 |
3.52 |
0.309 | |
| ALP (U/L) | 230.5 |
226.5 |
0.759 | |
| Hemoglobin A1c (%), (IQR) | 7.43 (6.47–8.33) | 7.27 (6.48–8.25) | 0.586 | |
| Triglycerides (mmol/L) | 1.41 |
1.55 |
0.313 | |
| Total cholesterol (mmol/L) | 4.32 |
4.04 |
0.062 | |
| HDL-C (mmol/L) | 1.09 |
1.15 |
0.240 | |
| LDL-C (mmol/L) | 2.91 |
2.83 |
0.436 | |
| hs-CRP (mg/L) | 8.41 |
8.19 |
0.643 | |
| Baseline TNT (ng/mL) | 3.54 |
3.32 |
0.280 | |
| Baseline CK-MB (IU/L) | 94.53 |
92.99 |
0.843 | |
| Medical therapies at discharge, n (%) | ||||
| Aspirin | 101 (100) | 275 (100) | ||
| P2Y12 inhibitor | 101 (100) | 275 (100) | ||
| ACEI or ARB | 68 (67.3) | 192 (69.8) | 0.643 | |
| Beta-blocker | 30 (29.7) | 62 (22.5) | 0.152 | |
| Statin | 78 (77.2) | 201 (73.1) | 0.416 | |
| Nitrate | 70 (69.3) | 187 (68.0) | 0.809 | |
Note: values are mean
Abbreviations: STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; LVEF, left ventricular ejection fraction; BMI, body mass index; CAD, coronary artery disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high sensitivity C-reactive protein; TNT, troponin-T; CK-MB, creatine kinase MB; ARB, angiotensin receptor blockers; ACEI, angiotensin-converting enzyme inhibitors; CKD, chronic kidney disease; ALP, alkaline phosphatase; CN, calcified nodule; PCI, percutaneous coronary intervention; UAP, unstable angina pectoris; ACS, acute coronary syndrome; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
| Variables | with CN (n = 101) | without CN (n = 83) | p value | |
| Age (years) | 63.57 |
62.06 |
0.193 | |
| Female, n (%) | 54 (60.7) | 54 (60.7) | ||
| Hypertension, n (%) | 61 (68.5) | 63 (70.8) | 0.872 | |
| Diabetes, n (%) | 45 (50.6) | 40 (44.9) | 0.061 | |
| Current smokers, n (%) | 26 (29.2) | 28 (31.5) | 0.702 | |
| Prior myocardial infarction, n (%) | 15 (16.9) | 15 (16.9) | ||
| Family history of CAD, n (%) | 16 (18.0) | 17 (19.1) | 0.802 | |
| History of dyslipidemia, n (%) | 29 (32.6) | 33 (37.1) | 0.721 | |
| LVEF (%) | 62.94 |
64.3 |
0.188 | |
| BMI, kg/m |
24.52 |
24.35 |
0.626 | |
| CKD (eGFR |
23 (25.8) | 22 (24.7) | 0.792 | |
| Hemodialysis, n (%) | 5 (5.6) | 5 (5.6) | ||
| Multivessel disease, n (%) | 40 (44.9) | 42 (47.2) | 0.705 | |
| A history of PCI, n (%) | 12 (13.5) | 10 (11.2) | 0.686 | |
| ACS presentation | 0.875 | |||
| STEMI, n (%) | 10 (11.2) | 9 (10.1) | ||
| NSTEMI, n (%) | 54 (60.7) | 53 (59.6) | ||
| UAP, n (%) | 25 (28.1) | 27 (30.3) | ||
| Calcium (mg/dL) | 9.18 |
9.15 |
0.812 | |
| Phosphorus (mg/dL) | 3.61 |
3.55 |
0.665 | |
| ALP (U/L) | 230.5 |
238.02 |
0.656 | |
| Hemoglobin A1c (%) (IQR) | 7.43 (6.47–8.32) | 7.29 (6.32–8.46) | 0.854 | |
| Triglycerides (mmol/L) | 1.41 |
1.61 |
0.253 | |
| Total cholesterol (mmol/L) | 4.32 |
3.99 |
0.078 | |
| HDL-C (mmol/L) | 1.09 |
1.11 |
0.728 | |
| LDL-C (mmol/L) | 2.91 |
2.83 |
0.557 | |
| hs-CRP (mg/L) | 8.41 |
8.74 |
0.617 | |
| Baseline TNT (ng/mL) | 3.54 |
3.15 |
0.171 | |
| Baseline CK-MB (IU/L) | 94.53 |
91.91 |
