- Academic Editors
†These authors contributed equally.
Background: Pediatric obesity is closely associated with
cardiometabolic comorbidities, but the role of sex in this relationship is less
investigated. We aimed to evaluate sex-related differences on cardiometabolic
risk factors and preclinical signs of target organ damage in adolescents with
overweight/obesity (OW/OB). Methods: The main cross-sectional study
included 988 adolescents (510 boys and 478 girls) with OW/OB aged 10–18 years.
In all youths clinical and biochemical variables were evaluated and an abdominal
echography was performed. Echocardiographic data for the assessment of left
ventricular mass (LVM) and relative wall thickness (RWT) were available in an
independent sample of 142 youths (67 boys and 75 girls), while echographic data
of carotid intima media thickness (cIMT) were available in 107 youths (59 boys
and 48 girls). Results: The three samples did not differ for age, body
mass index, and sex distribution. In the main sample, boys showed higher
waist-to-height ratio (WHtR) values (p
Notoriously, pediatric obesity represents an alarming phenomenon in industrialized countries from both a health and a socio-economic point of view. In addition, according to the World Health Organization, rate of children with obesity (OB) is expected to double by 2035 [1]. Noteworthy, this condition is closely associated with several comorbidities or preclinical signs of organ damage, such as prediabetes, hypertension, dyslipidemia, and fatty liver disease (FLD) [2]. This scenario is supposed to track into adulthood and produce serious long-term consequences on cardiometabolic health. Indeed, the early presence of obesity-related comorbidities may contribute to accelerate the risk of cardiovascular morbidity in adulthood [2].
The presence of cardiometabolic comorbidities has been largely studied in the pediatric population [3], but little is known about the influence of sex on this association. This aspect should not be overlooked in children, since it has been widely demonstrated that men are more frequently exposed to early morbidity and mortality for cardiovascular disease than females [4]. Given the well-documented protective role of oestrogens, this advantage is attenuated after menopause [5]. In addition, diseases associated to high cardiometabolic risk, such as type 2 diabetes [6] and FLD are more prevalent in males [7]. On the contrary, a higher risk of chronic kidney disease has been reported in females [8].
Given the high burden of cardiovascular risk in pediatric OB [9], it may be interesting to analyze in-depth whether and which kind of cardiovascular risk factors or preclinical signs of target organ damage are associated to a non-modifiable risk factor such as sex.
Based on these premises, we hypothesized that sex-related differences in cardiovascular risk might be present in adolescents with excess weight. Therefore, the aim of this study was to compare the traditional cardiovascular risk factors and preclinical signs of cardiac, vascular and renal impairment between adolescent boys and girls with overweight (OW) or OB.
This is a multicenter cross-sectional study that included 1562 youths with OW or OB. Participants were consecutively admitted to nine Italian endocrinology centers of the Pediatric Obesity Study Group within the Italian Society for Pediatric Endocrinology and Diabetology between 2016 and 2020 [10]. This study was conducted according to the principles expressed in the Declaration of Helsinki and approved by the Research Ethical Committee of University of Campania “Luigi Vanvitelli” (protocol code 834/2016). An informed consent was obtained from the parents of all participants before any procedure.
Exclusion criteria were: age
Two separate samples of 142 young people with OW or OB (67 boys and 75 girls) with echocardiographic evaluation performed in the Pozzuoli Hospital (Naples) [11], and 107 youths (59 boys and 48 girls) with carotid ultrasound performed in the Cardarelli Hospital of Naples [12] between 2003 and 2013, were included in the study.
Anthropometric parameters were measured according to standard methods by the
same trained physician in each center, as previously detailed [10]. Body mass
index (BMI) was calculated as the ratio of weight (Kg) and height (meters)
After an overnight fast, blood samples were taken for measurement of glucose,
insulin and lipids. Glycosylated hemoglobin A1c (HbA1c) was measured by
high-performance liquid chromatography in each center [10]. Homeostasis model
assessment of insulin-resistance (HOMA-IR) was calculated to estimate
insulin-resistance using the following formula: insulin (µU/mL)
Serum creatinine (mg/dL) was measured by kinetic colorimetric Jaffé method
in 293 youths and by enzymatic method in 695 youths. Estimated glomerular
filtration rate (eGFR) was calculated using Full Age Spectrum for height equation
(eGFRFAS
Biochemical tests were performed in the centralized laboratory of each center [10]. Each laboratory belongs to the National Health System and is certified according to International Standards ISO 9000 (http://www.iso9000.it/).
