- Academic Editor
Chronic kidney disease (CKD) and atrial fibrillation (AF) are associated with significant cardiovascular morbidity and mortality. Recent studies have highlighted an increased prevalence and incidence of AF in patients with CKD. This article aims to provide a comprehensive review of current management strategies and considerations of treating atrial fibrillation with concomitant CKD. Potential electrophysiological mechanisms between AF and CKD are explored. Current evidence and literature focusing on pharmacological rate and rhythm control along with procedural intervention is reviewed and presented. The management of AF and CKD together is complex, but particularly pertinent when considering the close cyclical relationship in the progression of both diseases.
Atrial Fibrillation (AF) is the most common sustained cardiac arrythmia with an
estimated prevalence of 1–2% in the general population [1]. In Europe the
number of adults affected by AF in 2010 was estimated to be 8.8 million (95% CI
6.5–12.3 million) which reflects 1.8% of the adult population aged
Although there are several traditional cardiovascular risk factors associated with chronic kidney disease (CKD), it is important to acknowledge the role of non-traditional risk factors, such as metabolic acidosis, oxidative stress, uraemia, chronic inflammation, anaemia, disrupted mineral bone homeostasis and chronic volume overload, in individuals with advanced CKD that do not respond to current recommended risk reduction strategies [4, 5, 6]. The presence of AF and CKD provides a clinical challenge with regards to pharmacological management, anti-thrombotic therapy, and whether to pursue a rate or rhythm control strategy. Up to 30% of patients diagnosed with AF have stage III-V CKD [7].
The initiation of AF is caused by a complex interaction between a trigger and substrate. It is the modification of the anatomical and/or electrical properties of the atria that gives rise to the underlying substrate. Cardiac chamber remodelling, in particular of the left atrium, which occurs secondary to sustained volume overload, elevated filling pressure and contractile dysfunction provides the substrate necessary for initiation, propagation and maintenance of AF [8]. Elevated atrial pressures may be found in patients with CKD due its association with hypertension. Therefore, the mechanical stress exerted on the atria, overtime, may result in electrophysiological remodelling which leads to the development of AF [9].
Overall, the development of AF in patients with CKD is multifaceted with other potentially relevant components including inflammation, renin-aldosterone-angiotensin-system (RAAS) activation, electrolyte abnormalities, anaemia and uraemia [10, 11, 12, 13].
The RAAS is an endocrine and paracrine system which has an important role in the regulation and modulation of renal, cardiovascular and pulmonary processes [14]. The RAAS cascade is also key in the progression of CKD [15]. Studies have previously suggested the integral role of RAAS in the pathogenesis of AF. Angiotensin II (ATII) plays a key role in fibroblast proliferation, matrix protein accumulation and subsequent interstitial fibrosis [16]. Furthermore, the elevation in left ventricular end diastolic pressure (LVEDP) caused by ATII will lead to a subsequent rise in left atrial pressure, particularly in patients with hypertension and heart failure [17, 18]. The secondary effects of atrial dilatation include alteration of ion channels and shortened refractory periods. This has been demonstrated in animal studies. Ravelli et al. [19] published a study in 1997 which demonstrated that in animal studies, increases in atrial pressure resulted in shortening of the atrial effective refractory periods (AERPs) and thus increased the susceptibility to AF. Termination of AF was observed on relieving the atrial stretch.
In patients with AF, heart failure and hypertension there may be prolonged
activation of the RAAS, resulting in elevated myocardial tissue levels of angiotensin converting enzyme (ACE).