0.784 | |
| Medical therapies at discharge , n (%) | ||||
| Aspirin | 89 (100) | 89 (100) | ||
| P2Y12 inhibitor | 89 (100) | 89 (100) | ||
| ACEI or ARB | 61 (68.5) | 63 (70.8) | 0.872 | |
| Beta-blocker | 24 (27.0) | 25 (28.1) | 0.839 | |
| Statin | 67 (75.3) | 64 (71.9) | 0.459 | |
| Nitrate | 62 (69.7) | 63 (70.8) | 0.903 | |
Note: values are mean
Abbreviations: PSM, propensity score matching; LVEF, left ventricular ejection fraction; BMI, body mass index; CAD, coronary artery disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high sensitivity C-reactive protein; TNT, troponin-T; CK-MB, creatine kinase MB; ARB, angiotensin receptor blockers; ACEI, angiotensin-converting enzyme inhibitors; CKD, chronic kidney disease; ALP, alkaline phosphatase; CN, calcified nodule; PCI, percutaneous coronary intervention; STEMI, ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; UAP, unstable angina pectoris; ACS, acute coronary syndrome; eGFR, estimated glomerular filtration rate; IQR, interquartile range.
Among the 376 patients with ACS who underwent imaging of 712 vessels for
analysis (48 left main, 252 left anterior descending [LAD], 186 left circumflex
coronary artery [LCx], and 226 right CA [RCA]), a total of 137 CN were detected
in 121 vessels in 101 patients. The prevalence of CN was 16.9% per artery (121
out of 712) and 26.9% per patient (101 out of 376). Specifically, there were 47
nodules in the LAD in 38 patients, 36 nodules in the LCx in 27 patients, and 54
nodules in the RCA of 36 patients. Notably, no CN were observed in the LMCA.
Overall, 16.7% of LAD vessels (42 out of 252), 19.9% of LCx vessels (34 out of
186), and 22.1% of RCA vessels (50 out of 226) contained only one CN. Multiple
CN were found in 11 CA (1.5%) among 9 patients (2.4%). Specifically, 2.0% of
LADs (5 out of 252), 1.1% of LCxs (2 out of 186), and 1.8% of RCAs (4 out of
226) exhibited multiple nodules. Only 4 CN (2.9%) showed evidence of moderate
calcium on angiography, 1 (0.7%) exhibited severe calcium, and 5 (3.6%)
appeared hazy on angiography. The remaining 127 CN (92.7%) appeared normal on
angiography. The number of coronary calcified nodules per patient was 1.4. The
average volume of calcified nodules for per vessel and per patient are 1.2 mm
The CSA of the lumen at the site of the CN was significantly larger compared to
the MLA site (8.53 mm
| Variables | Calcified nodules site (n = 137) | MLA site (n = 137) | p value |
| EEM CSA, mm |
15.53 (14.66–16.45) | 15.33 (14.48–16.46) | 0.467 |
| Lumen CSA, mm |
8.53 (8.27–8.81) | 7.29 (6.89–7.75) | |
| Plaque plus media CSA, mm |
7.54 (7.32–7.80) | 8.89 (8.46–9.20) | |
| Plaque burden, % | 44.80 (43.10–46.01) | 56.41 (54.55–57.96) | |
| Remodeling index (IQR) | 0.97 (0.96–0.98) | 0.98 (0.97–0.99) | 0.132 |
Abbreviations: MLA, minimum lumen cross-sectional area; EEM, external elastic membrane; CSA, cross-sectional area; IVUS, intravascular ultrasound; IQR, interquartile range.