Liver ultrasonography was performed by experienced radiologists in each center. The presence of FLD was based on the increased echogenicity (brightness) of the liver as compared to the renal cortex [15].
The echocardiographic data were collected with young people in the left lateral decubitus position, using a commercially available echocardiographic system with tissue Doppler (TD) capabilities (Power Vision 8000, Toshiba-Corp. Medical, Japan) equipped with variable frequency phased-array transducer (2.5–3.8 MHz), as elsewhere described [11].
The left ventricular mass (LVM) was calculated according to the American Society
of Echocardiography recommendations using M-mode whenever possible, or optimally
oriented 2-dimensional parasternal long-axis view. LVM (g) was indexed (LVMi)
using a simplified method proposed by Chinali et al. [16] and expressed
as LVM/[(height
Carotid intima media thickness (cIMT) was assessed by commercially available system equipped with a 7–13 MHz linear array probe was used for B-mode ultrasound evaluations. Quantitative B-mode ultrasound measurements of cIMT were obtained as mean of cIMT of near and far walls of both common carotid arteries of both carotid bulbs, as previously described [18].
OW or OB were defined using the Italian growth charts [19]. Visceral adiposity
was defined as WhtR
Hypertension was defined by criteria proposed by the American Academy of
Pediatrics based of BP
Fatty liver disease was defined as the presence of ultrasound detected hepatic steatosis and assessed as present or absent [12].
Mild reduced eGFR (MReGFR) was defined by a value of eGFRFAS
Left ventricular hypertrophy (LVH) was defined using the single cut-point
Variables with normal distribution were expressed as mean
The characteristics of the main sample are shown in Table 1. Despite similar age
and BMI, boys exhibited higher waist circumference and WHtR than girls
(p
| All | Boys | Girls | p value | |
| n | 988 | 510 | 478 | |
| Age, years | 12.9 |
12.8 |
13.0 |
0.063 |
| BMI (kg/m |
32.1 |
32.0 |
32.2 |
0.607 |
| BMI-z score | 2.39 |
2.36 |
2.42 |
0.183 |
| Waist circumference (cm) | 97.0 |
98.9 |
95.0 |
|
| Waist-to-height ratio | 0.616 |
0.623 |
0.609 |
|
| G |
88.6 |
89.5 |
87.6 |
0.002 |
| G |
111.5 |
112.3 |
110.7 |
0.226 |
| HbA1c (%) | 5.3 |
5.3 |
5.3 |
0.169 |
| HOMA-IR | 4.0 (2.7–6.0) | 3.9 (2.7–5.6) | 4.2 (2.7–6.2) | 0.221 |