There is a resultant up-regulation of ATII receptors which promote inflammatory
response and fibrosis. The atrial remodelling that then occurs provides the
substrate for sustaining AF. This cascade of events is summarised in Fig. 1 [20, 21, 22]. The atria appear to exhibit a greater susceptibility to fibrosis in
comparison to the ventricles through the involvement of three interconnected
pathways; RAAS, transforming growth factor
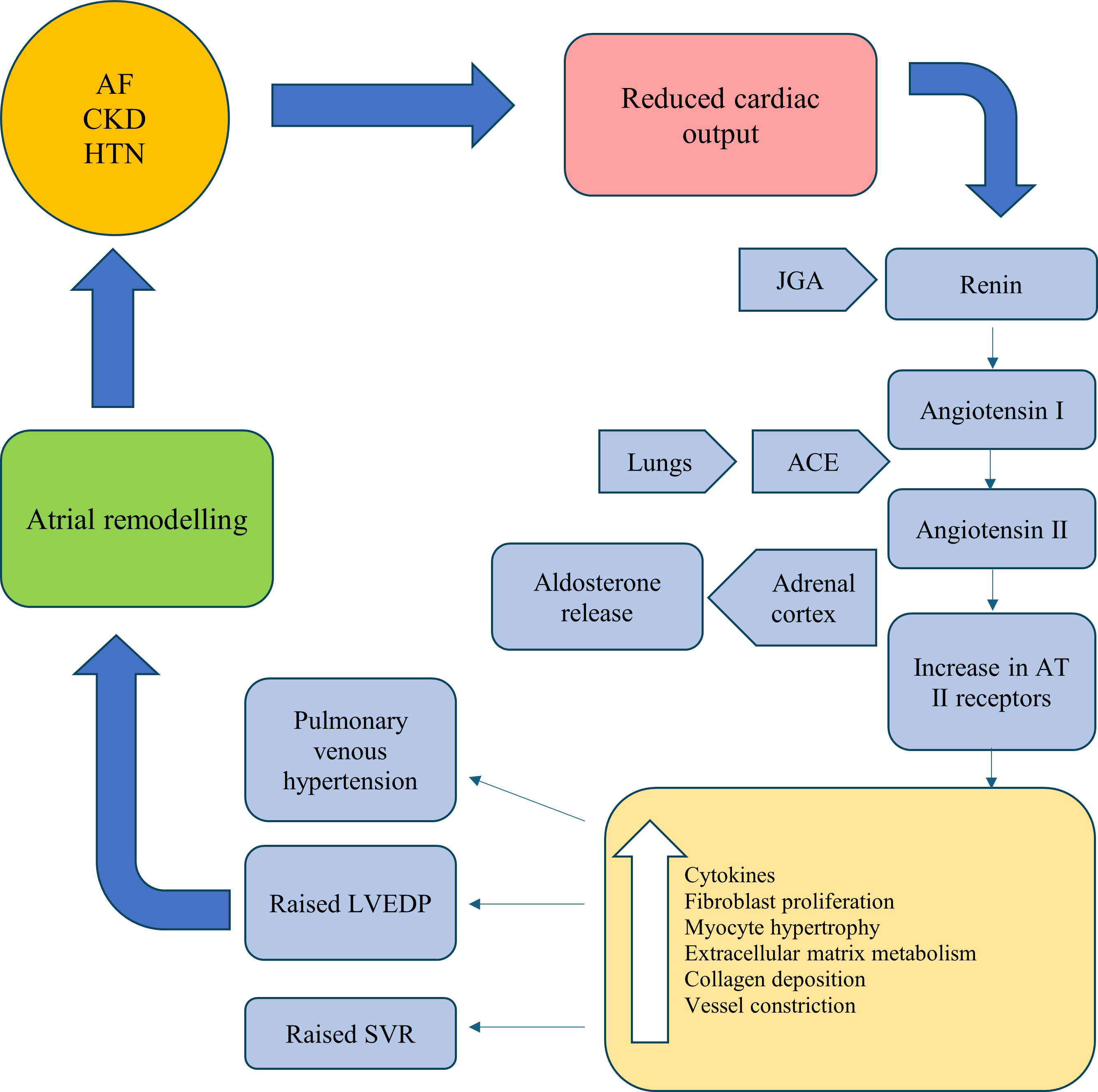 Fig. 1.
Fig. 1.A summary of the RAAS cascade in the pathogenesis of AF. AF, atrial Fibrillation; CKD, chronic kidney disease; HTN, hypertension; JGA, juxtaglomerular apparatus; AT, angiotensin; ACE, angiotensin converting enzyme; LVEDP, left ventricular end diastolic pressure; SVR, systemic vascular resistance; RAAS, renin-aldosterone-angiotensin-system.
Angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARBs) are among the most established and studied antihypertensive agents that provide renal and cardiovascular benefits for CKD patients [25, 26, 27, 28]. This is likely attributed to their established efficacy in favourably modifying the structure and function of the vasculature along with the inhibition of the ATII effect of cardiac myocytes, renal glomerular pericytes, and the vascular endothelium [29, 30, 31]. ACEi have additionally shown promising application and efficacy in the management of AF potentially though favourable effects on atrial electrical, structural and functional remodelling [32, 33, 34, 35]. A prospective study conducted by Boldt et al. [36] reported that patients with AF that were treated with ACEi, observed an attenuation of the atrial structural remodelling along with a preservation of atrial microcapillaries. Healey et al. [37] conducted a systematic review and meta-analysis where they concluded that ACEi and ARBs exhibited an efficacy in AF prevention, albeit in patients with systolic left ventricular dysfunction or LV hypertrophy. Overall, the current evidence base in literature gives support to the role and benefit of ACEi in reducing the incidence of AF and severity of atrial fibrosis.