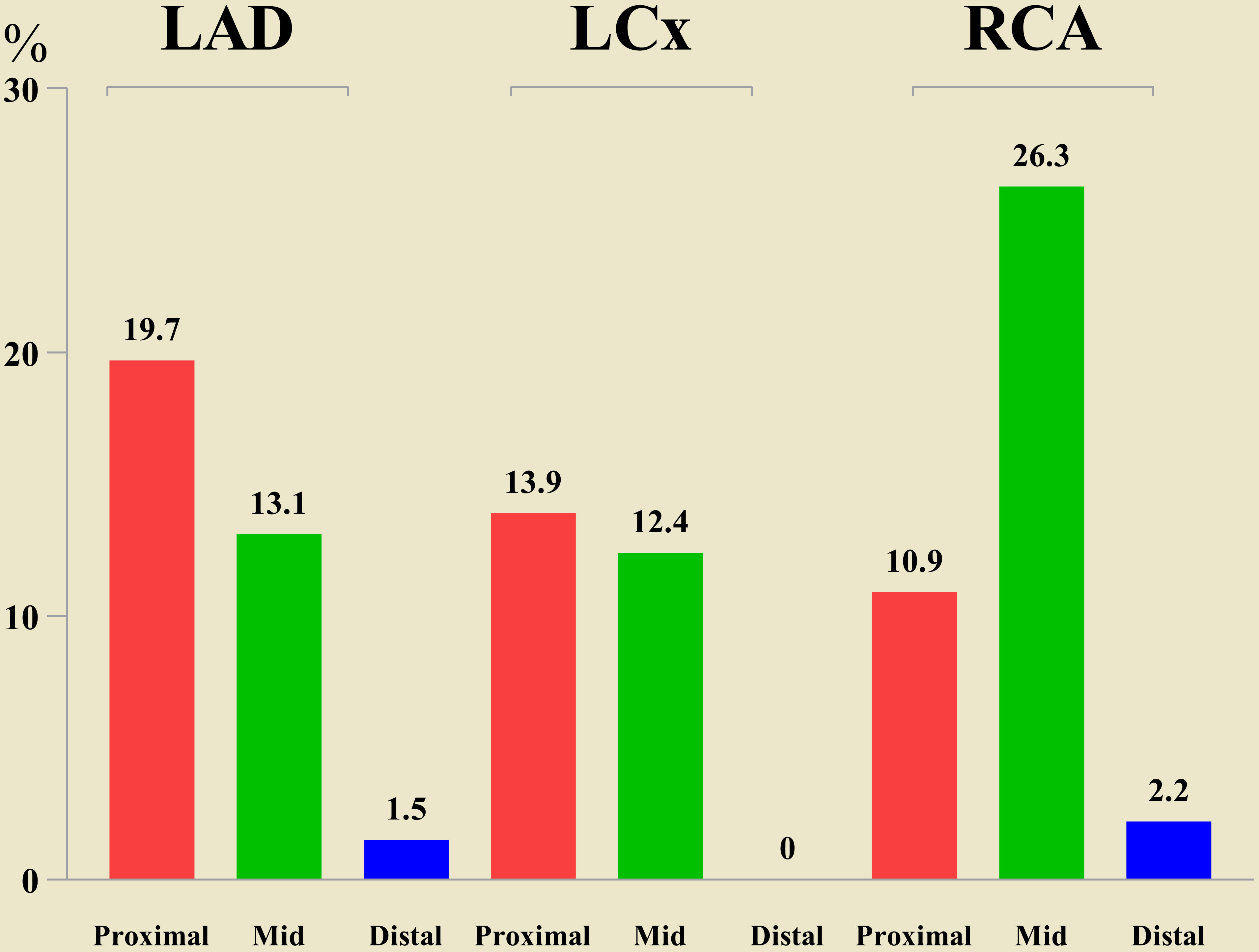 Fig. 3.
Fig. 3.Distribution of calcified nodule in the coronary arteries. Abbreviations: LAD, left anterior descending coronary artery; LCx, left circumflex coronary artery; RCA, right coronary artery.
| Variables | Calcified nodules (n = 101) | Without calcified nodules (n = 275) | p value |
| Total length analyzed, mm (IQR) | 184.43 (170.81–199.08) | 187.13 (171.45–202.22) | 0.371 |
| Nonculprit lesions, n | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 0.276 |
| Length of nonculprit lesions, mm (IQR) | 43.96 (34.53–55.22) | 45.26 (31.09–62.12) | 0.290 |
| Average EEM CSA, mm |
15.69 (13.13–17.58) | 15.43 (12.89–17.83) | 0.929 |
| Average lumen CSA, mm |
7.91 (7.34–8.52) | 7.95 (7.09–9.01) | 0.118 |
| Average P+M CSA, mm |
8.72 (7.97–9.65) | 9.04 (8.31–9.73) | 0.084 |
| Plaque burden, % | 47.76 (44.43–50.12) | 47.43 (43.89–50.51) | 0.339 |
| Reference diameter, mm | 3.10 |
3.13 |
0.236 |
| MLD, mm | 2.64 |
2.58 |
0.282 |
| MLA, mm |
2.81 |
2.89 |
0.233 |
| Reference area, mm |
13.94 |
14.32 |
0.480 |
Note: values are mean
Abbreviations: EEM, external elastic membrane; CSA, crosssectional area; P+M, plaque+media; IVUS, intravascular ultrasound; MLD, minimum lumen diameter; MLA, minimal lumenal area; IQR, interquartile range.