| Cholesterol (mg/dL) | 153.9 |
152.6 |
155.4 |
0.130 |
| HDL-C (mg/dL) | 47.0 |
47.2 |
46.8 |
0.524 |
| Triglycerides (mg/dL) | 80.5 (62.0–105.0) | 78.0 (60.8–102.0) | 84.5 (63.8–109.3) | 0.054 |
| TG/HDL-C ratio | 1.8 (1.3–2.5) | 1.7 (1.2–2.4) | 1.9 (1.3–2.5) | 0.051 |
| Systolic BP (mmHg) | 116.1 |
116.7 |
115.4 |
0.105 |
| Diastolic BP (mmHg) | 69.2 |
69.0 |
69.4 |
0.425 |
| eGFR (mL/min/1.73 m |
114.3 |
117.1 |
111.3 |
Data are expressed as mean
BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration
rate; G
When the categorical variables were considered, more boys than girls showed a
WhtR
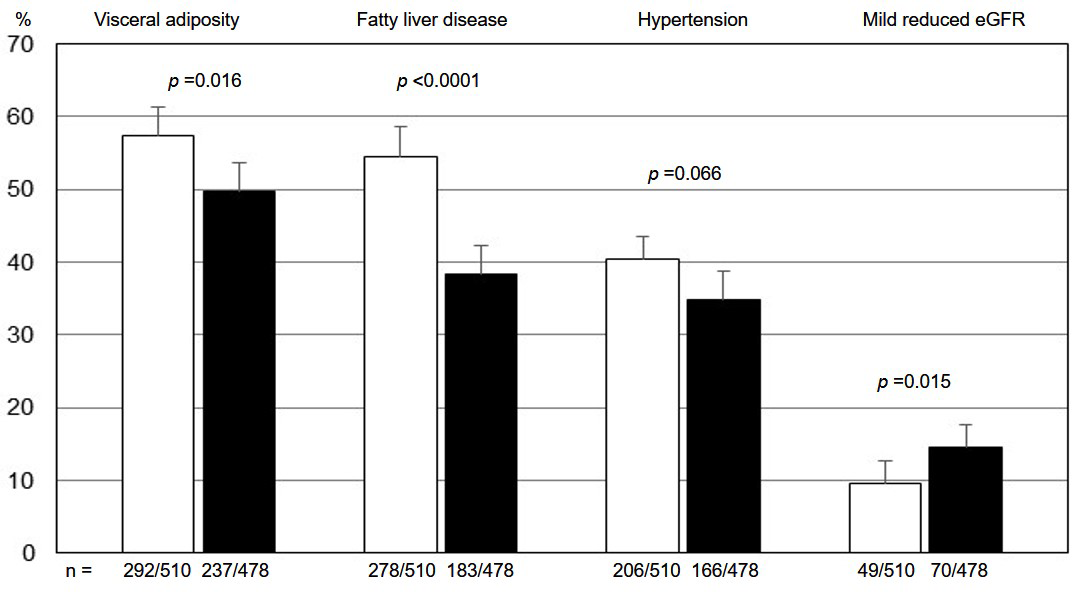 Fig. 1.
Fig. 1.Proportion (95% Cl) of youths with visceral adiposity, fatty liver disease, hypertension, and mild reduced estimated glomerular filtration rate in boys (white bars) and girls (black bars). eGFR, estimated glomerular filtration rate.
The main characteristics of the sample who underwent echocardiographic evaluation are reported in Table 2.
| All | Boys | Girls | p value | |
| n | 142 | 67 | 75 | |
| Age, years | 12.2 |
11.9 |
12.4 |
0.068 |
| BMI (kg/m |
29.8 |
29.5 |
30.1 |
0.493 |
| BMI-z score | 2.1 |
2.1 |
2.2 |
0.300 |
| Waist-to-height ratio | 0.623 |
0.626 |
0.620 |
0.545 |
| Cholesterol (mg/dL) | 162.5 |
157.7 |
166.8 |
0.123 |
| HDL-C (mg/dL) | 48.8 |
47.6 |
49.9 |
0.239 |
| Triglycerides (mg/dL) | 87.0 (59.0–107.0) | 86.0 (55.0–99.0) | 88.0 (63.0–120.0) | 0.115 |
| TG/HDL-C ratio | 1.8 (1.2–2.5) | 1.8 (1.0–2.4) | 1.7 (1.2–2.5) | 0.433 |
| Systolic BP (mmHg) | 109.7 |
110.2 |
109.3 |
0.603 |
| Diastolic BP (mmHg) | 66.5 |
68.2 |
65.0 |
0.009 |
| LVMi (g/m |
44.2 |
46.3 |
42.3 |
0.046 |
| RWT |
0.351 |
0.367 |
0.337 |
0.003 |
Data are expressed as mean
BMI, body mass index; BP, blood pressure; HDL-C, high-density lipoprotein
cholesterol; TG/HDL-C, triglycerides to high-density ipoprotein-cholesterol;
LVMi, left ventricular mass index; RWT
Despite no sex-related differences were observed for age and BMI, boys exhibited
higher values of LVMi (p = 0.046) and RWT
Boys exhibited a higher frequence of LVH (p = 0.015) or concentric LVH
(p = 0.002) (Supplementary Fig. 2). Multiple regression
analysis showed that LVMi was significantly and independently associated with
WHtR, TG/HDL-C ratio and and male sex (Supplementary Table 1). RWT
The features of youths who underwent a cIMT evaluation are reported in Table 3.