There is a strong association between AF and stroke secondary to cerebral
embolism [38]. Patients with CKD are at an increased risk of stroke and in those
patients the risk of stroke is thought to be 5–30 times higher, particularly if
they have end-stage kidney disease (ESKD) managed with maintenance dialysis [39, 40]. In patients with CKD, the prevalence of AF is very high when compared to the
general population. In patients with an estimated glomerular filtration rate (eGFR)
Airy et al. [44] concluded from their data that in non-dialysis dependent CKD concurrent AF has been associated with a higher all-cause cardiovascular mortality. Table 1 (Ref. [45, 46, 47, 48, 49]) summarises all-cause mortality reported by primary studies comparing outcomes in patients with atrial fibrillation and chronic kidney disease.
| All-cause mortality | ||||||||
| Study | Country | Study design | Sample size/n | Effect estimate | Comparison categories | Mean follow up/years | Results | p value |
| Hsu et al. [45] | Taiwan | Cohort study | 16,451 | HR (95% CI) | CKD: Prevalent AF vs Non-AF -Age |
4.72 |
1.98 (1.71–2.29) | |
| Hsu et al. [45] | Taiwan | Cohort study | 16,451 | HR (95% CI) | CKD: Incident AF vs Non-AF-Age |
4.72 |
2.07 (1.83–2.33) | 0.529 |
| Hsu et al. [45] | Taiwan | Cohort study | 16,451 | HR (95% CI) | CKD: Prevalent AF vs Non-AF-Age |
4.72 |
1.78 (1.68–1.88) | |
| Hsu et al. [45] | Taiwan | Cohort study | 16,451 | HR (95% CI) | CKD: Incident AF vs Non-AF-Age |
4.72 |
2.25 (2.12–2.4) | 0.529 |
| Olesen et al. [46] | Denmark | Retrospective study | 132,372 | HR (95% CI) | AF: Non-renal disease vs Non-ESRD renal disease | * | 2.37 (2.3–2.44) | |
| Olesen et al. [46] | Denmark | Retrospective study | 132,372 | HR (95% CI) | AF: Non-renal disease vs ESRD renal disease | * | 3.35 (3.13–3.58) | |
| Abbott et al. [47] | United States | Cohort study | 3374 | HR (95% CI) | Chronic dialysis patients: AF vs Sinus Rhythm | 2.92 |
1.54 (1.19–1.99) | |
| Banerjee et al. [48] | * | Prospective study | 5912 | HR (95% CI) | AF patients: eGFR 30–59 vs eGFR |
2.45 | 1.98 (1.66–2.35) | |
| Banerjee et al. [48] | * | Prospective study | 5912 | HR (95% CI) | AF patients: eGFR |
2.45 | 4.31 (3.27–5.68) | |
| Hung et al. [49] | Taiwan | Case-control study | 11,019 | HR (95% CI) | ESRD: AF vs Non-AF | **1.8, 3.3 | 1.36 (1.147–1.617) | 0.0004 |
| Hung et al. [49] | Taiwan | Case-control study | 11,019 | HR (95% CI) | Non-ESRD: AF vs Non-AF | **2.8, 4.4 | 1.838 (1.538–2.197) | |
HR, hazard ratio; ESRD, end stage renal disease; AF, atrial fibrillation; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
*Data was not identified on article.
**Data was reported as median follow-up duration for individual sub-cohorts
rather than mean follow-up duration.
The use of oral anticoagulants (OACs) in patients with CKD is controversial and
careful consideration must be taken when deciding to start this particular group
of patients on OAC. Balancing the risk of bleeding and clotting is a recurring
challenge in clinical practice, especially in individuals requiring renal
replacement therapy (Table 2 (Ref. [50]). Given that patients with AF and CKD
undergo changes in drug pharmacokinetics in addition to a greater propensity for
bleeding, the overall net benefit is difficult establish (Table 3 (Ref. [50])). A
Serum creatinine concentration
| Mechanisms of thrombosis in patients with CKD stage 3–5/non-dialysis (ND) |
| Hypercoagulability ( |
| Greater degree of higher coagulability compared to fibrinolysis |
| Stasis and turbulence of blood flow |
| Platelet dysfunction |
CKD, chronic kidney disease; ND, non-dialysis.
| Mechanisms of increased bleeding events in patients with CKD stage 5 on dialysis |
| Accumulation of uraemic toxins and Guanidnosuccinic acid |
| Abnormal platelet adhesion and aggregation |
CKD, chronic kidney disease.