| Variables | Calcified nodules (n = 101) | Without calcified nodules (n = 83) | p value |
| Total length analyzed, mm (IQR) | 184.43 (170.81–199.08) | 187.64 (173.08–200.88) | 0.129 |
| Nonculprit lesions, n | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 0.072 |
| Length of nonculprit lesions, mm (IQR) | 44.3.96 (34.51–55.22) | 49.82 (31.03–62.16) | 0.203 |
| Average EEM CSA, mm |
15.69 (13.13–17.58) | 14.87 (12.68–18.03) | 0.533 |
| Average lumen CSA, mm |
7.91 (6.54–9.32) | 8.04 (6.62–10.02) | 0.381 |
| Average P+M CSA, mm |
8.49 (7.21–10.43) | 9.02 (8.55–11.43) | 0.109 |
| Plaque burden, % | 47.32 (42.76–51.95) | 48.21 (43.29–53.21) | 0.441 |
| Reference diameter, mm | 3.1 |
3.18 |
0.514 |
| MLD, mm | 2.64 |
2.41 |
0.437 |
| MLA, mm |
2.81 |
2.93 |
0.741 |
| Reference area, mm |
13.94 |
14.44 |
0.578 |
Note: values are mean
Abbreviations: EEM, external elastic membrane; CSA, crosssectional area; PSM, propensity score matching; P+M, plaque+media; IVUS, intravascular ultrasound; MLD, minimum lumen diameter; MLA, minimal lumenal area; IQR, interquartile range.
By multivariate analysis, diabetes mellitus (DM) was found to have a positive correlation with MACE, but the existence of CN was found to have a negative association with MACE (Table 6). The presence of CN remained significantly associated with MACE after PSM (Table 7).
| Variables | HR | 95% CI | p value |
| Age | 1.019 | 0.980–1.058 | 0.350 |
| Diabetes mellitus | 2.216 | 1.246–3.940 | 0.007 |
| Prior myocardial infarction | 1.304 | 0.675–2.519 | 0.430 |
| CKD (eGFR |
1.144 | 0.619–2.113 | 0.668 |
| Calcified nodule | 0.341 | 0.140–0.829 | 0.018 |
| MLD | 1.075 | 0.892–1.296 | 0.449 |
Abbreviations: MACE, major adverse cardiovascular events; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; 95% CI, 95% confidence interval; HR, hazard ratio; MLD, minimum lumen diameter.
| Variables | HR | 95% CI | p value |
| Age | 1.022 | 0.964–1.083 | 0.474 |
| Diabetes | 1.665 | 0.715–3.878 | 0.238 |
| Prior myocardial infarction | 2.063 | 0.775–5.493 | 0.147 |
| CKD (eGFR |
1.563 | 0.586–4.167 | 0.372 |
| Calcified nodule | 0.275 | 0.108–0.703 | 0.007 |
| MLD | 1.272 | 0.953–1.699 | 0.103 |
Abbreviations: MACE, major adverse cardiovascular events; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; PSM, propensity score matching; 95% CI, 95% confidence interval; HR, hazard ratio; MLD, minimum lumen diameter.
Clinical outcomes and hazard ratios adjusted for PSM are summarized in Tables 8,9 and Fig. 4. Over the 19.35
| Variables | Calcified nodules (n = 101) | Without calcified nodules (n = 275) | p value |
| Nonculprit-lesion MACE | 6 (5.9) | 46 (16.7) | 0.046 |
| Cardiac-cause death | 5 (5.0) | 7 (2.5) | 0.198 |
| Recurrence of ACS | 5 (5.0) | 40 (14.5) | 0.049 |
| Rehospitalization | 2 (2.0) | 6 (2.2) | 0.482 |
Abbreviations: MACE, major adverse cardiovascular events which included cardiac-cause death, the recurrence of ACS resulting from the progression of non-culprit lesion, or rehospitalization due to unstable or progressive angina. ACS, acute coronary syndrome; 95% CI, 95% confidence interval; HR, hazard ratio.
