1. Introduction
Atherosclerosis (AS) is a chronic inflammatory disease. Systemic or localized
inflammation plays a central role in the onset and progression of AS, while
inflammatory markers have been shown to predict cardiovascular disease (CVD)
independently of traditional risk factors [1, 2, 3]. The downstream metabolites of
polyunsaturated fatty acids (PUFAs), such as arachidonic acid (AA),
prostaglandins (PG), thromboxanes (TXs), leukotriene (LTs), and other
inflammatory factors, have an important impact on the development of AS. PUFAs
and fatty acid desaturase (FADS), a key enzyme affecting its metabolism, play an
equally important role in the pathogenesis of AS [4, 5].
As one of the essential dietary fatty acids in the human body, the content of
PUFAs in the body reflects both the dietary intake and the fatty acid desaturase
activity [5]. Previous studies have found that the type and amount of PUFAs being
consumed are closely related to atherosclerotic cardiovascular disease (ASCVD).
Prospective, observational studies support the role of omega-3 PUFAs in the
primary prevention of ASCVD [6], although randomized controlled trials (RCTs)
have often reached neutral conclusions [7, 8]. The potential impact of the intake
of omega-6 PUFAs on ASCVD is also controversial, with previous studies suggesting
that higher intakes of omega-6 PUFAs (predominantly linoleic acid) are associated
with a lower risk of ASCVD [9, 10]. However, clinical studies have shown that
excessive intake of -6 PUFAs (predominantly linoleic acid) leads to
increased production of proinflammatory factors, which can lead to a higher risk
of developing ASVCD [11, 12]. Thus, the roles of the omega-3 and omega-6 PUFAs in
AS are complex and remain inconclusive. Studies on lipid metabolism disorders and
inflammatory responses due to the regulation of gene expression have shown that
there is as yet an undefined association between fatty acid desaturases, they are
regulated by the FADS gene cluster, and AS, whereby fatty acid desaturase
expression levels, as well as its activity, can differentially affect AS [13, 14, 15].
Based on existing studies, the effect of polyunsaturated fatty acid metabolism on
atherosclerosis remains in the exploratory stage. This article aims to illustrate
the relationship between polyunsaturated fatty acids, the regulation of fatty
acid desaturases by the fatty acid desaturase gene cluster, and atherosclerosis.
2. Classification of Polyunsaturated Fatty Acids
Fatty acids are components of cell membrane phospholipids with specific
functions, metabolism, and signaling roles. As a member of the fatty acid family,
PUFAs are a crucial nutrient for mammalian growth and development, they are
biologically active cellular components of membrane phospholipids, a substrate
for signaling molecules, and a direct regulator of gene expression that can
directly affect cellular function and the responsiveness of cells and tissues to
signals [16, 17]. Moreover, PUFAs can regulate inflammatory processes by
modulating signaling pathways [18, 19]. Fatty acids can be classified into
short-chain, medium-chain, and long-chain fatty acids according to the number of
carbon atoms they contain and into saturated fatty acids, monounsaturated fatty
acids, and polyunsaturated fatty acids according to the number of carbon–carbon
bonds they possess. PUFAs can be classified into two categories—-3
PUFA and -6 PUFA—according to the position of the double bond in
their chemical structure and the principle of counting the position of the first
double bond following the methyl carbon atom. The chemical structure of fatty
acids is usually expressed as the number of carbon atoms, double bonds, and the
position of the first double bond. For example, eicosapentaenoic acid (EPA) is
expressed as 20:5 -3, meaning it contains 20 carbon atoms, five double
bonds, and belongs to the -3 PUFA. In addition, fatty acids also have
the -coding system, which is different from the -coding
system because the double bond position is counted from the carboxyl carbon atom.
3. Endogenous Metabolism of Polyunsaturated Fatty Acids
The human body cannot synthesize the amount of PUFAs needed for the body’s
metabolism, meaning the -3 PUFA and -6 PUFA need to be
supplemented through the diet, thus, they are referred to as essential fatty
acids [20, 21]. The proportion of PUFAs in the diet is dominated by linoleic acid
(LA) and -linolenic acid (ALA), which are precursors of short-chain
PUFAs that can be converted to biologically active long-chain PUFAs by
5 and 6 desaturase and elongase enzymes. The metabolism and
metabolites of PUFAs will be summarized below according to the different types of
PUFAs.
3.1 Endogenous Metabolism and Metabolites of Omega-3 Polyunsaturated
Fatty Acids
ALA can be gradually converted to stearidonic acid (SDA), EPA, docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA) in
vivo by the action of the desaturase system and the elongation enzyme. The
downstream metabolites of EPA and DHA are physiologically crucial for the
organism. Specifically, EPA and DHA are catalyzed by cyclooxygenase (COX),
lipoxygenase (LOX), and cytochrome P450 oxidase (CYP450) to produce a series of
specialized pro-resolving mediators (SPMs). SPMs include separate families of
molecules: resolvins, protectins, and maresins. These act as stimulatory cell
agonists, arresting neutrophil infiltration and enhancing macrophage uptake of
apoptotic cells [22]. Resolvins can inhibit neutrophil infiltration, inhibit
platelet aggregation, and reduce the production of proinflammatory factors [23].
Protectins can promote the expression and activity of antiapoptotic proteins and
inhibit the expression and activity of proapoptotic proteins [24]. In addition,
EPA is catalyzed by COX to produce thromboxane A3, which inhibits platelet
aggregation, prostacyclin I3, which promotes vasodilatation, as well as
prostaglandin E3 and leukotriene LT5, which has anti-inflammatory properties.
Thus, both EPA and DHA metabolites can exert anti-inflammatory effects (Fig. 1).