The evidence base for OAC in AF patients with severe CKD has been relatively
scarce as many of the landmark RCTs that contributed to the evidence,
systematically excluded severe CKD [53, 54, 55, 56]. In addition, there was an
under-representation of patients in the moderate-to-severe CKD category. A
systematic review and meta-analysis of observational studies was conducted by
Chokesuwattanaskul et al. [57] reported that apixaban was associated
with a lower risk of major bleeding events compared to warfarin in patients with
advanced CKD and end stage renal disease (ESRD), while risk of thromboembolic events were overall similar.
The findings were also consistent with a retrospective matched-cohort study
conducted by Siontis et al. [58]. The overall uncertainty
surrounding OAC in AF and CKD is reiterated by a lack of consensus comparing
international guidelines. The Canadian Cardiovascular Society (CCS) 2014
guidelines indicated that VKA can be considered in patients exhibiting an eGFR
between 15–30 mL/min that are not established on renal replacement therapy (RRT) while they advise against
OAC for ESRD [59]. The American College of Cardiology/American Heart
Association/Heart Rhythm Society (ACC/AHA/HRS) guidelines advocated for NOAC
usage in CrCl down to 15 mL/min and VKA prescription irrespective of renal
function or RRT status [60]. The European Society of Cardiology (ESC) indicate
that OAC can be prescribed safely in CrCl
To conclude, at present, the evidence-based recommendations for anticoagulation in patients with AF and CKD indicate that there is a benefit in those with CKD stage 2–3 and there is consensus of net benefit for select patients with CKD stage 4, however, in patients with CKD stage 5 there is uncertainty and likely net harm and in these cases the decision to commence OAC should be taken on a case by case basis.
Patients with CKD or ESKD are often excluded from trials studying rate vs rhythm control and therefore there is a lack of evidence on how to manage AF in patients with CKD [63]. The decision to pursue a rate or rhythm control strategy in patients with CKD depends on their individual characteristics such as co-morbidities, duration of AF, symptom severity, contraindications to the use of anti-arrhythmic drugs (AADs) and the patient’s personal preference [64]. Overall the indications and considerations for a rhythm control strategy in CKD patients is similar to the general populations. There is a paucity of RCTs that have evaluated specific anti-arrhythmic strategies of rate vs. rhythm control in patients with CKD or ESRD. A post hoc analysis of the Global Utilization of Streptokinase and t-PA for Occluded Coronary Arteries (GUSTO) III trial reported that neither rate or rhythm control strategy significantly impacted short term or long-term mortality, irrespective of renal function.
The common drug classes administered for rate control include beta-blockers, non-dihydropyridine calcium channel blockers, and digoxin [65]. Water-soluble pharmacological agents are prone to accumulation in CKD due to impaired renal elimination and thus water-soluble beta-blocker therapies such as atenolol should typically be avoided [65]. Bisoprolol exhibits a mixed metabolism profile that may require dose adjustment based on the degree of renal impairment. Carvedilol is a lipophilic beta-blocker and exhibits minimal renal elimination with dose adjustments not considered to be required for CKD [66]. Digoxin is typically avoided in severe CKD as majority of the agent undergoes renal elimination [67]. The usage in CKD is complicated by a narrow therapeutic index, long half-life, and predisposition to arrhythmogenesis in the presence of abnormalities such as hypokalaemia which can occur during dialysis [68]. Yang et al. [67] conducted a population-based cohort study and reported an association of increased mortality with CKD. Although non-dihydropyridine calcium channel blockers such as Diltiazem and Verapamil can be used, these should be avoided in patients with left ventricular systolic dysfunction [12].
Rhythm control maybe the favoured option in patients where rate control is
difficult to achieve, the patient is young or there is evidence of tachycardia
mediated cardiomyopathy. There are several AADs which can be used in patients
with CKD, however, they must be prescribed with caution in view of renal
clearance as well as proarrhythmic risks in patients with structural heart
disease (see Table 4 (Ref. [69, 70, 71])). Amiodarone is among the most common
anti-arrhythmic agents used to treat AF and is neither eliminated through the
renal system or dialyzable. A large data set retrospective study conducted by
Ullal et al. [72] conveyed that amiodarone does not negatively affect
survival in patients with ESRD. The propensity for adverse events/organ toxicity
secondary to amiodarone in patients with CKD is currently yet to be established.