| Variables | Calcified nodules (n = 101) | Without calcified nodules (n = 83) | p value |
| Nonculprit-lesion MACE | 4 (4.0) | 15 (18.1) | 0.011 |
| Cardiac-cause death | 1 (1.0) | 1 (1.2) | 0.959 |
| Recurrence of ACS | 1 (1.0) | 4 (4.8) | 0.237 |
| Rehospitalization | 2 (2.0) | 3 (3.6) | 0.685 |
Abbreviations: MACE, major adverse cardiovascular events which included cardiac-cause death, the recurrence of ACS resulting from the progression of non-culprit lesion, or rehospitalization due to unstable or progressive angina. ACS, acute coronary syndrome; 95% CI, 95% confidence interval; HR, hazard ratio; PSM, propensity score matching.
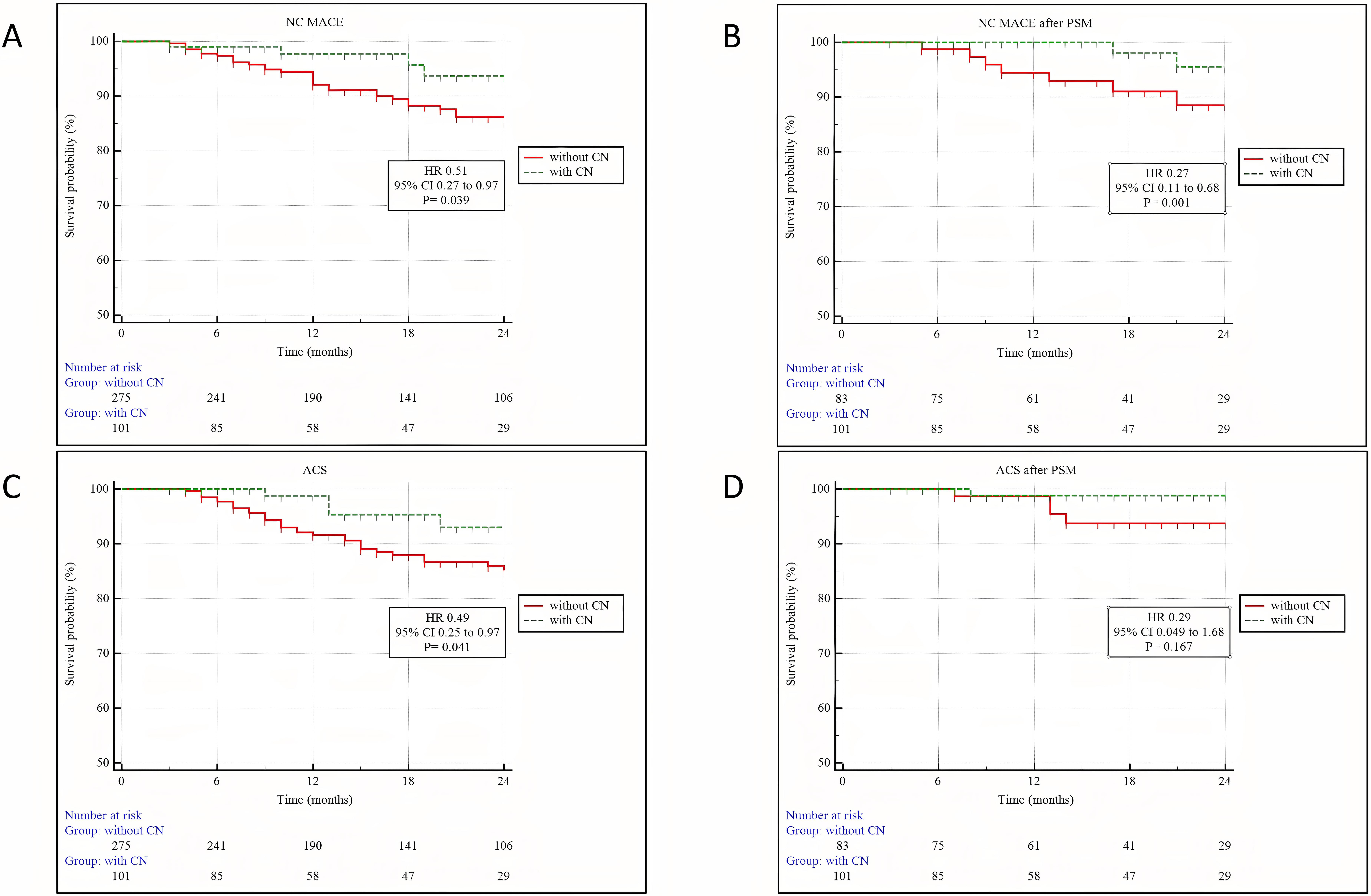 Fig. 4.