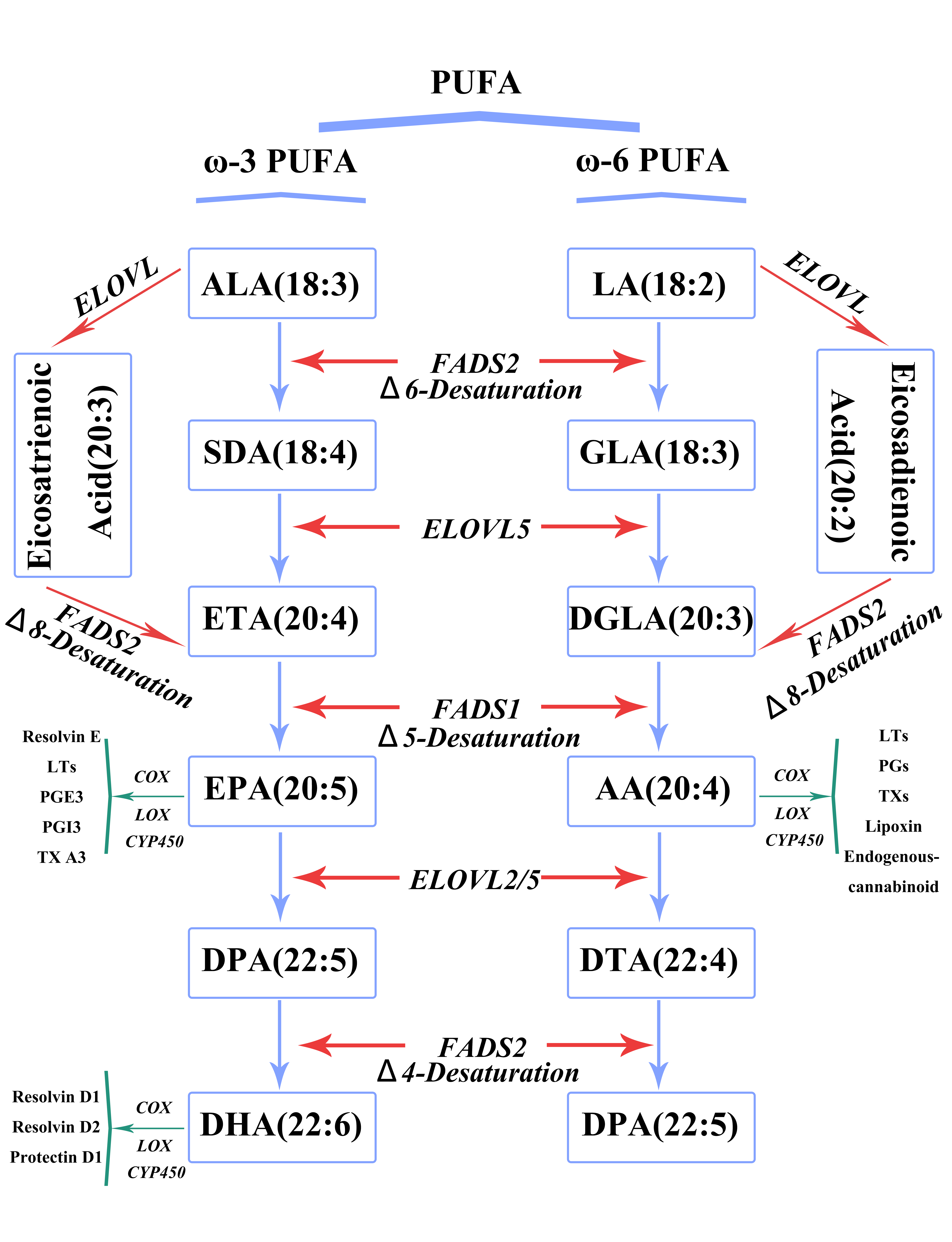 Fig. 1.
Fig. 1.
Classification and metabolism of PUFAs. ALA,
-linolenic acid; SDA, stearidonic acid; ETA, eicosatetraenoic acid;
EPA, eicosapentaenoic acid; DPA, docosapentaenoic acid; DHA, docosahexaenoic
acid; LA, linoleic acid; GLA, gamma-linoleic acid; DGLA, dihomogamma linoleic
acid; AA, arachidonic acid; DTA, docosatetraenoic acid; DPA, docosapentaenoic
acid; ELOVL, elongation of very long fatty acids; FADS, fatty acid desaturase;
COX, cyclooxygenase; LOX, lipoxygenase; CYP450, cytochrome p450; LT, leukotriene;
PGE3, prostaglandin E3; PGI3, prostacyclin I3; TX, thromboxane synthase; PUFAs, polyunsaturated fatty acids; FADS2, fatty acid desaturase 2.
3.2 Endogenous Metabolism and Metabolites of Omega-6 Polyunsaturated
Fatty Acids
LA can be converted to gamma-linolenic acid (GLA), double-high gamma-linolenic
acid (dihomo--linolenic acid, DGLA), arachidonic acid (AA), and
DPA in vivo, under the
action of the desaturase system and elongation enzyme. AA has essential
biological functions and is catalyzed by COX to produce prostaglandin E2 and
thromboxane A2, which promotes the inflammatory response, platelet aggregation,
and vasoconstriction. AA can also produce proinflammatory factors from the
leukotriene four-family, which, in the presence of LOX, play an essential role in
the development and maintenance of the inflammatory response. In addition, AA is
also catalyzed by LOX to produce lipoxin A4 (lipoxin) and lipoxin B4, which have
a pro-resolving role in the abrogation of inflammatory responses (Fig. 1).
However, in cardiovascular diseases, metabolites of AA exert deleterious effects
that are proinflammatory, prothrombotic, and proplatelet aggregation to promote
the development of atherosclerosis [25].