Boys showed similar age and BMI compared to girls, but they presented higher
values of cIMT (p = 0.011). cIMT values
| All | Boys | Girls | p value | |
| n | 107 | 59 | 48 | |
| Age, years | 12.1 |
12.3 |
11.9 |
0.220 |
| BMI (kg/m |
31.5 |
31.3 |
31.6 |
0.756 |
| BMI-z score | 2.3 |
2.3 |
2.4 |
0.484 |
| Waist-to-height ratio | 0.653 |
0.652 |
0.650 |
0.856 |
| Cholesterol (mg/dL) | 154.6 |
149.5 |
161.2 |
0.068 |
| HDL-C (mg/dL) | 44.9 |
44.5 |
45.4 |
0.628 |
| Triglycerides (mg/dL) | 86.0 (68.0–115.0) | 86.0 (64.5–109.8) | 87.5 (68.0–119.0) | 0.459 |
| TG/HDL-C ratio | 1.9 (1.4–2.8) | 2.1 (1.4–2.7) | 1.9 (1.5–3.0) | 0.695 |
| Systolic BP (mmHg) | 122.2 |
122.1 |
122.4 |
0.886 |
| Diastolic BP (mmHg) | 79.1 |
79.2 |
79.0 |
0.896 |
| cIMT (mm) | 0.50 |
0.52 |
0.49 |
0.011 |
Data are expressed as mean
BMI, body mass index; BP, blood pressure; cIMT, carotid intima media thickness;
TG/HDL-C, triglycerides to high-density lipoprotein-cholesterol.
The present study highlighted several sex-related differences in cardiovascular risk factors in adolescents with OW or OB, demonstrating that boys presented a higher degree of visceral adiposity, FLD, cardiac and vascular abnormalities compared to girls, while girls showed a higher risk of mild reduced glomerular function.
These findings are consistent with the sex-related cardiovascular risk described in non-elderly adults. Indeed, it is well established that males are considered at higher risk of cardiovascular morbidity and mortality than females, although this difference varies over time and geographically [26]. Due to the multifactorial nature of cardiometabolic risk, sex-related differences can be explained not only by the protective role of estrogens, but also by the complex interplay of several conditions, such as those correlated to lifestyle behaviours (e.g., smoking, alcohol consumption), or traditional cardiometabolic risk factors (such as visceral adiposity, dyslipidemia, and hypertension) which are more prevalent in non-elderly and non-diabetic males than females [26].
More complex and conflicting data are available on the role of sex as non
modifiable risk factor for cardiovascular mortality among adults with OW or OB.
For instance, the hazard ratio of cardiovascular death was higher in men with
obesity (BMI
Robust evidence has linked traditional pediatric cardiometabolic risk factors, such as increased adiposity, hypertension, hyperlipidemia, and risk factor clustering with subclinical cardiovascular disease [30]. The adverse cardiometabolic risk profile related to OB starting in early adolescence and even in early childhood has been also associated with fatal and not fatal cardiovascular events as early as 40 years of age [31].
The association between OB and cardiometabolic risk factors may begin at different stages of youth, depending on the degree of OB, cardiometabolic risk factors, and sex [32]. The emergence of sex-related differences in the trajectories of atherogenic lipids (apolipoprotein B containing very low-density lipoprotein and low density lipoprotein traits) and predictive biomarkers (glucose and HDL-C) for cardiometabolic diseases has been demonstrated in a prospective birth cohort study from childhood (age 7 years) to early adulthood (25 years) in United Kingdom [33]. Most changes of causal and predictive cardiometabolic traits were detrimental for males, and emerged only in late childhood and adolescence. It may be possible that multiple mediators such as adiposity, puberty timing, and other health behaviours might have played a role, but this aspect was not assessed in that study.