A prospective, nationwide registry of AF patients reported that Amiodarone was
the most commonly prescribed anti-arrhythmic in stage IV/V CKD (68.6%,
p
| Anti-arrhythmic drug | Metabolism/clearance | Caution |
| Propafenone | Liver metabolism/Renal excretion | Avoid in patients with heart failure & significant left ventricular hypertrophy (LVH). |
| Sotalol | Not metabolised/renally excreted | Pro-arrhythmic in CKD, hypomagnesia, hypokalaemia. Increased risk of Torsades de pontes (TdP) in patients on dialysis [70]. |
| Dialyzable – administer maintenance dose after dialysis. | ||
| Amiodarone | Liver metabolism/Biliary excretion | Thyroid dysfunction, pulmonary toxicity – even at low doses. |
| Flecanide | Minimal liver metabolism/renal excretion | Avoid in patients with severe CKD due to increased risk of toxicity [71]. |
| Avoid in patients with significant structural heart disease [69]. |
AADs, antiarrhythmic drugs; CKD, chronic kidney disease; AF, atrial fibrillation.
A rhythm control strategy using direct current cardioversion (DCCV) has limited and inconsistent evidence. One study assessing patients with CKD and post-myocardial infarction AF concluded that 70% of patients with CKD and managed with DCCV were discharged in sinus rhythm, compared to 84% who had preserved renal function [75]. Schmidt et al. [76] also observed that patients with AF and moderately or severely impaired renal function were more likely to have recurrence. In contrast to previously mentioned studies, Reinecke et al. [7] reported findings from a large nationwide prospective registry and indicated that the success rate of restored sinus rhythm was very similar with 79.5–82.9% of patients successfully treated with DCCV irrespective of their renal function. In addition, Schmidt et al. [76] reported that patients with moderate renal impairment showed an increase in eGFR where sinus rhythm was maintained for 1 month post DCCV. Although DCCV may be considered for highly symptomatic or relatively recent onset AF, it is typically insufficient to maintain normal sinus rhythm in patients with long standing persistent AF, permanent AF and/or severe LA dilation, thus long term anti-arrhythmic medications, catheter ablation or a pace and ablate strategy may be considered depending on the patients’ symptoms and/or the presence heart failure.
Although catheter ablation is a well-established management option for rhythm
control in AF, the evidence base and effect of CKD on outcomes in patients with
CKD who have an ablation is limited. There are several predictors of recurrence
of AF in patients undergoing catheter ablation (CA) such as enlarged left atrium
(LA) and persistent AF [77]. Several studies have analysed the impact of impaired
renal function on CA. Chao et al. [78] looked at 232 patients
who underwent CA and concluded that in patients with PAF, a reduced eGFR was
associated with a higher recurrence rate. Naruse et al. [79] studied 221
patients with CKD (defined as an eGFR
Overall, it appears that the evidence base for catheter ablation in patients with AF and CKD is limited. While there appears to be potentially some benefit when successfully conducted, recurrence rates especially in increasing severities of CKD, are significant and considerable. As a result, further robust evaluation into outcomes corresponding to specific CKD patient groups might be beneficial to optimise patient selection.
Managing patients with concomitant AF and CKD is complex. The limited evidence base for managing these patients can present a challenge to the physician when considering management options. There is a close cyclical relationship between AF and CKD and the progression of both diseases [84]. As stated in the ESC guidelines a shared decision-making process is required between the physician and the patient [61]. This pertains to all aspects of AF management in patients with CKD, including weighing up the risks and benefits of OAC, pursuing a rate or rhythm control strategy and deciding upon CA where there is likely to be of clinical benefit.
Study conception and design – BS, ZV, GAN, JOB; Acquisition of data– BS, AM; Analysis and interpretation of data – BS, AM; Drafting of manuscript – BS, AM, IK, KLH; Critical revision of manuscript– BS, AM, IK, ZV, JOB, GAN. KLH was involved in the study conception and design along with drafting of manuscript and critical revision of manuscript; IK was involved in the acquisition of data along with analysis and; interpretation of data and drafting of manuscript and critical revision of manuscript; JOB was involved in the study conception and design along with critical revision of manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
Not applicable.
G.A.N. has been supported by a British Heart Foundation Programme Grant (RG/17/3/32774) and the Medical Research Council Biomedical Catalyst Developmental Pathway Funding Scheme (MR/S037306/1).
G.A.N. has been supported by a British Heart Foundation Programme Grant (RG/17/3/32774) and the Medical Research Council Biomedical Catalyst Developmental Pathway Funding Scheme (MR/S037306/1).
The authors declare no conflict of interest.
Publisher’s Note: IMR Press stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