4. Effect of Polyunsaturated Fatty Acid Intake on Atherosclerotic
Cardiovascular Disease
The role of PUFA intake in ASCVD has been controversial [6, 8, 26]. Although it
has been demonstrated that -3 PUFAs can lower blood triglyceride levels
and -6 PUFAs can lower blood total cholesterol levels, the results of
the effects of -3 and -6 PUFAs on ASCVD in clinical practice
have been inconsistent. Although clinical guidelines point to a positive effect
from the use of icosapent ethyl and EPA in preventing ASCVD [27, 28, 29], there is a
high degree of clinical heterogeneity in the design of previous studies and the
final results. There is no solid evidence for using PUFAs to effectively treat or
prevent ASCVD in patients with different backgrounds [30]. Therefore, the effects
on ASCVD following the intake of PUFAs will be briefly summarized, as well as the
reasons for the different results among the various studies.
4.1 Effect of Omega-3 Polyunsaturated Fatty Acids on Atherosclerotic
Cardiovascular Disease
The controversy over the effect of -3 PUFAs on ASCVD lies in the fact
that different clinical studies have yielded different results. REDUCE-IT, a
multicenter RCT that included and followed more than 8000 patients with
cardiovascular disease for almost five years, showed that compared to the
placebo, patients who ingested EPA (4 g/d) underwent a significant reduction in
the risk of cardiovascular death and nonfatal risk of myocardial infarction [31].
However, two similar RCTs (OMEMI (Omega-3 Fatty acids in Elderly with Myocardial Infarction), STRENGTH study (the Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia)) concluded that the intake of
omega-3 PUFAs (EPA+DHA) did not reduce the risk of nonfatal myocardial
infarction, stroke, or cardiovascular death [32, 33]. A systematic evaluation of
the effects of -3 PUFAs on cardiovascular health showed that although
the increased intake of EPA and DHA lowered plasma triglycerides and increased
high-density lipoprotein (HDL) levels, they did not reduce the incidence of
coronary artery disease (CAD), stroke, or other ASCVD events, nor the risk of
death. In addition, increased intake of ALA reduces the risk of death from CAD,
and increased intake of ALA has a preventive effect on ASCVD [34, 35, 36]. An RCT of
the CVD population in the United States showed that increased omega-3 PUFAs
(EPA+DHA) did not significantly reduce the risk of major cardiovascular events
(myocardial infarction, stroke, and cardiovascular death) [8]. Circulating DHA,
total omega-3, LA, and total omega-6 concentrations had no protective effect on
the risk of cardiovascular disease in a Mendelian randomization study from the UK
Biobank [37].
Although the above studies yielded different results, an analysis of these
results from the different studies found that the composition of -3
PUFAs being consumed by the patients was different in each study, which may be
one of the reasons for the controversy over the effects of -3 PUFAs on
ASCVD. It has been suggested that EPA intake alone may be more effective in
reducing the risk of cardiovascular disease than EPA+DHA [38], that serum EPA
levels may need to reach a certain threshold to exert a preventive effect on
ASCVD, and that a high dose (1 g/d) significantly reduces the risk of
cardiovascular events compared to low-dose EPA levels [39, 40]. In addition, when
EPA was combined with DHA, higher DHA levels attenuated the preventive effect of
EPA on ASCVD [30]. Therefore, the intake of EPA in -3 PUFAs and EPA
blood levels in the study population may be important reasons for the observed
variability in the effects of -3 PUFAs on ASCVD [41]. At present, the
effect of omega-3 PUFAs on atherosclerosis remains a focus of clinical research.
An ongoing RCT (NCT05365438) from Korea will assess the effects of combination
therapy using atorvastatin and omega-3 PUFAs (EPA+DHA) compared with atorvastatin
and ezetimibe combination therapy in diabetes mellitus type 2 (T2DM) patients with asymptomatic carotid
atherosclerosis. The progression of carotid intima-media thickness and carotid
artery plaques will be evaluated by three-dimensional (3D) carotid ultrasound. Another ongoing RCT
(NCT05725486) from Croatia will investigate the influence of n-3 PUFAs enriched
chicken on vascular and endothelial functions in a population of healthy young
subjects and active athletes. Specifically, whether the intake of omega-3 PUFAs
affects lipid profiles, oxidative stress, and inflammation. Further elucidating
the mechanisms of vascular protection for omega-3 PUFAs may lead to new
interventions for atherosclerosis in clinical practice.
4.2 Effect of Omega-6 Polyunsaturated Fatty Acids on Atherosclerotic
Cardiovascular Disease
The effect of the intake of -6 PUFAs on ASCVD is equally
controversial. A meta-analysis involving 44 prospective cohort studies showed
that higher LA intake was associated with a reduced risk of death from CAD. It
supported the potential long-term benefits of -6 PUFAs in reducing the
risk of cardiovascular disease [9]. In addition, replacing a saturated fatty acid
diet with an -6 PUFA (LA) may reduce the risk associated with CAD [42].
However, a systematic evaluation of the effects of -6
PUFAs on cardiovascular health suggests that increased intake of -6
PUFAs (LA+GLA) may reduce total cholesterol levels in the blood but does not
significantly affect the risk of cardiovascular disease or mortality; therefore,
the potential benefits in terms of reduced myocardial infarction remain to be
demonstrated [43]. Another meta-analysis of randomized controlled trials showed
that increasing the intake of omega-6 PUFAs (LA, GLA, DGLA, and AA) did not
affect the incidence of myocardial infarction, stroke, CAD, and mortality [44].
An RCT studying the effects following the intake of different types of PUFAs from
vegetable oils on cardiovascular disease in a population of hypercholesterolemic
adults in China showed that after one year of measuring the intake of oleic acid
(saturated fatty acid)-rich peanut oil, LA-rich corn oil, and ALA-rich blended
oils, on fasting lipids, glucose, insulin concentration, and high sensitivity
C-reactive protein levels of the different populations, the intake of different
fatty acids did not affect cardiovascular risk factors [45].