Fig. 4.Kaplan–Meier analysis of cardiac outcomes between patients with and without CN. (A) NC MACE. (B) NC MACE after PSM. (C) ACS. (D) ACS after PSM. Abbreviations: NC MACE, nonculprit-lesion major adverse cardiovascular events; PSM, propensity score matching; ACS, acute coronary syndrome; 95% CI, 95% confidence interval; HR, hazard ratio; CN, calcified nodules.
The following are the key conclusions of the current study addressing CN in non-culprit lesions: (1) CN was detected in 16.9% of arteries and in 26.9% of patients with ACS in non-culprit lesions; (2) non-culprit CN was more commonly found in the mid-portion of the RCA and at bifurcation sites; (3) non-culprit CN exhibited a negative association with MACE; (4) patients with non-culprit CN had a significantly lower incidence of MACE.
Based on pathologic examination, CN, protruding into the lumen exhibited thin
fibrous cap derived from disruptive calcified nodules, filled a non- or occlusive
platelet/fibrin thrombus [1]. IVUS criteria for calcified nodules showed a
luminal surface with irregular, protruding, and convex appearing lesions [11].
Using optical coherence tomography (OCT), CN was defined as a lesion with a
disruptive fibrous cap in associated with a calcified plaque, protruding calcium,
superficial calcification, and the presence of extensive calcification [13].
Near-infrared spectroscopy (NIRS) findings shown NIRS-CN was associated with
convex calcium deposits with a maximum lipid core burden index of 4 mm
(MaxLCBI
Advanced age, DM, and other risk factors have been recognized as contributing factors to the development of CA calcification [21, 22]. Additionally, we discovered that age and DM were independently associated with the development of CN in this study, while there was no difference between the two groups in baseline characteristics after PSM. Previous studies concentrated on the clinical features of patients with CN at the culprit lesion. CN has been linked to CABG, and it is thought that changes in vascular shear stress caused by decreased native CA flow may lead to the development of CN [20]. Kobayashi et al. [3] found that CN was observed more frequently by OCT in older individuals and those with DM than in those without CN. Additionally, Sugane et al. [19] also found that CN patients were more likely to have coronary risk factors such as CKD, continuous hemodialysis, and a history of PCI. Given that decreased kidney function can result in abnormal calcium and phosphorus metabolism, as well as the release of calcification-related proteins and inflammatory cytokines, these CKD-related variables could play a major role in the development of CN [23].
There is a scarcity of information on the distribution of non-culprit CN. However, our observations indicate that the mid segment of the RCA is more frequently affected by non-culprit CNs. Mechanical stress caused by coronary hinge motion during cardiac pulsations may lead to the formation of eruptive CN, and the mid segment of the RCA is more vulnerable to this impact caused by cardiac motion [1]. The axial position of non-culprit CN was investigated in 185 patients in a recent study [24], which demonstrated that non-culprit CNs were predominantly located in the proximal segments of the LAD and LCx. Similarly, in an OCT study [17] involving 3231 consecutive patients, the location of eruptive or noneruptive CNs was analyzed, revealing a tendency for CNs to cluster within the proximal segments of the LAD and the proximal to mid segments of the RCA. Another OCT study by Lee et al. [16] reported a similar finding, with CN tending to cluster in the ostium or mid portion of the RCA. Interestingly, in our study, CN were predominantly located at the bifurcating regions of coronary arteries. Notably, in patients with CABG, 85.7% of CNs were found within 5 mm to the LMCA bifurcation, which could potentially impact the delivery and expansion of balloons and stents [20]. Pathology investigations have thoroughly detailed the spatial distribution of atherosclerosis within a coronary bifurcation, finding atherosclerotic plaque formation on the lateral walls but largely undamaged flow [25]. CN lesions with fibrous layer elements have the highest degree of calcification relative to plaque area of any vulnerable plaque subtype and are thought to be related with healed fibroatheromas [26]. Based on these concepts, we can speculate on the mechanisms underlying CN development in bifurcating regions.