Of note, we did not find any sex-related difference in the traditional
cardiometabolic risk factors, either when they were considered as continuous or
categorical variables, in our sample of adolescents with OW or OB, except for
higher values of fasting plasma glucose in boys and a slightly higher frequence
of impaired glucose tolerance in girls. Moreover, a significally higher WHtR
values and frequence of individuals with WHtR
Similarly to visceral adiposity, we found a higher frequence of FLD in boys than girls. The close relationship of FLD with cardiometabolic risk in children with OB has been largely demonstrated [34, 35, 36]. As observed in adults [37, 38, 39], accumulating pediatric evidence described non-alcoholic FLD as a condition with sexual dimorphism, with a higher prevalence in boys than in girls [40, 41, 42]. This might be attributable to the predominant visceral distribution of adipose tissue in males that is associated with insulin resistance and free fatty acids flux, leading to FLD development [39]. On the other hand, females have a prevalent subcutaneous fat distribution and leptin production that prevents from visceral fatty tissue accumulation in cooperation with estrogens, which may play a protective role against liver fat accumulation. However, the role of sex steroids on metabolic impairments is still complex and needs to be further elucidated [41, 42, 43].
In the wide perspective of cardiometabolic burden of pediatric obesity, the obesity-related glomerulopathy has recently gained remarkable attention [44, 45]. Similarly to adults [46], emerging data demonstrated that children with OB are at higher risk of kidney damage (expressed as renal function decline with or without hypertension and/or proteinuria) [47, 48, 49, 50]. Of note, cardiometabolic parameters have been closely associated to kidney injury, suggesting an intimate link between renal function and OB, but also with the obesity-related dysmetabolic state [45, 51, 52].
In line with adult evidence reporting that females are more susceptible to chronic kidney disease development than males [53, 54], we demonstrated for the first time sex-related differences for mild renal injury in adolescents with OB.
It is well established that LVH or abnormal LV geometry act as risk factors for
cardiovascular morbidity and mortality in adult populations [55, 56]. The
assessment of LVH depends on the cut-off used to calculate LV mass or on the
presence of hypertension, which is the principal determinant of LVH [13]. In any
case, using the method proposed by Chinali et al. [16], that indexes the
LVM for height
With regard to cIMT as a marker of preclinical atherosclerosis in adolescents, studies regarding the impact of sex are limited and not conclusive. In addition, the lack of robust normative tables makes difficoult the interpretation of cIMT [56]. We observed a higher prevalence of high cIMT in boys than girls, but the finding that approximately 76% of youths with OW/OB had cIMT levels above 90th percentile [25] highlights the difficulty of interpreting cIMT in adolescents with OW or OB from a clinical point of view.
This study has some limitations that should be acknowledged. Firstly, the cross sectional design does not allow to understand whether the sex-related differences in the traditional cardiometabolic risk factors might increase in the following years. Secondly, information about pubertal stage, family history or lifestyle behaviours were not available to assess their possible influence on the sex prevalence of visceral adiposity, hypertension, fatty liver disease, and preclinical signs of target organ damage. On the other hand, study strengths include the multicenter study design and the large and well-phenotyped sample allowing to show significant sex-differences in the cardiovascularrisk profile of children with obesity.
In conclusion, this cross-sectional study conducted in adolescents with OW/OB confirmed our hypothesis that sex-related differences in cardiovascular risk might be present in adolescents with OW/OB. Boys exhibited a higher degree of visceral adiposity, hypertension, FLD, LVH, and cIMT than girls. Conversely, a higher prevalence of MReGFR was detectable in girls. These findings mirror the cardiovascular risk profile observed in non-elederly and non-diabetic adults. Considering that cardiovascular risk might occur early in life, the role of a non modifiable factor such as sex should be considered for early cardiovascular risk stratification.
Worthy of note, relevant clinical and prognostic implications could be also drawn. Indeed, a better understanding of sex-differences related to cardiometabolic health of children with OW/OB might enhance the effectiveness of obesity prevention interventions by significantly improving the challenging management of these at-risk patients in clinical practice.
Therefore, more research efforts are needed to expand knowledge about sex-differences in the context of pediatric OB. As a matter of fact, this could pave the way for an insightful approach of personalized medicine through targeted strategies for children with OB.
BP, blood pressure; BMI, body mass index; cIMT, carotid intima media thickness;
eGFRFAS
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
PDB and GV designed the research study. ADS, MRL, DC, MW, EMdG, AM, CM, MFF, EM, VC, FF, GM, NM, AI performed the research. PDB analyzed the data. PDB, ADS, and GV wrote the manuscript. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
The research was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Research Ethical Committee of University of Campania “Luigi Vanvitelli” (protocol code 834/2016) and an informed parental consent was obtained before any procedure.
Not applicable.
This research received no external funding.
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