Although some studies supported beneficial outcomes for cardiovascular disease
following an increased intake of -6 PUFAs, several studies still
produced conflicting results. The intake of -6 PUFAs may negatively
impact ASCVD by causing an increase in downstream metabolites, such as
proinflammatory 2-series prostaglandins and 4-series leukotriene. However, it is
difficult to show a direct effect of -6 PUFAs on ASCVD when the impact
of -6 PUFA metabolites on ASCVD is studied [42]. Therefore, more
rigorous RCTs are needed to elucidate whether -6 PUFAs play a
preventive or promotional role in ASCVD.
The competitive inhibition of enzymes between -3 and -6
PUFAs makes balancing the intake ratio between both -3 and -6
PUFAs complex. In addition, PUFA metabolism is also affected by genetic factors,
which lead to alterations in the activity of fatty acid desaturase and the
subsequent conversion of its products. Therefore, focusing only on the intake of
a particular PUFA without considering the proportion of -3/-6
PUFAs in the diet and the influence of genetic factors on PUFA metabolism makes
it difficult to explain the variability in the results from these studies.
5. Fatty Acid Desaturase and FADS Gene Cluster
5.1 Function and Classification of Fatty Acid Desaturases
The primary function of fatty acid desaturases is to dehydrogenate and introduce
a double bond between the carbon atoms of the fatty acyl chain. In humans,
membrane-bound fatty acid desaturases are known as “front-end” desaturases, and
introduce a nascent double bond between an existing double bond, usually between
the carboxyl group and the ninth carbon atom of the terminal methyl group, with
front-end desaturation occurring at the 4, 5, 6,
and 8 positions, while they are responsible for the endogenous
biosynthesis of PUFAs. Therefore, fatty acid desaturases are categorized into
four different types based on the location where desaturation occurs, namely,
4 fatty acid desaturases, 5 fatty acid desaturases,
6 fatty acid desaturases, and 8 fatty acid desaturases [46].
5.2 Structure of the Fatty Acid Desaturase Gene
Cluster
The fatty acid desaturase (FADS) gene cluster that encodes fatty acid desaturase
is located on human chromosome 11 (11q12-13.1) [47]. Data from the National Center of Biotechnology Information (NCBI) database
demonstrates that FADS1, FADS2, and FADS3 are composed
of 13 exons and 11 introns, and the total lengths of FADS1,
FADS2, and FADS3 are 17.2, 39.1, and 18.7 kb, respectively
(Fig. 2). FADS1 encodes 5 fatty acid desaturase;
FADS2 encodes 4, 6, and 8 fatty acid
desaturase; FADS3 encodes 9 and 13 fatty acid
desaturase. Since the gene clusters have the same location and similar
structures, it is hypothesized that they have evolved based on gene duplication,
meaning they have acquired substrate specificity [46, 48, 49].
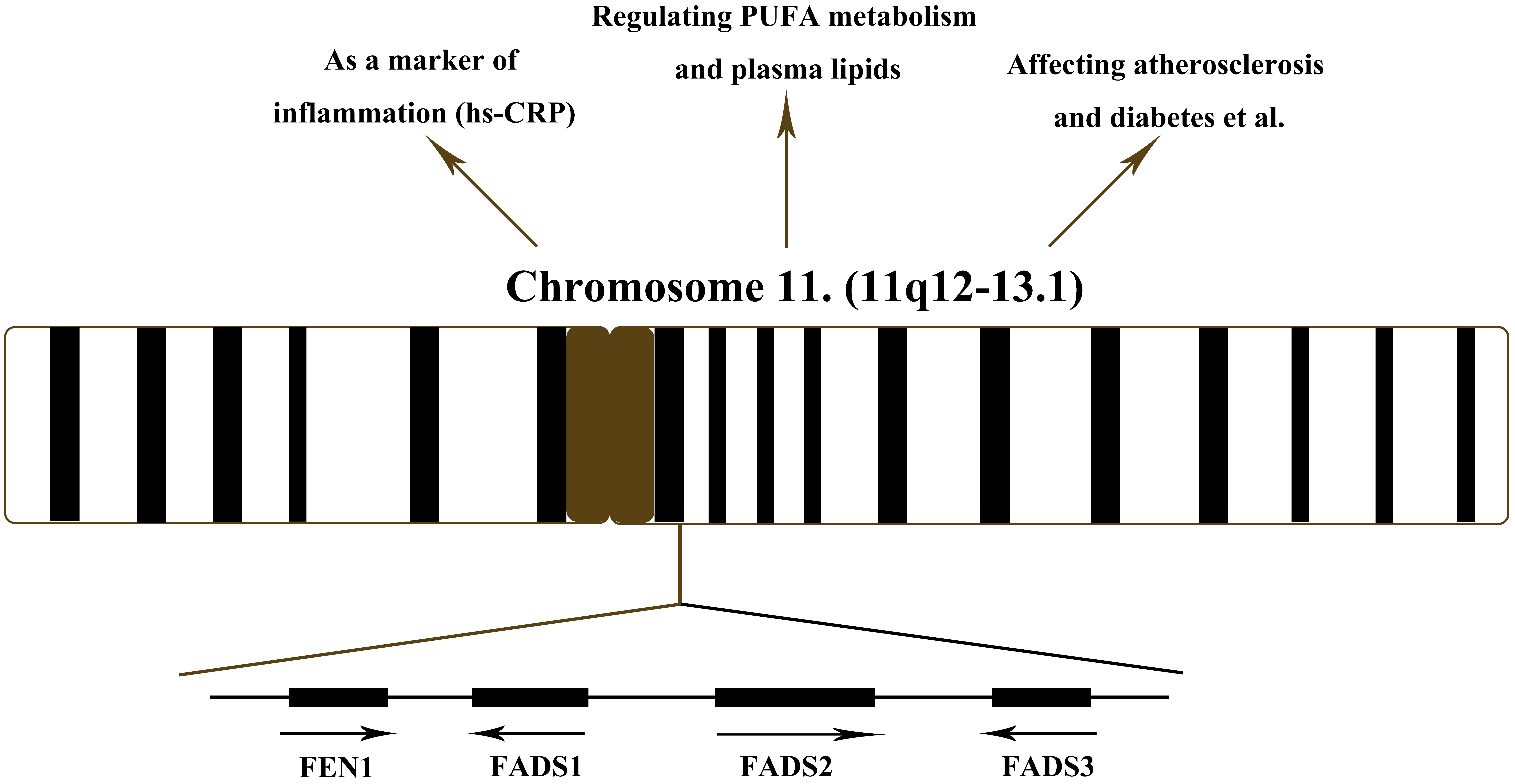 Fig. 2.