In the age of drug-eluting stents (DES), severely CLs with CN are anticipated to have a negative impact on PCI results [4]. Target lesion revascularization (TLR) is more frequently required following PCI of lesions with severe calcification compared to those without [27]. Previous research has found TLR rates ranging from 20.0% to 38.0% after 2 years, mainly in unselected CLs and especially in the group with eruptive CN [3, 4, 28, 29]. Morofuji et al. [4] discovered that CN was present in half of the severely CLs that required rotational atherectomy, and that CN was related with worse adverse outcomes after a 5-year follow-up period. The recurrence of CN within the implanted DES was responsible for more than 80% of TLR at the CN lesion [19]. A recent OCT study found a 2-year cumulative rate of target lesion failure (TLF) caused primarily by clinically induced TLR. This study indicated that eruptive CN morphology has a different influence on long-term clinical outcomes when compared to non-eruptive CN morphology [17]. Recently, a previous OCT study [30] demonstrated that patients with eruptive CN had a remarkably higher 2-year incidence of cumulative MACE compared with the calcified protrusion and superficial calcific sheet groups. This finding suggest that eruptive CNs in culprit lesions with ACS patients are more frequently to impact clinical adverse outcomes after PCI. However, it remains unclear whether CN in non-culprit lesions with ACS patients is considered as the reason for adverse outcomes. There have been no previous systematic studies using intracoronary imaging modalities that have shown an influence of CN in non-culprit lesions. Xu et al. [24] found that CN in non-culprit lesions of ACS patients resulted in better clinical outcomes over a 3-year follow-up period, which is consistent with our current investigation. Surprisingly, no deaths, cardiac arrests, or myocardial infarctions occurred in the CN group. While one pathology group has described culprit CN as a rare cause of coronary thrombosis [1], non-culprit CN may represent precursor lesions similar to thin-cap fibroatheromas (TCFs). It is important to note that CNs do not always cause thrombosis, and TCFs do not always cause plaque rupture (PR). In the current study, we found the CN group have less non-culprit lesion MACEs compared with the non-CN group. We hypothesize that CNs could be the result of plaque rupture (PR), thrombosis, and subsequent healing rather than the cause of poor outcomes. Furthermore, CNs in non-culprit lesions may help to stabilize the lesion rather than being the cause of adverse events.
This study has several limitations. First, it was a retrospective single-center study, which may have introduced an element of selection bias. Additionally, the number of lesions with CN was relatively small, limiting the generalizability of the findings. Another limitation is the lack of pathological assessments for non-culprit lesions with and without CN. Although IVUS is commonly used for evaluating coronary calcification, its resolution may not be sufficient to visualize small nodular calcifications. Furthermore, it is important to consider that intensive medical care in compliant patients may have mitigated the occurrence of MACE during the follow-up period, potentially influencing the study outcomes. Therefore, a larger, prospective, randomized study is needed to further investigate and validate our findings.
The occurrence of CN at non-culprit lesions in patients with ACS was prevalent and caused fewer adverse clinical outcomes.
CN, calcified nodule; ACS, acute coronary syndrome; IVUS, intravascular ultrasound; PSM, propensity score matching; MACE, major adverse cardiovascular event; CI, confidence intervals; HR, hazard ratio; PCI, percutaneous coronary intervention; TLR, target lesion revascularization; IRS, refractory in-stent restenosis; STEMI, ST-segment elevation myocardial infarction; NSTEMI, nonST-segment elevation myocardial infarction; uAP, unstable angina pectoris; MLA, minimum lumen area; QCA, quantitative coronary angiography; LM, left main; MLA, minimum lumen area; EEM, external elastic membrane; CSA, cross-sectional area; PB, plaque burden; IQRs, interquartile ranges; CABG, coronary artery bypass grafting; OCT, optical coherence tomography; CKD, chronic kidney disease.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
XW and HH had the idea for the paper, reviewed and edited it critically for important intellectual content. HBH and LW performed the literature search and analysis. XW, MXW, HH, JC, ZL and LW substantially contributed to the conception of the paper, drafted and critically revised the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The current study was carried out in accordance with the tenets mentioned in the Helsinki Declaration and was approved by the Ethical Board of Xiangtan Central Hospital (approval number: X20201228). Prior to the commencement of the research, our team obtained written informed consent from each patient.
We are grateful to Bo Chen for their secretarial assistance.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