Fig. 2.
Function of FADS cluster and location of FADS cluster in
chromosome 11. FEN1, flap endonuclease 1; FADS1, fatty acid desaturase 1; FADS2,
fatty acid desaturase 2; FADS3, fatty acid desaturase 3; PUFA, polyunsaturated
fatty acid; hs-CRP, high-sensitivity C-reactive protein.
5.3 Effect of Variants in the Fatty Acid Desaturase Gene Cluster on
the Metabolic Activity of the Organism
Fatty acid desaturase is a critical enzyme in PUFA metabolism, and gene
polymorphism in FADS affects the activity and function of fatty acid
desaturase [50, 51], which in turn affects metabolic activities in the body, such
as lipid concentrations, cardiovascular disease risk, pregnancy, cognitive
function, Alzheimer’s disease, overweight, and type 2 diabetes mellitus
[20, 52, 53].
The first exploratory study of variants in the FADS gene cluster found that
single nucleotide polymorphisms (SNPs) in FADS1 and FADS2affect the composition of serum phospholipid fatty acids in healthy adults, as
evidenced by changes in the levels of LA, DGLA, AA, and DPA [54]. Other studies
have obtained consistent results. Mid-FADS gene cluster variants in Caucasian
populations are associated with higher levels of precursor PUFAs and lower levels
of AA and EPA, less inflammation, and lower risks of cardiovascular disease
[55, 56]. A recent cohort study on FADS SNPs and fetal growth and
development in pregnant women in China’s Han population showed that pregnant
women with the FADS1/rs174448G,
FADS3/rs174455T, and FADS3/rs174464A allele
should be supplemented with DHA-rich -3 PUFAs exogenously during
pregnancy due to the blockage of the endogenous synthesis of DHA [57].
These findings suggest that FADS gene cluster variants have a direct impact on
PUFA metabolism, which in turn affects the risk of inflammation and
cardiovascular disease, and that FADS gene cluster variants in pregnant women
have a direct effect on PUFA levels in breast milk, which in turn affects infant
growth and development. Several studies on the association between variants in
the FADS gene cluster and child development have shown that the FADS
SNPs are strongly associated with the synthesis of DHA and AA in vivo
and can influence intelligence quotient (IQ) as well as cognitive ability in infants and young children,
as shown by the fact that genetic restriction of endogenous PUFA synthesis
results in poorer cognitive development in infants fed formulas that do not
provide DHA and AA. This developmental deficit can be eliminated when infants are
fed AA. European legislation mandating the addition of AA and DHA to infant
formula may address developmental deficiencies due to insufficient synthesis of
endogenous PUFAs in infants or mutations in the FADS gene cluster [20].
A case–control study reported that the
FADS1/rs174556 genotype significantly increased the
susceptibility to Alzheimer’s disease by regulating the efficiency of AA
synthesis in -6 PUFAs [58]. However, this study had a small sample size
and did not analyze AA derivatives in detail. Another case–control study
reported an association between FADS1/rs174556,
FADS2/rs174617, and obesity, by demonstrating that plasma
levels of omega-6 PUFAs and AA were higher in overweight and obese patients;
however, the difference in -3 PUFA levels was not significant. The
study concluded that mutations in the FADS1/FADS2 locus could
cause metabolic disorders and increase the risk of cardiovascular disease [59].
Genome-wide association studies (GWAS) in European and Asian populations have
shown that FADS1/rs174546 is associated with reduced
5 fatty acid desaturase activity, obesity, and the risk of insulin
resistance [60]. A study of the genetic etiology of type 2 diabetes mellitus
showed that people with the FADS1/rs174546G allele had higher
fasting insulin levels and higher HOMA-IR (an indicator to assess the level of
insulin resistance) and that the increase in Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was more pronounced with
elevated plasma DGLA and AA levels [61]. FADS1/rs174547 effects
on dyslipidemia have been reported in many races, and a study of the Chinese
adult population showed that FADS1/rs174547 was significantly
associated with high triglyceride levels in men and negatively associated with
low density lipoprotein (LDL) cholesterol levels in women, thereby suggesting that it may be a sex-specific
SNP locus [62].
The FADS gene cluster affects a variety of diseases by regulating the metabolism
of PUFAs. Genetic variants in the FADS gene cluster are associated with an
increased risk of dyslipidemia, obesity, and insulin resistance. These outcomes
are strongly associated with atherosclerotic disease and produce a state of
hypersecretion of proinflammatory cytokines, which increases the body’s
susceptibility to atherosclerotic disease.
5.4 Effect of Fatty Acid Desaturase Gene Cluster Variation on
Atherosclerotic Cardiovascular Disease
Previous studies on the association between differences in dietary PUFAs and the
metabolism of PUFAs in humans and ASCVD suggest that both -3 and
-6 PUFAs and their downstream metabolites can affect ASCVD. However,
there are relatively few studies on the effect of the FADS gene cluster on ASCVD.
Genetic studies have shown that variants in FADS1, encoding the
5 fatty acid desaturase, and FADS2, encoding the 6
fatty acid desaturase, are most directly genetically linked to plasma PUFA levels
and that the FADS gene cluster is the most important locus for influencing PUFA
metabolism [56, 63, 64, 65]. Therefore, the FADS SNP can alter the
accumulation of PUFAs and directly affect ASCVD. Genetic studies have provided a
theoretical foundation for exploring the impact of the FADS gene cluster on the
pathogenesis of ASCVD. Several studies have found a strong association between
FADS gene cluster variants and ASCVD (Table 1, Ref. [10, 51, 66, 67, 68, 69, 70, 71, 72, 73]). For
example, a Mendelian randomization study of European and American populations
showed that plasma ALA and LA levels were higher in the FADS1/rs174547 sub-allele population and that this population
was less likely to have CAD, stroke, and aortic stenosis, thereby suggesting that
the FADS1 SNP drives the association between plasma levels of PUFAs and
ASCVD [66]. An RCT on the effect of the FADS2 SNP on left ventricular
remodeling after acute myocardial infarction (AMI) demonstrated that six months
of high doses of -3 PUFAs after an AMI resulted in a significant
attenuation of adverse left ventricular remodeling, noninfective myocardial
fibrosis, and amelioration of the FADS2 rs1535GG-imposed
hyperinflammatory response [51]. A case–control study in the Han Chinese population in northern China showed a strong association between the
FADS3 SNP and CAD. The recessive G allele of FADS3
rs1000778 was associated with a higher risk of CAD, whereas the minor
AA allele was associated with a lower risk of CAD; however, the plasma
cholesterol and triglyceride levels remained similar between the two genotypes
[67]. Aortic stenosis contributes to cardiovascular mortality and morbidity, and
recent studies found that FADS1 SNP is associated with the risk of
aortic stenosis. A GWAS of 44703 participants in the Genetic Epidemiology
Research on Adult Health and Aging (GERA) cohort, one of the largest collections
of aortic stenosis in the world, demonstrated that the
FADS1/FADS2 locus variants (rs174547) are associated
with aortic stenosis, while higher levels of AA and a higher ratio of AA/LA were
associated with increased odds of calcification of the aortic valve leaflets
[68]. A study of the relationship between localized PUFA in
aortic valves and FADS genotypes by expression quantitative loci (eQTL)
found that the minor C allele of rs174547, which corresponds to
the protective genotype for aortic stenosis, was associated with higher
FADS2 mRNA levels in calcified valve tissues, whereas FADS1
mRNA and other transcripts in proximity of the SNP were unaltered. In contrast,
the 5 desaturase activity and the 6 desaturase activity were
decreased. The authors concluded that the association between the FADS1
genotype and lower risk for aortic stenosis may implicate DHA and DHA-derived
specialized pro-resolving mediators that contribute to a protective effect [74].
In addition, the diet–gene interaction for ASCVD is crucial. A recent
cross-sectional population-based cohort study demonstrated that differential
associations between the FADS1 locus variant and carotid–femoral pulse
wave velocity (for assessing atherosclerosis) were observed depending on the
intake of omega-3 PUFAs, with a high intake of omega-3 PUFAs attenuating the
FADS1 locus variant-dependent associations. This suggests that the high
intake of omega-3 PUFAs (EPA/DHA) may compensate for an unfavorable
FADS1 locus genotype [75].
Table 1.Studies on the association between FADS gene cluster variation
and atherosclerotic cardiovascular disease.
| First author (year) |
Study type |
Intakes/evaluation indicators |
SNP |
Outcomes |
Results |
| Baylin (2007) [69] |
Case–control |
ALA levels in plasma and adipose tissue |
FADS2 promoter deletion |
MI |
No significant effect |
| Kwak (2011) [70] |
Case–control |
Plasma PUFAs and total cholesterol levels |
FADS1 rs174537 |
CAD |
FADS1 rs174537 T allele decreased plasma total cholesterol, AA/LA ratio, and decreased the risk of CAD |
| Li (2013) [71] |
Case–control |
Δ6 fatty acid desaturase activity (AA/LA) |
FADS1 rs174537 |
CAD |
The FADS1 rs174537T allele population has lower Δ6 fatty acid desaturase activity and reduced CAD risk; however, the G allele has increased Δ6 fatty acid desaturase activity and increased CAD risk |
| Hellstrand (2014) [72] |
Cohort |
LA, ALA intakes |
FADS1 rs174546 |
ASCVD |
Negative association of dietary ALA: LA ratio or ALA intake with ASCVD in sub-allele T carriers |
| Liu (2015) [73] |
Case–control |
EPA, DHA intakes |
FADS1 rs174547 |
CAD |
Lower dietary intake of EPA or DHA individuals associated with a higher risk of CAD |
| Wu (2017) [67] |
Case–control |
Blood lipid level |
FADS3 rs1000778 |
CAD |
The secondary allele AA was associated with a lower risk of CAD, whereas the recessive allele G was associated with a higher risk of CAD |
| Marklund (2019) [10] |
Meta |
Plasma LA, AA levels |
FADS1 rs174547 |
ASCVD |
LA was negatively associated with ASCVD in carriers of the common allele in purebloods and not in carriers of the minor allele |
| Yuan (2019) [66] |
Mendelian randomization |
Plasma fatty acid levels |
FADS1 rs174547 |
CAD, stroke, AS |
The FADS1 rs174547 sub-allele is negatively associated with ASCVD |
| Kwong (2019) [51] |
RCT |
ω-3 PUFAs intakes |
FADS2 rs1535 |
Left ventricular remodeling after AMI |
A high omega-3 PUFAs diet ameliorates the heightened inflammatory response associated with FADS2 rs1535GG, significantly attenuating adverse left ventricular remodeling and non-infarcted myocardial fibrosis |
| Chen (2020) [68] |
GWAS |
Plasma LA, AA levels |
FADS1/FADS2 rs174547 |
Aortic valve stenosis and calcification |
FADS1/FADS2 locus variants are associated with aortic stenosis and calcification, and AA level is strongly associated with aortic stenosis |
RCT, randomized controlled trial; GWAS, genome-wide association study; MI,
myocardial infarction; CAD, coronary artery disease; ASCVD, atherosclerotic
cardiovascular disease; AMI, acute myocardial infarction; AS, aortic valve
stenosis; FADS, fatty acid desaturase; ALA, -linolenic acid; PUFAs,
polyunsaturated fatty acids; AA, arachidonic acid; LA, linoleic acid; EPA,
eicosapentaenoic acid; DHA, docosahexaenoic acid; SNP, single nucleotide polymorphism.
The effect of FADS gene cluster variants on ASCVD is mainly due to regulating
the metabolism of PUFAs in the body, by altering fatty acid desaturase activity.
In addition, exogenous supplementation of PUFAs can reverse the adverse effects
of certain FADS gene cluster variants, suggesting that certain FADS
gene-deficient disorders can be treated by increasing the intake of PUFAs that
can prevent or alter the course of cardiovascular diseases.
5.5 Exploratory Studies of the Effects of FADS1 on Atherosclerosis
Various clinical data indicate that the FADS gene cluster has an essential
effect on PUFAs metabolism, ASCVD, and glucose metabolism. Since FADS1
encodes 5 fatty acid desaturase and its metabolites, EPA and AA play
essential roles in the inflammatory response and atherosclerosis. In a study
investigating the effect of FADS1 on atherosclerosis and its mechanism
of action, Powell et al. [14] showed that under high-fat dietary
conditions, FADS1 knockout mice had weight loss, improved blood glucose,
and reduced atherosclerotic plaques compared with wild-type mice. This study
hypothesized that the low expression of FADS1 is associated with a
reduced inflammatory response in the arterial wall, which is mainly manifested as
a reduced AA/LA ratio in plasma and adipose tissue, and plays a positive role in
preventing AS. Shuichi et al. [76] found that oral administration of a
5 fatty acid desaturase inhibitor to apolipoprotein E (ApoE) KO mice on a
high-fat dietary background resulted in a reduction in atherosclerotic plaques, a
decrease in the levels of AA and DHA, and an increase in the levels of DGLA. This
finding is in general agreement with Powell’s conclusions. Gromovsky et
al. [15] used antisense oligonucleotides (ASOs) to specifically knockdown
FADS1 in the liver, adipose, and reticuloendothelial systems of
low density lipoprotein receptor (LDLR) KO mice. However, they produced a different result, whereby the
specific knockdown of the FADS1 in LDLR KO mice aggravated
atherosclerosis and the hepatic inflammatory response, which differed from the
previous two studies. The authors highlight several possibilities for their
differing conclusions. The study by Powell et al. [14] did not validate
the hepatic levels of FADS1 expression. Second, the Powell et
al. [14] mouse model produced sub-alleles; therefore, the functionality of
FADS1 was not completely lost. In addition, the different genetic
backgrounds of the two mice may be another reason for the inconsistent results.
The study also noted that a diet of -3 PUFAs (ALA+SDA) reduced the area
of the atherosclerotic plaque in the aortic root of the mice compared with a
saturated fatty acid diet.
Studies have shown that mice of different genetic backgrounds present different
outcomes after FADS1 knockdown and that the percentage of PUFAs in the
diet affects the survival and progression of atherosclerosis in mice. The
downstream metabolites and signal transduction pathways regulated by
FADS1 have yet to be thoroughly studied. Whether FADS1 can
directly affect AS by affecting the production of inflammatory factors, such as
PGs, LTs, and TXs is worthy of further
exploration.
6. Fatty Acid Metabolism in Macrophages and Atherosclerosis
Monocytes/macrophages play a crucial role in the development and progression of
ASCVD and the deterioration of advanced lesions. Intimal infiltration and
modification of plasma-derived lipoproteins and their uptake, mainly by
macrophages, with the ensuing formation of lipid-filled foam cells, can initiate
atherosclerotic lesion formation and alter the efferocytotic removal of apoptotic
cells and foam cells, which contributes to the progression of atherosclerotic
lesions [77]. One of the properties of macrophages is the ability to dynamically
regulate PUFAs metabolism during the acute phase of the inflammatory
stimulus-response and inflammatory regression. This dynamic regulation of PUFAs
metabolism may contribute to macrophage plasticity, in particular by controlling
the balance between pro- and anti-inflammatory mediators [78]. Therefore, PUFAs
metabolism in macrophages is closely related to atherosclerosis.
Liver X receptors (LXRs) are nuclear receptors that can participate in
regulating cholesterol homeostasis and fatty acid metabolism and are essential in
controlling inflammation and innate immune responses. A recent study
investigating the treatment of human primary monocyte–macrophages with LXR
agonists found that PUFAs synthesis was affected by the significant induction of
5 and 6 desaturases (FADS1 and FADS2,
respectively) after LXR agonist treatment alongside the induction of acyl-CoA
synthase long-chain family member 3 (ACSL3) and the fatty acid elongase 5
(ELOVL5). In addition, LXR agonist treatment of ApoE-/- mice led to significant
changes in the PUFAs profile in atherosclerotic arteries with increases in both
the AA/LA and the DHA/EPA ratios, while also being associated with the decreased
expression of proinflammatory genes, such as Cox2 and Il1. Therefore,
local production of PUFAs and derived lipid mediators in macrophages triggered by
LXR within the atheromatous plaque could affect inflammation and the development
of atherosclerosis [79]. Another study demonstrated that PUFAs
can alter the micro RNA (miRNA) profiles of macrophages. When macrophages are enriched with
either DHA or AA, they alter the expression of many miRNAs closely associated
with inflammation, thus, suggesting that PUFAs are regulators of macrophage
phenotypes and the inflammatory response [80]. Macrophages can sense internal and
environmental changes and subsequently adapt their phenotype. This sequence is
commonly named macrophage activation (classical or alternative) or polarization,
and the most common are M1 and M2 polarization. In vitro stimulation of
macrophages with interferon-gamma (IFNg) and lipopolysaccharide (LPS) leads to M1
polarization, while interleukin-4 (IL-4) or IL-13 treatment induces alternative
activation of M2 polarization. M1 macrophages are characterized by a
proinflammatory phenotype with intense bactericidal activities, while M2
macrophages are involved in the resolution of inflammation and in tissue
remodeling and repair. The current view is that an unbalanced interplay between
M1 and M2 macrophages could contribute to atherogenesis [81, 82]. Fatty acid
synthesis in macrophages is activated during M1 polarization, and excess fatty
acid and triglyceride synthesis promote foam cell formation with proatherogenic
effects [83]. A study demonstrated that peroxisome proliferator-activated
receptor gamma (PPARg), a nuclear receptor activated by fatty acid derivatives
that control fatty acid oxidation, was required for M2 polarization [84]. A
recent study found that FADS1 knockdown in macrophages was associated
with a tendency toward M1 and away from M2 polarization. Specifically,
FADS1 knockdown resulted in augmented LPS-driven proinflammatory gene
expression yet was associated with diminished IL-4-driven alternative activation
gene signatures. Therefore, FADS1 reciprocally regulates M1 and M2
polarization programs in the macrophage [15]. The metabolism of fatty acids in
macrophages involves M1 and M2 polarization processes, while FADS1
affects the metabolism of PUFAs in macrophages, affecting the dynamic balance
between proinflammatory and pro-resolving mediators. Future studies should focus
on how macrophages regulate their metabolism of PUFAs, and more studies are
needed to assess the impact of FADS1 on atherosclerosis development
under various metabolic conditions.
7. Conclusions
Atherosclerosis results from a multifactorial combination of factors. The
development and progression of atherosclerosis are associated with PUFAs, fatty
acid desaturase, and FADS gene cluster variation (Fig. 3). Clinical trials to
establish the necessary -3 to -6 ratio for optimum CVD health
should consider ethnic background, genetic predisposition, biochemical markers,
and dietary habits. The effects of dietary intake of -3 PUFAs and
-6 PUFAs on atherosclerotic cardiovascular disease are controversial
and need to be validated in more rigorous randomized controlled trials. Moreover,
it is imperative to understand the differences between the impact of the
formulation and the distinct effects of the -3/-6 PUFAs on
lipid oxidation, inflammation, membrane structure/organization, cholesterol
domain formation, and endothelial cell function. FADS gene cluster variants have
an essential impact on atherosclerotic cardiovascular diseases and may be
potential therapeutic targets to prevent and treat these diseases. FADS
genotypes may be helpful for future stratification and targeting of dietary
recommendations in individuals carrying the FADS minor allele. PUFAs and
their metabolites are regulated by various factors, including FADS gene clusters
and dietary background; thus, future in-depth studies of the association between
PUFA and atherosclerosis will enrich the knowledge of the pathogenesis of
atherosclerosis and provide new therapies for its treatment.
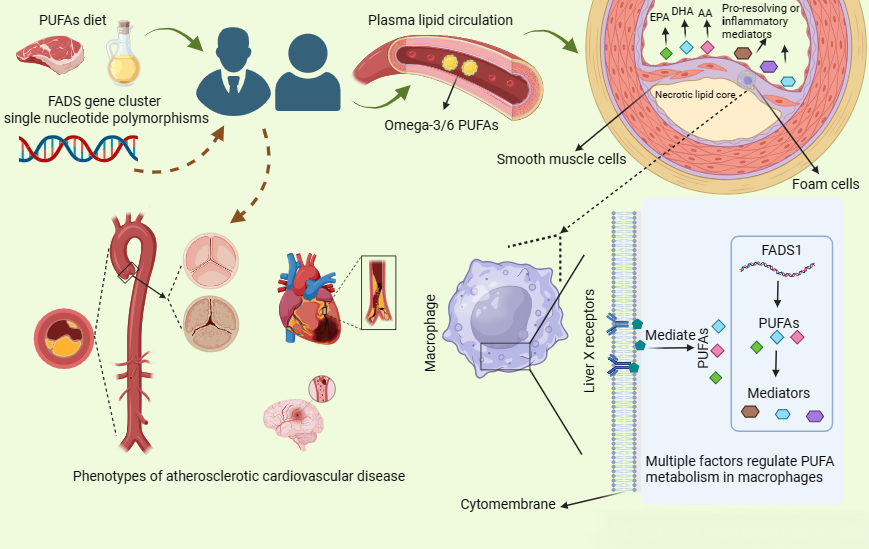 Fig. 3.
Fig. 3.
The progression of atherosclerosis is associated with PUFAs
intake, fatty acid desaturase, and FADS gene cluster variation. Intake of
polyunsaturated fatty acids has an important impact on atherosclerosis, and the
effect of FADS gene cluster variants on ASCVD, mainly by regulating the
metabolism of PUFAs in the body but more precisely by altering fatty acid
desaturase activity. LXRs and FADS1 expression regulate the metabolism
of PUFAs in macrophages, while the metabolites of PUFAs in macrophages are also
involved in the formation and progression of atherosclerosis. PUFAs,
polyunsaturated fatty acids; FADS, fatty acid desaturase; ASCVD, atherosclerotic
cardiovascular disease; LXRs, Liver X receptors; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; AA, arachidonic acid.
Author Contributions
QLL drafted the manuscript and participated in its design and so on. ZXL, DW and
SW conceived of the study, and participated in its coordination and helped to
draft the manuscript and so on. All authors read and approved the final
manuscript. All authors have participated sufficiently in the work and agreed to
be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
Not applicable.
Acknowledgment
We would like to express our gratitude to all those who helped us during the
writing of this manuscript. Thanks to all the peer reviewers for their opinions
and suggestions.
Funding
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
 Fig. 1.
Fig. 1. Fig. 2.
Fig. 2. Fig. 3.
Fig. 3.